Effects of Storage Conditions on the Flavor Stability of Fried Pepper (Zanthoxylum bungeanum) Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemicals
2.3. Fried Pepper Oil Sample Preparation
2.4. Pepper Oil with Different Storage Conditions
- (1)
- The No. 1 sample was only treated with UV irradiation. The pepper oil (100 g) sample was stored in a transparent and sealed glass bottle, and nitrogen (99.9%) was filled into the bottle to discharge the air. Then, the glass bottles were placed in an incubator and exposed under four ultraviolet lights with peak emission at 254 nm at a distance of 10 cm for 30 days under a power of 10 W. The temperature of the incubator was controlled at 25 °C.
- (2)
- The No. 2 sample was only treated with oxygen exposure. The pepper oil (100 g) sample was stored in a transparent and sealed glass bottle with wrapped aluminum foil. Then, the glass bottles were placed in an incubator at 25 °C. Oxygen was injected into the glass bottles every 2 days.
- (3)
- The No. 3 sample that was simultaneously treated with UV irradiation and oxygen exposure. The subsequent procedures followed those for sample 1. Oxygen was injected into the glass bottles every 2 days.
- (4)
- The No. 4 sample (newly prepared pepper oil) was directly analyzed.
2.5. Separation of Volatile Compounds
2.6. GC-MS Analysis and Chiral GC-MS Analysis
2.7. Identification and Quantitation
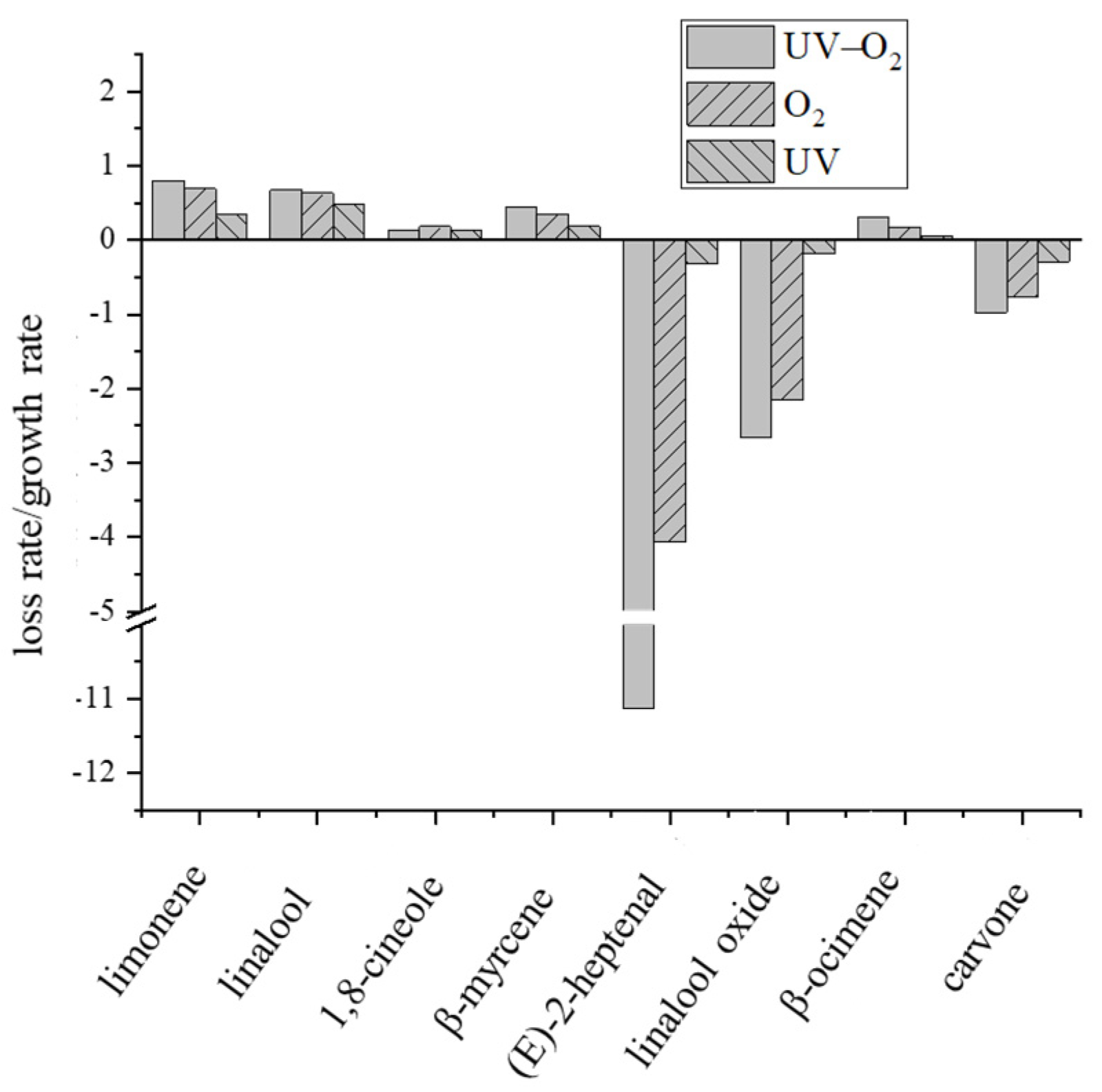
2.8. Loss or Grow Rate of Key Volatile Compounds
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effects of Different Storage Conditions on Enantiomer Ratios of Chiral Compounds
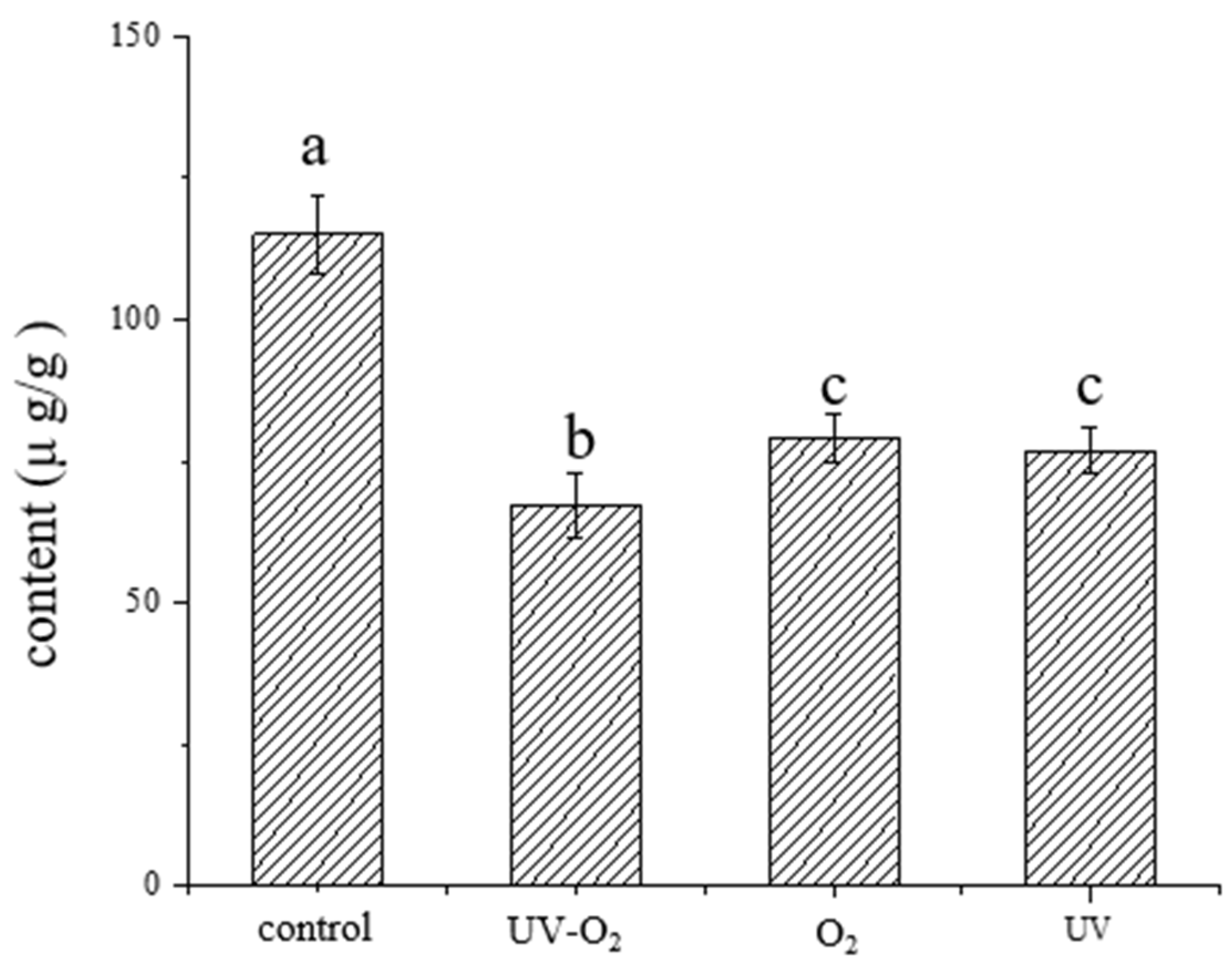
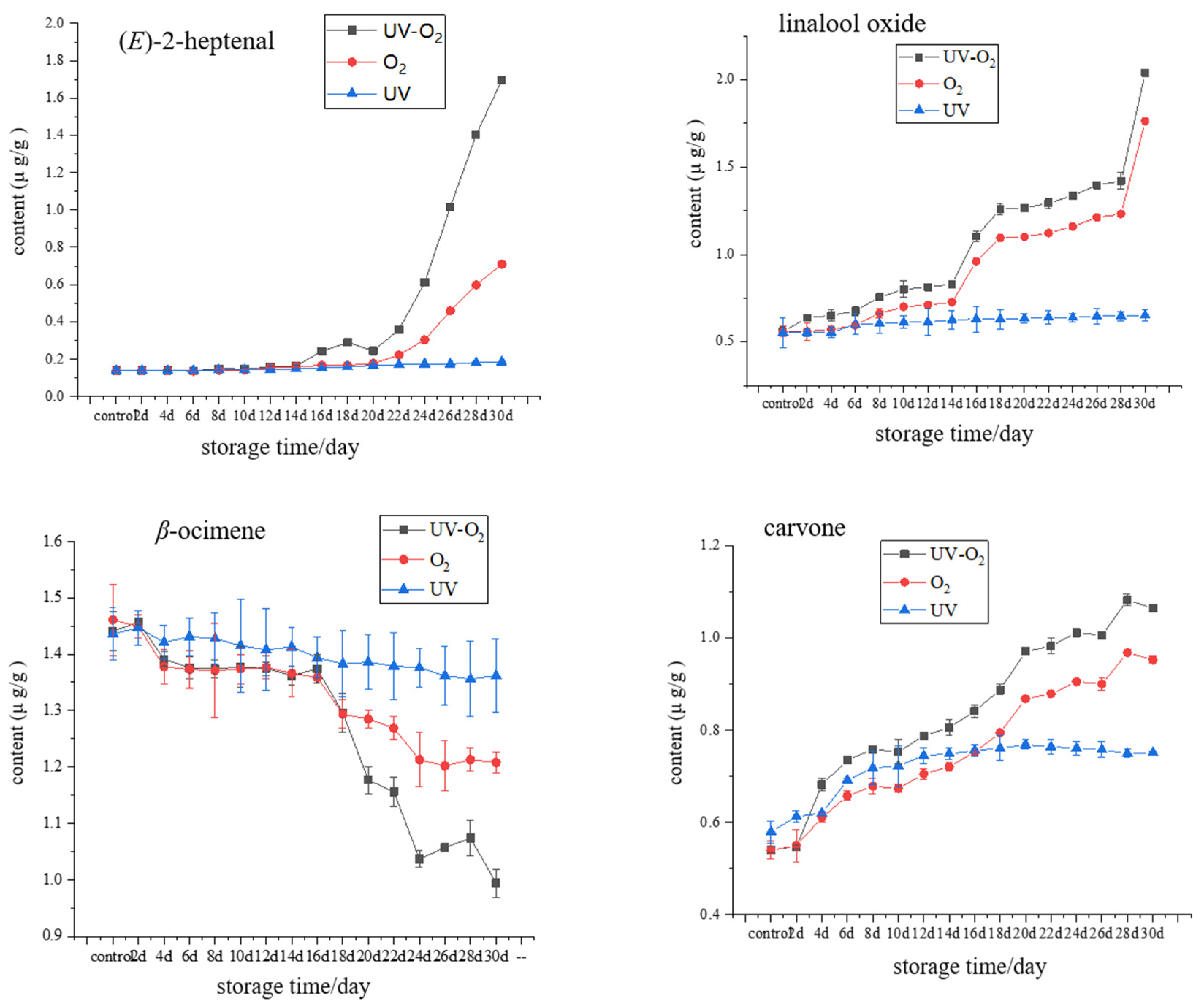
3.2. Changes of Key Aroma Compounds under Different Storage Conditions
