Bioactive Potential of Minor Italian Olive Genotypes from Apulia, Sardinia and Abruzzo
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Olive Sampling
2.2. Genetic Characterization
2.3. Oil Extraction and Characterization
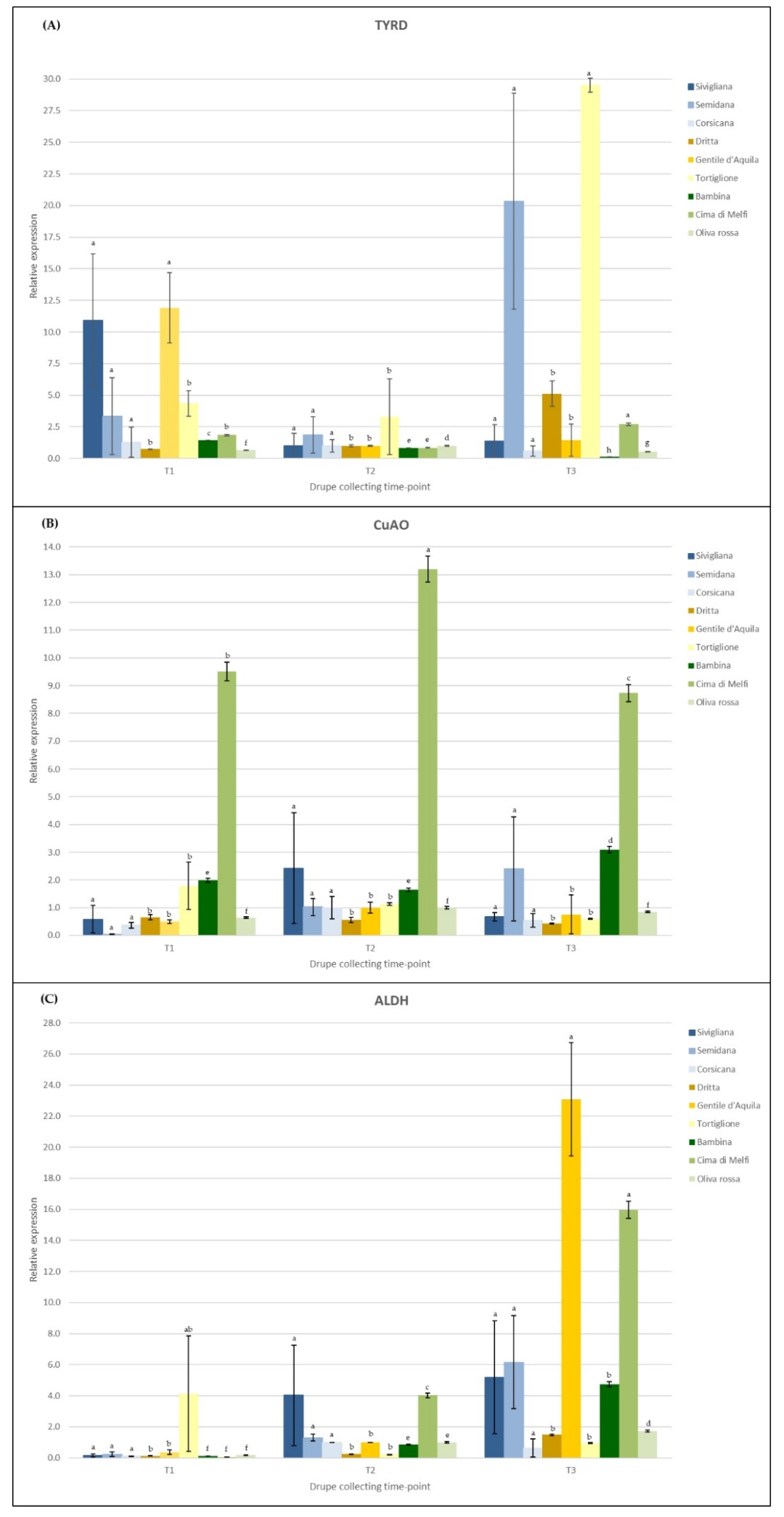
2.4. Polyphenolic Compound Gene Expression
2.5. Statistical Analysis
3. Results
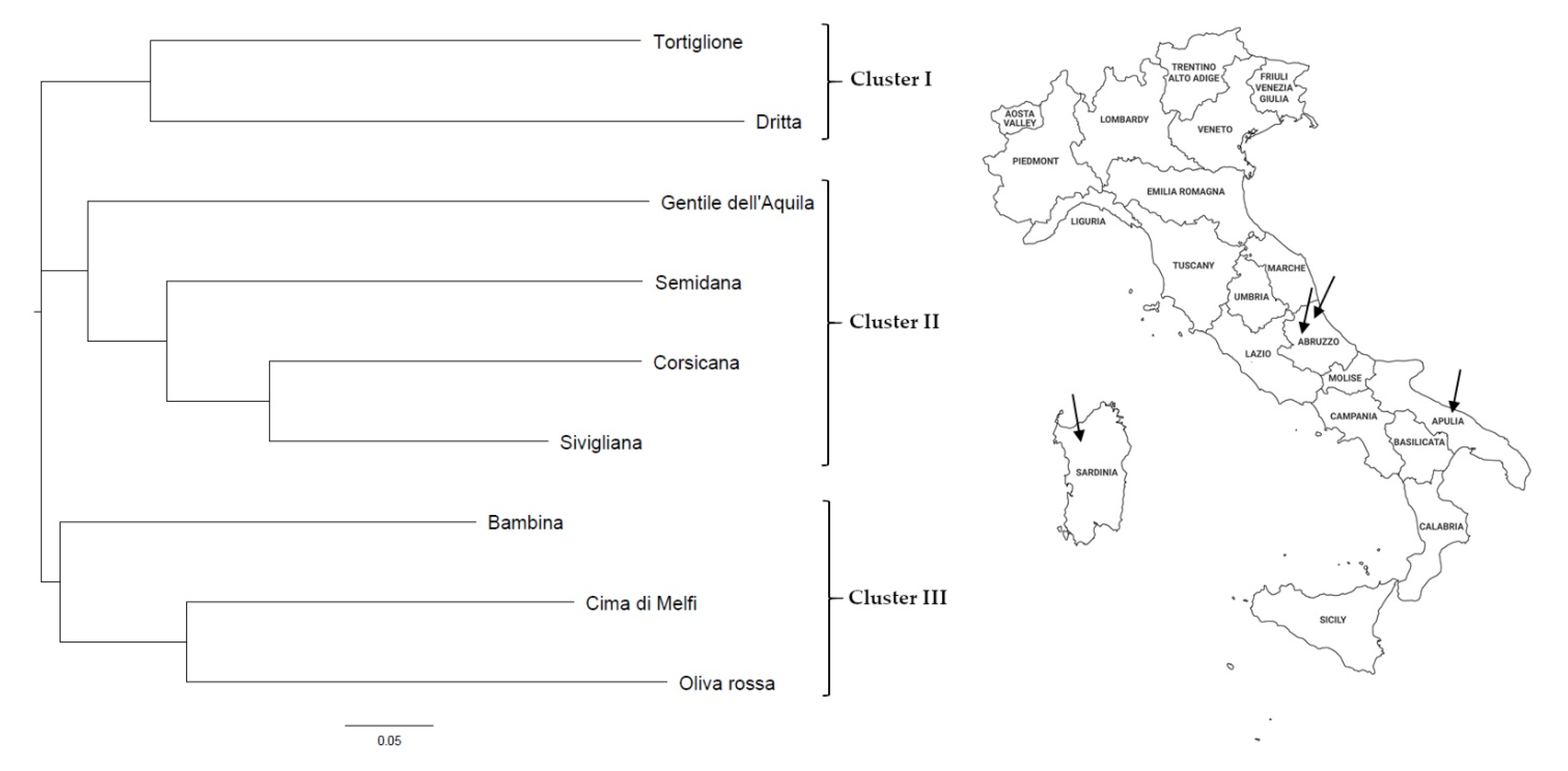
3.1. Genetic Characterization
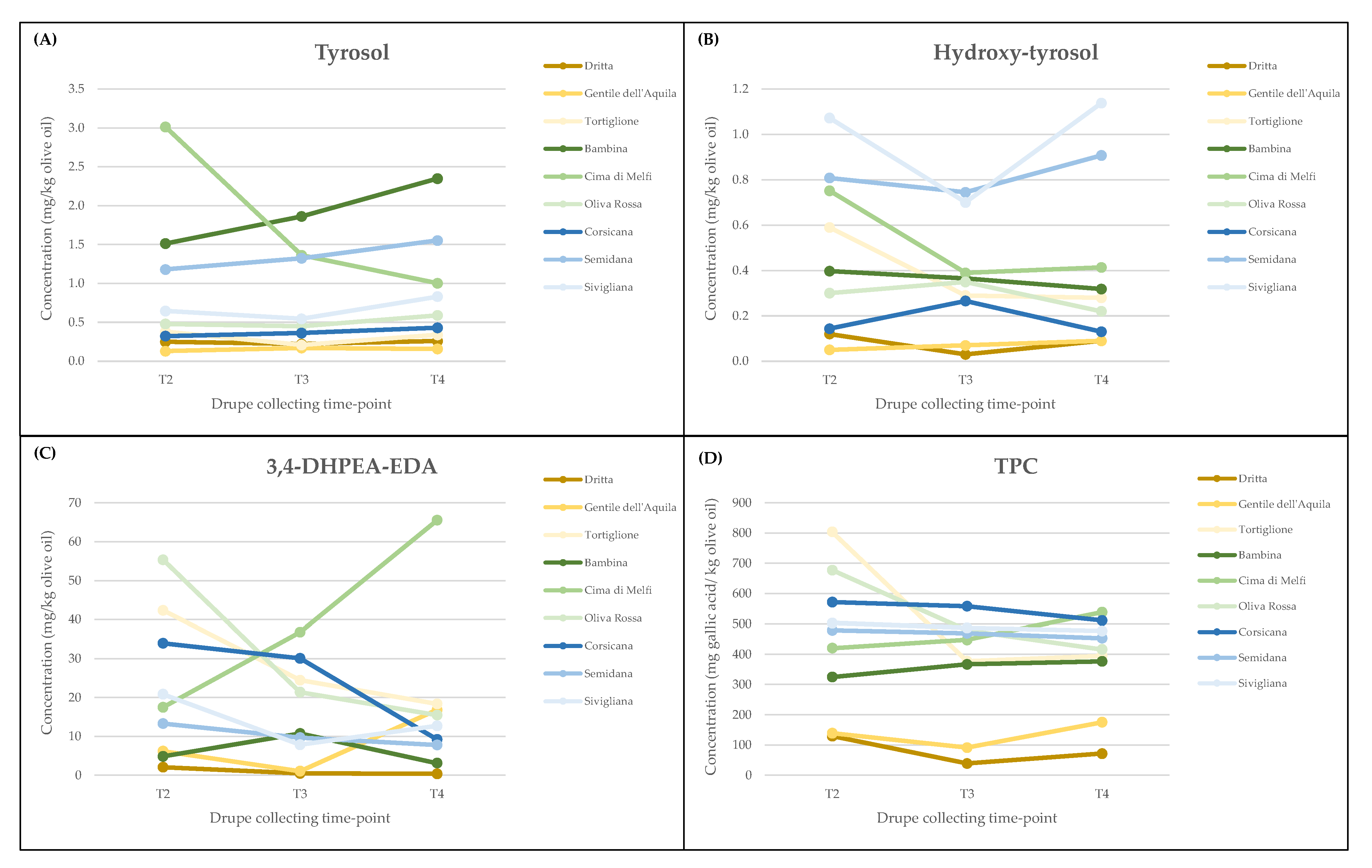
3.2. Monovarietal Olive Oil Phenolic Content
3.3. Gene Expression Analysis
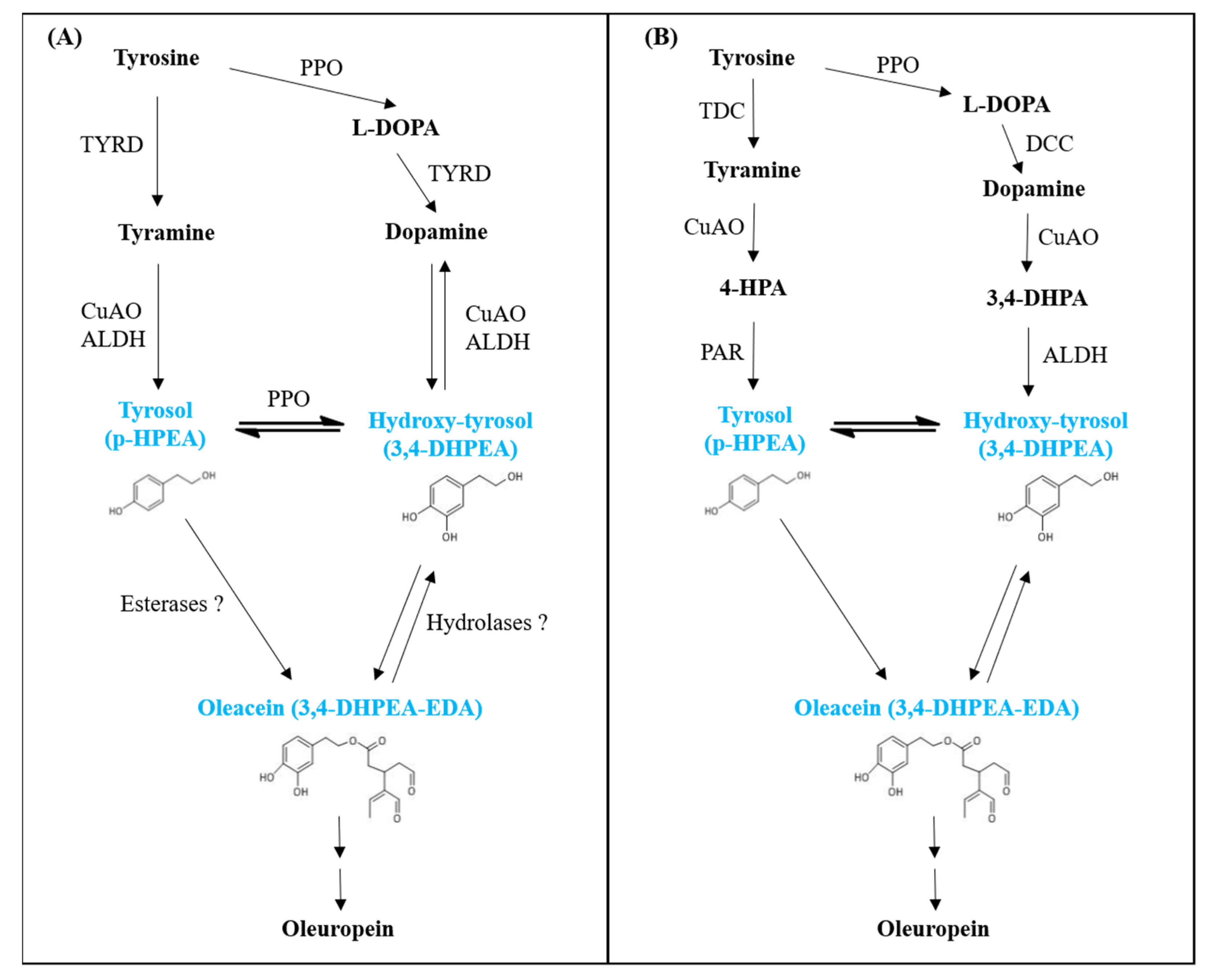
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Muzzalupo, I. Olive Germplasm–Italian Catalogue of Olive Varieties; InTech: Rijeka, Croatia, 2012; p. 430. [Google Scholar] [CrossRef]
- Bartolini, G.; Cerreti, S.; Briccoli Bati, C.; Stefani, F.; Zelasco, S.; Perri, E.; Petruccelli, R. Oleadb: Worldwide database for the management of genetic resources of olive (Olea europaea L.). In Proceedings of the X National Congress on Biodiversity, Rome, Italy, 3–5 September 2014; pp. 18–23. [Google Scholar]
- Miazzi, M.M.; di Rienzo, V.; Mascio, I.; Montemurro, C.; Sion, S.; Sabetta, W.; Vivaldi, G.A.; Camposeo, S.; Caponio, F.; Squeo, G.; et al. REGEROP: An integrated project for the recovery of ancient and rare olive germplasm. Front. Plant Sci. 2020, 11, 73. [Google Scholar] [CrossRef]