3.3. Investigation of the Volatile Changes under UV–O2 Storage Condition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Epifano, F.; Curini, M.; Carla Marcotullio, M.; Genovese, S. Searching for novel cancer chemopreventive plants and their products: The genus Zanthoxylum. Curr. Drug Targets 2011, 12, 1895–1902. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Yoshihara, K.; Hirose, Y. Constituents of fruit oil from Japanese pepper. Bull. Chem. Soc. Jpn. 1968, 41, 1945–1950. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, S.; Ohsuka, A.; Kotake, M.; Sakai, T. Constituents of leaf oil from Japanese pepper. Bull. Chem. Soc. Jpn. 1968, 41, 1950–1953. [Google Scholar] [CrossRef]
- Jiang, L.; Kubota, K. Formation by mechanical stimulus of the flavor compounds in young leaves of Japanese pepper (Xanthoxylum piperitum dc.). J. Agric. Food Chem. 2001, 49, 1353–1357. [Google Scholar] [CrossRef]
- Jiang, L.; Kubota, K. Differences in the volatile components and their odor characteristics of green and ripe fruits and dried pericarp of Japanese pepper (Xanthoxylum piperitum, DC.). J. Agric. Food Chem. 2004, 52, 4197–4203. [Google Scholar] [CrossRef] [PubMed]
- Yang, X. Aroma constituents and alkylamides of red and green Huajiao (Zanthoxylum bungeanum and Zanthoxylum schinifolium). J. Agric. Food Chem. 2008, 56, 1689–1696. [Google Scholar] [CrossRef]
- Sun, J.; Sun, B.G.; Ren, F.Z.; Chen, H.T.; Zhang, N.; Zhang, Y.Y. Characterization of key odorants in Hanyuan and Hancheng fried pepper (Zanthoxylum bungeanum) oil. J. Agric. Food Chem. 2020, 68, 6403–6411. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, S.; Grauwet, T.; Kebede, B.T.; Hendrickx, M. Study of chemical changes in pasteurised orange juice during shelf-life: A fingerprinting-kinetics evaluation of the volatile fraction. Food Res. Int. 2015, 75, 295–304. [Google Scholar] [CrossRef]