- Rotondi, A.; Cultrera, N.G.; Mariotti, R.; Baldoni, L. Genotyping and evaluation of local olive varieties of a climatically disfavoured region through molecular, morphological and oil quality parameters. Sci. Hortic. 2011, 130, 562–569. [Google Scholar] [CrossRef]
- Muzzalupo, I.; Vendramin, G.G.; Chiappetta, A. Genetic biodiversity of Italian olives (Olea europaea L.) germplasm analyzed by SSR markers. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- Mousavi, S.; Mariotti, R.; Regni, L.; Nasini, L.; Bufacchi, M.; Pandolfi, S.; Baldoni, L.; Proietti, P. The first molecular identification of an olive collection applying standard simple sequence repeats and novel expressed sequence tag markers. Front. Plant Sci. 2017, 8, 1283. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, L.; Busconi, M. Recent developments in olive Olea europaea L.) genetics and genomics: Applications in taxonomy, varietal identification, traceability and breeding. Plant Cell Rep. 2017, 36, 1345–1360. [Google Scholar] [CrossRef] [PubMed]
- El Bakkali, A.; Essalouh, L.; Tollon, C.; Rivallan, R.; Mournet, P.; Moukhli, A.; Hayat Zaher, H.; Mekkaoui, A.; Hadidou, A.; Sikaoui, L.; et al. Characterization of worldwide olive germplasm banks of Marrakech (Morocco) and Córdoba (Spain): Towards management and use of olive germplasm in breeding programs. PLoS ONE 2019, 14, e0223716. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, C.; Miazzi, M.M.; Pasqualone, A.; Fanelli, V.; Sabetta, W.; di Rienzo, V. Traceability of PDO olive oil ‘‘terra di Bari’’ using high resolution melting. J. Chem. 2015, 2015. [Google Scholar] [CrossRef]