- Sun, H.; Ni, H.; Yang, Y.Y.; Wu, L.; Cai, H.N.; Xiao, A.F.; Chen, F. Investigation of sunlight-induced deterioration of aroma of Pummelo (Citrus maxima) essential oil. J. Agric. Food Chem. 2014, 62, 11818–11830. [Google Scholar] [CrossRef]
- Cheng, H.X.; Yuan, Y.L.; Hu, L.L.; Qiu, P.; Chen, H.F.; Dou, J.W.; Dong, S.Y. Effect of storage conditions on limonene and linalool of Zanthoxylum bungeanum seed oil. Food Sci. 2014, 35, 258–261. [Google Scholar]
- Yuan, F.; He, F.; Qian, Y.L.; Zheng, J.; Qian, M.C. Aroma stability of lemon-flavored hard iced tea assessed by chirality and aroma extract dilution analysis. J. Agric. Food Chem. 2016, 64, 5717–5723. [Google Scholar] [CrossRef]
- He, F.; Qian, Y.P.L.; Qian, M.C. Flavour and chiral stability of lemon-flavored hard tea during storage. Food Chem. 2017, 239, 622–630. [Google Scholar] [CrossRef]
- Ruiz del Castillo, M.L.; Caja, M.M.; Herraiz, M. Use of the enantiomeric composition for the assessment of the authenticity of fruit beverages. J. Agric. Food Chem. 2003, 51, 1284–1288. [Google Scholar] [CrossRef] [PubMed]
- Bonnländer, B.; Cappuccio, R.; Liverani, F.S.; Winterhalter, P. Analysis of enantiomeric linalool ratio in green and roasted coffee. Flavor Frag. J. 2006, 21, 637–641. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Lan Phi, N.T.; Sawamura, M. Compositional changes in Yuzu (Citrus junos) steam-distilled oil and effects of antioxidants on oil quality during storage. Food Sci. Technol. Res. 2010, 16, 51–58. [Google Scholar] [CrossRef][Green Version]