- Sabetta, W.; Miazzi, M.M.; di Rienzo, V.; Fanelli, V.; Pasqualone, A.; Montemurro, C. Development and application of protocols to certify the authenticity and the traceability of Apulian typical products in olive sector. Riv. Ital. Sost. Grasse 2017, 94, 37–43. [Google Scholar]
- Xanthopoulou, A.; Ganopoulos, I.; Bosmali, I.; Tsaftaris, A.; Madesis, P. DNA fingerprinting as a novel tool for olive and olive oil authentication, traceability, and detection of functional compounds. In Olives and Olive Oil as Functional Foods: Bioactivity, Chemistry and Processing; Shahidi, F., Kiritsakis, A., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 587–601. [Google Scholar]
- Gomes, S.; Breia, R.; Carvalho, T.; Carnide, V.; Martins-Lopes, P. Microsatellite High-Resolution Melting (SSR-HRM) to track olive genotypes: From field to olive oil. J. Food Sci. 2018, 8, 2415–2423. [Google Scholar] [CrossRef]
- Piarulli, L.; Savoia, M.A.; Taranto, F.; D’Agostino, N.; Sardaro, R.; Girone, S.; Gadaleta, S.; Fucilli, V.; De Giovanni, C.; Montemurro, C.; et al. A robust DNA isolation protocol from filtered commercial olive oil for PCR-based fingerprinting. Foods 2019, 8, 462. [Google Scholar] [CrossRef]
- International Olive Council (IOC). 2019. Available online: https://www.internationaloliveoil.org (accessed on 5 April 2021).
- Uylaşer, V.; Yildiz, G. The historical development and nutritional importance of olive and olive oil constituted an important part of the Mediterranean diet. Crit. Rev. Food Sci. Nutr. 2014, 54, 1092–1101. [Google Scholar] [CrossRef]
- Tuck, K.L.; Hayball, P.J. Major phenolic compounds in olive oil: Metabolism and health effects. J. Nutr. Biochem. 2002, 13, 636–644. [Google Scholar] [CrossRef]
- Harwood, J.L.; Yaqoob, P. Nutritional and health aspects of olive oil. Eur. J. Lipid Sci. Technol. 2002, 104, 685–697. [Google Scholar] [CrossRef]
- Stark, A.H.; Madar, Z. Olive oil as a functional food: Epidemiology and nutritional approaches. Nutr. Rev. 2002, 60, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Piroddi, M.; Albini, A.; Fabiani, R.; Giovannelli, L.; Luceri, C.; Natella, F.; Rosignoli, P.; Rossi, T.; Taticchi, A.; Servili, M.; et al. Nutrigenomics of extra-virgin olive oil: A review. BioFactors 2017, 43, 17–41. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, G.K.; Keast, R.S.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.; Smith, A.B.; Breslin, P.A.S. Ibuprofen-like activity in extra-virgin olive oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef] [PubMed]
- Servili, M.; Esposto, S.; Fabiani, R.; Urbani, S.; Taticchi, A.; Mariucci, F.; Selvaggini, R.; Montedoro, G.F. Phenolic compounds in olive oil: Antioxidant, health and organoleptic activities according to their chemical structure. Inflammopharmacology 2009, 17, 1–9. [Google Scholar] [CrossRef]
- Lucas, L.; Russell, A.; Russell, K. Molecular mechanisms of inflammation. Anti-inflammatory benefits of virgin olive oil and the phenolic compound oleocanthal. Curr. Pharm. Des. 2011, 17, 754–768. [Google Scholar] [CrossRef]
- Yubero-Serrano, E.M.; Lopez-Moreno, J.; Gomez-Delgado, F.; Lopez-Miranda, J. Extra virgin olive oil: More than a healthy fat. Eur. J. Clin. Nutr. 2019, 72, 8–17. [Google Scholar] [CrossRef]
- De la Torre-Carbot, K.; Chávez-Servín, J.L.; Jaúregui, O.; Castellote, A.I.; Lamuela-Raventós, R.M.; Nurmi, T.; Poulsen, H.E.; Gaddi, A.V.; Kaikkonen, J.; Zunft, H.F.; et al. Elevated circulating LDL phenol levels in men who consumed virgin rather than refined olive oil are associated with less oxidation of plasma LDL. J. Nutr. 2010, 140, 501–508. [Google Scholar] [CrossRef]
- Calabriso, N.; Massaro, M.; Scoditti, E.; D’Amore, S.; Gnoni, A.; Pellegrino, M.; Storelli, C.; De Caterina, R.; Palasciano, G.; Carluccio, M.A. Extra virgin olive oil rich in polyphenols modulates VEGF-induced angiogenic responses by preventing NADPH oxidase activity and expression. J. Nutr. Biochem. 2016, 28, 19–29. [Google Scholar] [CrossRef]
- Storniolo, C.E.; Rosello-Catafau, J.; Pintó, X.; Mitjavila, M.T.; Moreno, J.J. Polyphenol fraction of extra virgin olive oil protects against endothelial dysfunction induced by high glucose and free fatty acids through modulation of nitric oxide and endothelin-1. Redox Biol. 2014, 2, 971–977. [Google Scholar] [CrossRef]
- Lockyer, S.; Rowland, I.; Spencer, J.P.E.; Yaqoob, P.; Stonehouse, W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomised controlled trial. Eur. J. Nutr. 2017, 56, 1421–1432. [Google Scholar] [CrossRef]
- Moreno, J.J. Effect of olive oil minor components on oxidative stress and arachidonic acid mobilization and metabolism by macrophages RAW 264.7. Free Radic. Biol. Med. 2003, 35, 1073–1081. [Google Scholar] [CrossRef]
- Martin, M.A.; Ramos, S.; Granado-Serrano, A.B.; Rodríguez-Ramiro, I.; Trujillo, M.; Bravo, L.; Goya, L. Hydroxy-tyrosol induces antioxidant/ detoxificant enzymes and Nrf2 translocation via extracellular regulated kinases and phosphatidylinositol-3-kinase/protein kinase B pathways in HepG2 cells. Mol. Nutr. Food Res. 2010, 54, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Giordano, E.; Davalos, A.; Visioli, F. Chronic hydroxy-tyrosol feeding modulates glutathione-mediated oxido-reduction pathways in adipose tissue: A nutrigenomic study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Orak, H.H.; Karamac, M.; Amarowicz, R.; Orak, A.; Penkacik, K. Genotype-related differences in the phenolic compound profile and antioxidant activity of extracts from olive (Olea europaea L.) leaves. Molecules 2019, 24, 1130. [Google Scholar] [CrossRef] [PubMed]
- Caponio, F.; Gomes, T.; Pasqualone, A. Phenolic compounds in virgin olive oils: Influence of the degree of olive ripeness on organ-oleptic characteristics and shelf-life. Eur. Food Res. Technol. 2001, 212, 329–333. [Google Scholar] [CrossRef]
- Siliani, S.; Mattei, A.; Innocenti, L.B.; Zanoni, B. Bitter taste and phenolic compounds in extra virgin olive oil: An empirical relationship. J. Food Qual. 2006, 29, 431–441. [Google Scholar] [CrossRef]
- Beltrán, G.; Ruano, M.T.; Jiménez, A.; Uceda, M.; Aguilera, M.P. Evaluation of virgin olive oil bitterness by total phenol content analysis. Eur. J. Lipid Sci. Technol. 2007, 109, 193–197. [Google Scholar] [CrossRef]