- Rouseff, R.L.; Ruiz Perez-Cacho, P.; Jabalpurwala, F. Historical review of citrus flavor research during the past 100 years. J. Agric. Food Chem. 2009, 57, 8115–8124. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, A.J.; Saura, D.; Lorente, J.; Carbonell-Barrachina, Á.A. Limonene, linalool, a-terpineol, and terpinen-4-ol as quality control parameters in mandarin juice processing. Eur. Food Res. Technol. 2006, 222, 281–285. [Google Scholar] [CrossRef]
- Haleva-Toledo, E.; Naim, M.; Zehavi, U.; Rouseff, R.L. Formation of α-terpineol in citrus Juices, model and buffer solutions. J. Food Sci. 1999, 64, 838–841. [Google Scholar] [CrossRef]
- Njoroge, S.M.; Ukeda, H.; Sawamura, M. Changes in the volatile composition of Yuzu (Citrus junos Tanaka) cold-pressed oil during storage. J. Agric. Food Chem. 1996, 44, 550–556. [Google Scholar] [CrossRef]
- Ohta, H.; Tonohara, K.; Watanabe, A.; Iino, K.; Kimura, S. Flavor specificities of Satsuma mandarin juice extracted by a new-type screw press extraction system. Agric. Biol. Chem. 1982, 46, 1385–1386. [Google Scholar]
- Yang, Y.; Jin, G.J.; Wang, X.J.; Kong, C.L.; Liu, J.B.; Tao, Y.S. Chemical profiles and aroma contribution of terpene compounds in Meili (Vitis vinifera L.) grape and wine. Food Chem. 2019, 284, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Xie, B.J.; Zhang, Y.; Zhang, Y.; Fan, G.; Yao, X.L.; Pan, S.Y. Characterization of aroma active compounds in fruit juice and peel oil of jin chen sweet orange fruit (Citrus sinensis (L.) osbeck) by GC-MS and GC-O. Molecules 2008, 13, 1333–1344. [Google Scholar] [CrossRef] [PubMed]

| Compound | Odor Description [7] |
|---|---|
| S-(-)-limonene | lemon-like |
| R-(+)-limonene | orange-like |
| R-(-)-linalool | woody and lavender-like |
| S-(+)-linalool | sweet, floral, petitgrain-like |
| S-(+)-carvone | caraway-like |
| R-(-)-carvone | spearmint-like |
| 2R, 5R-linalool oxide | floral |