- Pedan, V.; Popp, M.; Rohn, S.; Nyfeler, M.; Bongartz, A. Characterization of phenolic compounds and their contribution to sensory properties of olive oil. Molecules 2019, 24, 2041. [Google Scholar] [CrossRef]
- Serrilli, A.M.; Padula, G.; Bianco, A.; Petrosino, L.; Ripa, V. Searching new olive (Olea europaea L.) cultivars: Analysis of phenolic fraction in olive selections derived from a breeding program. Adv. Hort. Sci. 2008, 22, 104–109. [Google Scholar]
- Riachy, E.M.; Priego-Capote, F.; Rallo, L.; Luque-de Castro, M.D.; León, L. Phenolic composition of virgin olive oils from cross breeding segregating populations. Eur. J. Lipid Sci. Tech. 2012, 114, 542–551. [Google Scholar] [CrossRef]
- Pérez Rubio, A.G.; León, L.; Sanz, C.; Rosa, R.D.L. Fruit Phenolic Profiling: A New Selection Criterion in Olive Breeding Programs. Front. Plant Sci. 2018, 9, 241. [Google Scholar] [CrossRef] [PubMed]
- Alagna, F.; Mariotti, R.; Panara, F.; Caporali, S.; Urbani, S.; Veneziani, G.; Esposto, S.; Taticchi, A.; Rosati, A.; Rao, R.; et al. Olive phenolic compounds: Metabolic and transcriptional profiling during fruit development. BMC Plant Biol. 2012, 12, 162. [Google Scholar] [CrossRef]
- Alagna, F.; Geu-Flores, F.; Kries, H.; Panara, F.; Baldoni, L.; O’Connor, S.E.; Osbourn, A. Identification and characterization of the iridoid synthase involved in oleuropein biosynthesis in olive (Olea europaea L.) fruits. J. Biol. Chem. 2015, 291, 5542–5554. [Google Scholar] [CrossRef] [PubMed]
- Mougiou, N.; Trikka, F.; Trantas, E.; Ververidis, F.; Makris, A.; Argiriou, A.; Vlachonasios, K.E. Expression of hydroxy-tyrosol and oleuropein biosynthetic genes are correlated with metabolite accumulation during fruit development in olive, Olea europaea, cv Koroneiki. Plant Physiol. Biochem. 2018, 128, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Guodong, R.; Jianguo, Z.; Xiaoxia, L.; Ying, L. Identification of putative genes for polyphenol biosynthesis in olive fruits and leaves using full-length transcriptome sequencing. Food Chem. 2019, 300, 125246. [Google Scholar] [CrossRef]
- Guodong, R.; Zhang, J.; Liu, X.; Li, X.; Wang, C. Combined Metabolome and Transcriptome Profiling Reveal Optimal Harvest Strategy Model Based on Different Production Purposes in Olive. Foods 2021, 10, 360. [Google Scholar] [CrossRef]
- Bandino, G.; Mulas, M.; Sedda, P.; Moro, C. Le varietà di Olivo Della Sardegna; Consorzio Interprovinciale per la Frutticoltura di Cagliari, Oristano e Nuoro: Cagliari, Italy, 2001; p. 256. [Google Scholar]
- Deiana, P.; Santona, M.; Dettori, S.; Molinu, M.G.; Dore, A.; Culeddu, N.; Azara, E.; Naziri, E.; Tsimidou, M.Z. Can all the Sardinian varieties support the PDO “Sardegna” virgin olive oil? Eur. J. Lipid Sci. Technol. 2019, 121, 1800135. [Google Scholar] [CrossRef]
- Lombardo, N.; Alessandrino, M.; Godino, G.; Madeo, A.; Ciliberti, A.; Pellegrino, M. Caratterizzazione della crescita, produttività, inoliazione e qualità dell’olio di 10 cultivar di olivo autoctone abruzzesi. In Proceedings of the National Congress “Maturazione e Raccolta Delle Olive: Strategie e Tecnologie Per Aumentare la Competitività in Olivicoltura”, ARSSA, Regione Abruzzo Alanno, Italy, Alanno (PE), Italy, 1 April 2006; p. 238. [Google Scholar]
- Albertini, E.; Torricelli, R.; Bitocchi, E.; Raggi, L.; Marconi, G.; Pollastri, L.; Di Minco, G.; Battistini, A.; Papa, R.; Veronesi, F. Structure of genetic diversity in Olea europaea L. cultivars from central Italy. Mol. Breed. 2011, 27, 533–547. [Google Scholar] [CrossRef]
- Squeo, G.; Difonzo, G.; Silletti, R.; Paradiso, V.M.; Summo, C.; Pasqualone, A.; Caponio, F.C. Bambina, una varietà minore pugliese: Profilo di maturazione, composizione delle drupe e caratterizzazione chimica dell’olio vergine. Riv. Ital. Sost. Grasse 2019, 94, 143–149. [Google Scholar]
- Squeo, G.; Silletti, R.; Mangini, G.; Summo, C.; Caponio, F. The potential of Apulian olive biodiversity: The case of Oliva Rossa Virgin Olive Oil. Foods 2021, 10, 369. [Google Scholar] [CrossRef] [PubMed]
- Spadoni, A.; Sion, S.; Gadaleta, S.; Savoia, M.A.; Piarulli, L.; Fanelli, V.; Di Rienzo, V.; Taranto, F.; Miazzi, M.M.; Montemurro, C.; et al. A simple and rapid method for genomic DNA extraction and microsatellite analysis in tree plants. J. Agric. Sci. Technol. 2019, 21, 1215–1226. [Google Scholar]
- Sefc, K.M.; Lopes, M.S.; Mendonça, D.; Rodrigues Dos Santos, M.; Laimer Da Câmara Machado, M.; Da Câmara Machado, A. Identification of microsatellite loci in olive (Olea europaea L.) and their characterization in Italian and Iberian olive trees. Mol. Ecol. 2000, 9, 1171–1193. [Google Scholar] [CrossRef]
- Carriero, F.; Fontanazza, G.; Cellini, F.; Giorio, G. Identification of simple sequence repeats (SSRs) in olive (Olea europaea L.). Theor. Appl. Genet. 2002, 104, 301–307. [Google Scholar] [CrossRef] [PubMed]