| 2S, 5S-linalool oxide | floral |
| Storage Conditions a | Compound | Enantiomeric Ratio | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| con b | 2 d c | 4 d | 6 d | 8 d | 10 d | 12 d | 14 d | 16 d | 18 d | 20 d | 22 d | 24 d | 26 d | 28 d | 30 d | ||
| UV | S-(-)-limonene | 60 | 60 | 60 | 59 | 58 | 58 | 55 | 57 | 55 | 55 | 55 | 55 | 53 | 53 | 52 | 52 |
| R-(+)-limonene | 40 | 40 | 40 | 41 | 42 | 42 | 45 | 43 | 45 | 45 | 45 | 45 | 47 | 47 | 48 | 48 | |
| 2R, 5R-linalool oxide | 53 | 51 | 51 | 51 | 51 | 50 | 51 | 51 | 51 | 51 | 50 | 50 | 50 | 50 | 50 | 49 | |
| 2S, 5S-linalool oxide | 47 | 49 | 49 | 49 | 49 | 50 | 49 | 49 | 49 | 49 | 50 | 50 | 50 | 50 | 50 | 51 | |
| R-(-)-linalool | 10 | 10 | 11 | 11 | 11 | 11 | 11 | 11 | 11 | 12 | 12 | 12 | 12 | 13 | 14 | 15 | |
| S-(+)-linalool | 90 | 90 | 89 | 89 | 89 | 89 | 89 | 89 | 89 | 88 | 88 | 88 | 88 | 87 | 86 | 85 | |
| R-(-)-carvone | 55 | 56 | 56 | 56 | 56 | 56 | 56 | 55 | 55 | 54 | 54 | 54 | 54 | 52 | 52 | 52 | |
| S-(+)-carvone | 45 | 44 | 44 | 44 | 44 | 44 | 44 | 45 | 45 | 46 | 46 | 46 | 46 | 48 | 48 | 48 | |
| O2 | S-(-)-limonene | 60 | 59 | 57 | 57 | 57 | 55 | 55 | 55 | 54 | 53 | 53 | 52 | 52 | 52 | 51 | 51 |
| R-(+)-limonene | 40 | 41 | 43 | 43 | 43 | 45 | 45 | 45 | 46 | 47 | 47 | 48 | 48 | 48 | 49 | 49 | |
| 2R, 5R-linalool oxide | 53 | 53 | 53 | 53 | 53 | 52 | 52 | 52 | 52 | 52 | 52 | 52 | 51 | 51 | 51 | 50 | |
| 2S, 5S-linalool oxide | 47 | 47 | 47 | 47 | 47 | 48 | 48 | 48 | 48 | 48 | 48 | 48 | 49 | 49 | 49 | 50 | |
| R-(-)-linalool | 10 | 10 | 11 | 11 | 11 | 11 | 11 | 11 | 11 | 11 | 12 | 12 | 12 | 13 | 13 | 13 | |
| S-(+)-linalool | 90 | 90 | 89 | 89 | 89 | 89 | 89 | 89 | 89 | 89 | 88 | 88 | 88 | 87 | 87 | 87 | |
| R-(-)-carvone | 55 | 54 | 53 | 53 | 53 | 53 | 53 | 52 | 52 | 52 | 52 | 52 | 52 | 52 | 52 | 51 | |