- De La Rosa, R.; James, C.M.; Tobutt, K.R. Using microsatellites for paternity testing in olive progenies. Hort. Sci. 2004, 39, 351–354. [Google Scholar] [CrossRef]
- Pasqualone, A.; Montemurro, C.; Summo, C.; Sabetta, W.; Caponio, F.; Blanco, A. Effectiveness of microsatellites DNA markers in checking the identity of PDO extra virgin olive oil. J. Agric. Food Chem. 2007, 55, 3857–3862. [Google Scholar] [CrossRef] [PubMed]
- Baldoni, L.; Cultrera, N.G.; Mariotti, R.; Ricciolini, C.; Arcioni, S.; Vendramin, G.G.; Buonamici, A.; Porceddu, A.; Sarri, V.; Ojeda, M.A.; et al. A consensus list of microsatellite markers for olive genotyping. Mol. Breed. 2009, 24, 213–231. [Google Scholar] [CrossRef]
- Boucheffa, S.; Miazzi, M.M.; di Rienzo, V.; Mangini, G.; Fanelli, V.; Tamendjari, A.; Pignone, D.; Montemurro, C. The coexistence of oleaster and traditional varieties affects genetic diversity and population structure in Algerian olive (Olea europaea L.) germplasm. Gen. Res. Crop Evol. 2017, 64, 379–390. [Google Scholar] [CrossRef]
- Di Rienzo, V.; Sion, S.; Taranto, F.; D’Agostino, N.; Montemurro, C.; Fanelli, V.; Sabetta, W.; Boucheffa, S.; Tamendjari, A.; Pasqualone, A.; et al. Genetic flow among olive populations within the Mediterranean basin. PeerJ 2018, 6, e5260. [Google Scholar] [CrossRef] [PubMed]
- Caponio, F.; Catalano, P. Hammer crushers vs disk crushers: The influence of working temperature on the quality and preservation of virgin olive oil. Eur. Food Res. Technol. 2001, 213, 219–224. [Google Scholar] [CrossRef]
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gómez-Caravaca, A.M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Lercker, G. Phenolic molecules in virgin olive oils: A survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decade Alessandra. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef] [PubMed]
- Zago, L.; Squeo, G.; Bertoncini, E.I.; Difonzo, G.; Caponio, F. Chemical and sensory characterization of Brazilian virgin olive oils. Food Res. Int. 2019, 126, 108588. [Google Scholar] [CrossRef] [PubMed]
- International Olive Council (IOC). Determination of Biophenols in Olive Oils by HPLC; COI/ T.20/Doc. No 29/Rev.1; International Olive Council: Madrid, Spain, 2017. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Baldoni, L.; Tosti, N.; Ricciolini, C.; Belaj, A.; Arcioni, S.; Pannelli, G.; Germanà, M.A.; Mulas, M.; Porceddu, A. Genetic structure of wild and cultivated olives in the central Mediterranean basin. Ann. Bot. 2006, 98, 935–942. [Google Scholar] [CrossRef]
- Dίez, C.M.; Trujillo, I.; Martinez-Uriroz, N.; Barranco, D.; Rallo, L.; Marfil, P.; Gaut, B.S. Olive domestication and diversification in the Mediterranean basin. New Phytol. 2015, 206, 436–447. [Google Scholar] [CrossRef]
- Erre, P.; Chessa, I.; Munoz-Diez, C.; Belaj, A.; Rallo, L.; Trujillo, I. Genetic diversity and relationships between wild and cultivated olives (Olea europaea L.) in Sardinia as assessed by SSR markers. Genet. Resour. Crop Evol. 2010, 57, 41–54. [Google Scholar] [CrossRef]
- Covas, M.I.; Ruiz-Gutiérrez, V.; De La Torre, R.; Kafatos, A.; Lamuela-Raventós, R.M.; Osada, J.; Visioli, F. Minor components of olive oil: Evidence to date of health benefits in humans. Nutr. Rev. 2006, 64 (Suppl. 4), S20–S30. [Google Scholar] [CrossRef]
- The European Parliament and The Council of the European Union. Commission Regulation (EU) No 1924/2006 of 20 December 2006 on Nutrition and Health Claims Made on Foods. Off. J. Eur. Union 2006, 1924. Available online: https://eur-lex.europa.eu/eli/reg/2006/1924/oj#document1 (accessed on 5 April 2021).
- Commission Regulation (EU) No 432/2012 of 16 May 2012 Establishing a List of Permitted Health Claims Made on Foods, Other than Those Referring to the Reduction of Disease Risk and to Children’s Development and Health. Available online: https://eur-lex.europa.eu/eli/reg/2012/432/oj (accessed on 5 April 2021).
- Malik, N.S.A.; Bradford, J.M. Recovery and stability of oleuropein and other phenolic compounds during extraction and processing of olive (Olea europaea L.) leaves. J. Food Agric. Environ. 2008, 6, 8–13. [Google Scholar]
- Baiano, A.; Terracone, C.; Viggiani, I.; Del Nobile, M.A. Effects of cultivars and location on quality, phenolic content and antioxidant activity of extra-virgin olive oils. J. Am. Oil Chem. Soc. 2013, 90, 103–111. [Google Scholar] [CrossRef]
- Miho, H.; Díez, C.M.; Mena-Bravo, A.; Sánchez de Medina, V.; Moral, J.; Melliou, E.; Magiatis, P.; Rallo, L.; Barranco, D.; Priego-Capote, F. Cultivar influence on variability in olive oil phenolic profiles determined through an extensive germplasm survey. Food Chem. 2018, 266, 192–199. [Google Scholar] [CrossRef]