| S-(+)-carvone | 45 | 46 | 47 | 47 | 47 | 47 | 47 | 48 | 48 | 48 | 48 | 48 | 48 | 48 | 48 | 49 | |
| UV–O2 | S-(-)-limonene | 60 | 58 | 58 | 57 | 55 | 56 | 57 | 55 | 55 | 55 | 54 | 53 | 50 | 50 | 50 | 48 |
| R-(+)-limonene | 40 | 42 | 42 | 43 | 45 | 44 | 43 | 45 | 45 | 45 | 46 | 47 | 50 | 50 | 50 | 52 | |
| 2R, 5R-linalool oxide | 53 | 53 | 53 | 52 | 52 | 51 | 51 | 51 | 51 | 51 | 50 | 50 | 50 | 50 | 50 | 49 | |
| 2S, 5S-linalool oxide | 47 | 47 | 47 | 48 | 48 | 49 | 49 | 49 | 49 | 49 | 50 | 50 | 50 | 50 | 50 | 51 | |
| R-(-)-linalool | 10 | 10 | 11 | 11 | 11 | 11 | 11 | 12 | 12 | 12 | 12 | 12 | 12 | 13 | 13 | 14 | |
| S-(+)-linalool | 90 | 90 | 89 | 89 | 89 | 89 | 89 | 88 | 88 | 88 | 88 | 88 | 88 | 87 | 87 | 86 | |
| R-(-)-carvone | 55 | 55 | 55 | 55 | 54 | 54 | 54 | 54 | 54 | 54 | 54 | 53 | 53 | 52 | 52 | 51 | |
| S-(+)-carvone | 45 | 45 | 45 | 45 | 46 | 46 | 46 | 46 | 46 | 46 | 46 | 47 | 47 | 48 | 48 | 49 | |
| Volatile | Concentration (μg/g) | Identification b | |
|---|---|---|---|
| con a | 30 Days | ||
| sabinene | 0.254 ± 0.04 a | 0.245 ± 0.05 a | MS, RI, Std |
| β-myrcene | 13.273 ± 1.03 a | 7.259 ± 0.23 b | MS, RI, Std |
| limonene | 68.498 ± 1.42 a | 13.456 ± 0.86 b | MS, RI, Std |
| β-ocimene | 1.441 ± 0.05 a | 0.994 ± 0.06 b | MS, RI, Std |
| 1,8-cineole | 4.213 ± 0.21 a | 3.611 ± 0.05 b | MS, RI, Std |
| δ-3-carene | 1.428 ± 0.09 a | 2.413 ± 0.09 b | MS, RI, Std |
| p-cymene | 0.294 ± 0.02 a | 1.901 ± 0.1 b | MS, RI, Std |
| terpinolene | 0.102 ± 0.01 a | 0.196 ± 0.06 a | MS, RI, Std |
| (E)-limonene oxide | 0.342 ± 0.06 a | 1.005 ± 0.09 b | MS, RI, Std |
| 1-octen-3-one | n.d. | 0.48 ± 0.05 | MS, RI, Std |
| (E)-2-heptenal | 0.14 ± 0.01 a | 1.697 ± 0.07 b | MS, RI, Std |
| 6-methyl-5-hepten-2-one | n.d. | 1.452 ± 0.04 | MS, RI, Std |