- Bruno, L.; Picardi, E.; Pacenza, M.; Chiappetta, A.; Muto, A.; Gagliardi, O.; Muzzalupo, I.; Pesole, G.; Bitonti, M.B. Changes in gene expression and metabolic profile of drupes of Olea europaea L. cv Carolea in relation to maturation stage and cultivation area. BMC Plant Biol. 2019, 19, 428. [Google Scholar] [CrossRef]
- Talhaoui, N.; Gómez-Caravaca, A.M.; León, L.; De la Rosa, R.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Pattern of Variation of Fruit Traits and Phenol Content in Olive Fruits from Six Different Cultivars. J. Agric. Food Chem. 2015, 63, 10466–10476. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Palmero, D.; Romero-Segura, C.; García-Rodríguez, R.; Hernández, M.L.; Vaistij, F.E.; Graham, I.A.; Pérez, A.G.; Martínez-Rivas, J.M. An oleuropein b-glucosidase from olive fruit is involved in determining the phenolic composition of Virgin Olive Oil. Front. Plant Sci. 2017, 8, 1902. [Google Scholar] [CrossRef]
- Servili, M.; Selvaggini, R.; Esposto, S.; Taticchi, A.; Montedoro, G.; Morozzi, G. Health and sensory properties of virgin olive oil hydrophilic phenols: Agronomic and technological aspects of production that affect their occurrence in the oil. J. Chromatogr. A 2004, 1054, 113–127. [Google Scholar] [CrossRef]
- Paiva-Martins, F.; Fernandes, J.; Rocha, S.; Nascimento, H.; Vitorino, R.; Amado, F.; Santos-Silva, A. Effects of olive oil polyphenols on erythrocyte oxidative damage. Mol. Nutr. Food Res. 2009, 53, 609–616. [Google Scholar] [CrossRef]
- Artajo, L.S.; Romero, M.P.; Motilva, M.J. Transfer of phenolic compounds during olive oil extraction in relation to ripening stage of the fruit. J. Sci. Food Agric. 2006, 86, 518–527. [Google Scholar] [CrossRef]
- Squeo, G.; Difonzo, G.; Summo, C.; Crecchio, C.; Caponio, F. Study of the influence of technological coadjuvants on enzyme activities and phenolic and volatile compounds in virgin olive oil by a response surface methodology approach. LWT 2020, 133, 109887. [Google Scholar] [CrossRef]
- Cecchi, L.; Migliorini, M.; Cherubini, C.; Giusti, M.; Zanoni, B.; Innocenti, M.; Mulinacci, N. Phenolic profiles, oil amount and sugar content during olive ripening of three typical Tuscan cultivars to detect the best harvesting time for oil production. Food Res. Int. 2013, 54, 1876–1884. [Google Scholar] [CrossRef]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D. Discrimination of olive oils and fruits into cultivars and maturity stages based on phenolic and volatile compounds. J. Agric. Food Chem. 2005, 53, 8054–8062. [Google Scholar] [CrossRef] [PubMed]
- Cirilli, M.; Caruso, G.; Gennai, C.; Urbani, S.; Frioni, E.; Ruzzi, M.; Servili, M.; Gucci, R.; Poerio, E.; Muleo, R.M. The Role of Polyphenoloxidase, Peroxidase, and β-Glucosidase in Phenolics Accumulation in Olea europaea L. Fruits under Different Water Regimes. Front. Plant Sci. 2017, 8, 717. [Google Scholar] [CrossRef]
- Ortega-Garcia, F.; Blanco, S.; Peinado, M.A.; Peragon, J. Polyphenol oxidase and its relationship with oleuropein concentration in fruits and leaves of olive (Olea europaea) cv. “Picual” trees during fruit ripening. Tree Physiol. 2008, 28, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Morelló, J.R.; Romero, M.P.; Ramo, T.; Motilva, M.J. Evaluation of L-phenylalanine ammonia-lyase activity and phenolic profile in olive drupe (Olea europaea L.) from fruit setting period to harvesting time. Plant Sci. 2005, 168, 65–72. [Google Scholar] [CrossRef]
- Ryan, D.; Antolovich, M.; Herlt, T.; Prenzler, P.D.; Lavee, S.; Robards, K. Identification of phenolic compounds in tissues of the novel olive cultivar hardy’s mammoth. J. Agric. Food Chem. 2002, 50, 6716–6724. [Google Scholar] [CrossRef]
- Facchini, P.J.; De Luca, V. Differential and tissue-specific expression of a gene family for tyrosine/dopa decarboxylase in opium poppy. J. Biol. Chem. 1994, 269, 26684–26690. [Google Scholar] [CrossRef]
- Saimaru, H.; Orihara, Y. Biosynthesis of acteoside in cultured cells of Olea europaea. J. Nat. Med. 2010, 64, 139–145. [Google Scholar] [CrossRef]
| Sampling Time-Point | Drupe Status | Performed Analysis |
|---|---|---|
| T1 | yellow-green | Gene expression |
| T2 | turning | Gene expression + oil biochemical characterization |
| T3 | almost dark | Gene expression + oil biochemical characterization |
| T4 | fully dark | Oil biochemical characterization |
| Cultivar | Microsatellite Marker | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DCA03 | DCA05 | DCA09 | DCA13 | DCA15 | DCA17 | DCA18 | GAPU45 | GAPU71b | GAPU101 | EMO90 | EMOL | |||||||||||||
| Corsicana (SA) | 232 | 253 | 198 | 208 | 162 | 182 | 120 | 124 | 246 | 257 | 109 | 115 | 177 | 183 | 181 | 181 | 127 | 144 | 192 | 200 | 186 | 188 | 190 | 198 |
| Semidana (SA) | 245 | 253 | 202 | 206 | 162 | 174 | 122 | 140 | 246 | 257 | 117 | 117 | 177 | 179 | 183 | 185 | 130 | 144 | 192 | 218 | 186 | 188 | 190 | 198 |
| Sivigliana (SA) | 243 | 253 | 206 | 208 | 162 | 174 | 120 | 120 | 246 | 257 | 113 | 117 | 179 | 183 | 181 | 181 | 127 | 144 | 198 | 200 | 188 | 194 | 190 | 198 |
| Tortiglione (AB) | 229 | 245 | 198 | 206 | 186 | 186 | 120 | 120 | 246 | 246 | 113 | 113 | 177 | 185 | 195 | 195 | 124 | 127 | 192 | 192 | 188 | 198 | 198 | 198 |
| Dritta (AB) | 229 | 229 | 208 | 212 | 186 | 194 | 120 | 120 | 246 | 257 | 113 | 113 | 177 | 177 | 181 | 185 | 127 | 127 | 190 | 206 | 186 | 190 | 192 | 192 |
| Gentile dell’Aquila (AB) | 243 | 249 | 206 | 206 | 162 | 162 | 120 | 120 | 246 | 246 | - | - | 179 | 181 | 183 | 183 | 144 | 144 | 200 | 206 | 188 | 194 | 198 | 198 |
| Bambina (AP) | 243 | 253 | 198 | 206 | - | - | 120 | 120 | 246 | 266 | 117 | 117 | 177 | 177 | 183 | 185 | 127 | 144 | - | - | 188 | 188 | 198 | 198 |
| Cima di Melfi (AP) | 243 | 253 | 198 | 206 | 184 | 184 | 120 | 120 | 246 | 246 | 143 | 143 | 177 | 181 | 181 | 185 | 124 | 144 | 182 | 182 | 190 | 190 | 192 | 198 |
| Oliva Rossa (AP) | 239 | 253 | 206 | 206 | 184 | 194 | 120 | 122 | 246 | 273 | 143 | 143 | 177 | 181 | 185 | 185 | 127 | 144 | 182 | 218 | 188 | 188 | 200 | 214 |
| Region | Genotype | Drupe Collecting Time-Point | Hydroxy-Tyrosol (3,4-DHPEA) | Tyrosol (p-HPEA) | Oleacein (3,4-DHPEA-EDA) | TPC |
|---|---|---|---|---|---|---|
| Sardinia | Corsicana | T2 | 0.14 ± 0.12 d | 0.32 ± 0.02 f | 33.95 ± 0.21 a | 572 ± 3 a |
| Corsicana | T3 | 0.27 ± 0.01 d | 0.36 ± 0.02 f | 30.06 ± 0.17 b | 558 ± 1 a | |
| Corsicana | T4 | 0.13 ± 0.01 d | 0.43 ± 0.01 ef | 9.20 ± 0.43 e | 511 ± 1 b | |
| Semidana | T2 | 0.81 ± 0.28 bc | 1.18 ± 0.17 b | 13.32 ± 3.95 d | 479 ± 23 de | |
| Semidana | T3 | 0.74 ± 0.19 c | 1.32 ± 0.08 b | 9.69 ± 0.61 e | 469 ± 9 ef | |
| Semidana | T4 | 0.91 ± 0.15 abc | 1.55 ± 0.12 a | 7.78 ± 0.67 e | 453 ± 3 f | |
| Sivigliana | T2 | 1.07 ± 0.20 ab | 0.65 ± 0.10 d | 20.83 ± 1.46 c | 503 ± 10 bc | |
| Sivigliana | T3 | 0.70 ± 0.15 c | 0.55 ± 0.04 de | 7.88 ± 0.55 e | 487 ± 6 cd | |
| Sivigliana | T4 | 1.14 ± 0.29 a | 0.83 ± 0.04 c | 12.72 ± 1.46 d | 477 ± 10 de | |
| Apulia | Bambina | T2 | 0.40 ± 0.06 a | 1.51 ± 0.06 c | 4.85 ± 0.52 b | 358 ± 15 b |
| Bambina | T3 | 0.36 ± 0.06 a | 1.86 ± 0.07 b | 10.75 ± 1.76 a | 406 ± 10 a | |
| Bambina | T4 | 0.32 ± 0.09 a | 2.35 ± 0.13 a | 3.12 ± 1.83 b | 392 ± 6 a | |
| Cima di Melfi | T2 | 0.75 ± 0.01 a | 3.01 ± 0.01 a | 17.49 ± 1.89 c | 386 ± 5 c | |
| Cima di Melfi | T3 | 0.39 ± 0.06 b | 1.36 ± 0.01 b | 36.79 ± 2.76 b | 502 ± 20 b | |
| Cima di Melfi | T4 | 0.41 ± 0.05 b | 1.00 ± 0.05 c | 65.52 ± 12.26 a | 730 ± 11 a | |
| Oliva Rossa | T2 | 0.30 ± 0.05 ab | 0.48 ± 0.02 b | 55.34 ± 2.07 a | 677 ± 47 a | |
| Oliva Rossa | T3 | 0.35 ± 0.05 a | 0.45 ± 0.01 b | 21.36 ± 3.46 b | 478 ± 5 b | |
| Oliva Rossa | T4 | 0.22 ± 0.06 b | 0.59 ± 0.04 a | 15.46 ± 1.77 c | 416 ± 19 c | |
| Abruzzo | Dritta | T2 | 0.12 ± 0.02 c | 0.25 ± 0.01 b | 3.71 ± 0.24 de | 129 ± 27 d |
| Dritta | T3 | 0.03 ± 0.01 c | 0.23 ± 0.02 b | 0.50 ± 0.09 e | 39 ± 2 f | |
| Dritta | T4 | 0.09 ± 0.03 c | 0.26 ± 0.04 b | 1.41 ± 0.08 e | 71 ± 9 e | |
| Gentile dell’Aquila | T2 | 0.05 ± 0.01 c | 0.17 ± 0.03 d | 6.19 ± 1.37 d | 123 ± 38 d | |
| Gentile dell’Aquila | T3 | 0.07 ± 0.02 c | 0.13 ± 0.05 cd | 1.09 ± 0.51 e | 106 ± 26 de | |
| Gentile dell’Aquila | T4 | 0.09 ± 0.01 c | 0.16 ± 0.02 cd | 16.80 ± 1.35 c | 176 ± 15 c | |
| Tortiglione | T2 | 0.59 ± 0.12 a | 0.38 ± 0.07 a | 42.37 ± 3.78 a | 804 ± 68 a | |
| Tortiglione | T3 | 0.29 ± 0.08 b | 0.21 ± 0.02 b | 24.44 ± 3.72 b | 377 ± 30 b | |
| Tortiglione | T4 | 0.28 ± 0.06 b | 0.34 ± 0.01 a | 18.34 ± 1.61 c | 395 ± 6 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabetta, W.; Mascio, I.; Squeo, G.; Gadaleta, S.; Flamminii, F.; Conte, P.; Di Mattia, C.D.; Piga, A.; Caponio, F.; Montemurro, C. Bioactive Potential of Minor Italian Olive Genotypes from Apulia, Sardinia and Abruzzo. Foods 2021, 10, 1371. https://doi.org/10.3390/foods10061371
Sabetta W, Mascio I, Squeo G, Gadaleta S, Flamminii F, Conte P, Di Mattia CD, Piga A, Caponio F, Montemurro C. Bioactive Potential of Minor Italian Olive Genotypes from Apulia, Sardinia and Abruzzo. Foods. 2021; 10(6):1371. https://doi.org/10.3390/foods10061371
Chicago/Turabian StyleSabetta, Wilma, Isabella Mascio, Giacomo Squeo, Susanna Gadaleta, Federica Flamminii, Paola Conte, Carla Daniela Di Mattia, Antonio Piga, Francesco Caponio, and Cinzia Montemurro. 2021. "Bioactive Potential of Minor Italian Olive Genotypes from Apulia, Sardinia and Abruzzo" Foods 10, no. 6: 1371. https://doi.org/10.3390/foods10061371
APA StyleSabetta, W., Mascio, I., Squeo, G., Gadaleta, S., Flamminii, F., Conte, P., Di Mattia, C. D., Piga, A., Caponio, F., & Montemurro, C. (2021). Bioactive Potential of Minor Italian Olive Genotypes from Apulia, Sardinia and Abruzzo. Foods, 10(6), 1371. https://doi.org/10.3390/foods10061371