| cyclohexanone | n.d. | 0.04 ± 0.006 | MS, RI, Std |
| 1-hexanol | n.d. | 0.552 ± 0.07 | MS, RI, Std |
| (E,Z)-2,6-dimethyl-2,4,6-octatriene | 0.524 ± 0.05 a | 0.959 ± 0.09 b | MS |
| octanal | 0.23 ± 0.02 a | 0.574 ± 0.04 b | MS, RI, Std |
| (E,E)-2,4-hexadienal | n.d. | 0.486 ± 0.03 | MS, RI, Std |
| perillen | 0.042 ± 0.001 | n.d. | MS, RI, Std |
| (E)-2-octenal | n.d. | 2.916 ± 0.12 | MS, RI, Std |
| 1-methyl-4-(1-methylethenyl)-benzene | 0.064 ± 0.003 a | 0.169 ± 0.03 b | MS, RI, Std |
| linalool oxide | 0.558 ± 0.02 a | 2.04 ± 0.08 b | MS, RI, Std |
| 1-octen-3-ol | 0.028 ± 0.003 a | 1.219 ± 0.14 b | MS, RI, Std |
| 1-heptanol | n.d. | 1.19 ± 0.11 | MS, RI, Std |
| acetic acid | 0.9 ± 0.04 a | 5.808 ± 0.17 b | MS, RI, Std |
| piperenone | 0.036 ± 0.002 a | 0.068 ± 0.005 a | MS, RI, Std |
| (E,E)-2,4-heptadienal | 0.208 ± 0.02 a | 1.353 ± 0.14 b | MS, RI, Std |
| 4-ethylcyclohexanol | n.d. | 0.079 ± 0.006 | MS, RI, Std |
| benzaldehyde | n.d. | 0.038 ± 0.002 | MS, RI, Std |
| 4-methylcyclohex-3-en-1-one | n.d. | 0.245 ± 0.01 | MS |
| menthol | n.d. | 0.109 ± 0.007 | MS, RI, Std |
| p-ment-8-en-1-ol | 0.066 ± 0.004 a | 0.174 ± 0.003 b | MS |
| linalool | 12.414 ± 0.32 a | 4.049 ± 0.25 b | MS, RI, Std |
| linalyl acetate | 6.8 ± 0.14 a | 2.519 ± 0.13 b | MS, RI, Std |
| 1-octanol | n.d. | 0.39 ± 0.09 | MS, RI, Std |
| 5-methyl furfural | 0.082 ± 0.003 | n.d. | MS, RI, Std |
| caryophyllene | 0.048 ± 0.004 a | 0.036 ± 0.004 a | MS, RI, Std |
| 6-methyl-3,5-heptadien-2-one | 0.03 ± 0.001 a | 0.06 ± 0.006 a | MS, RI, Std |
| dihydrocarvone | n.d. | 0.479 ± 0.001 | MS |
| terpinen-4-ol | 0.718 ± 0.008 | n.d. | MS, RI, Std |
| 5-ethyl-2(5H)-furanone | n.d. | 2.122 ± 0.07 | MS |
| (Z)-p-2,8-menadien-1-ol | 0.094 ± 0.003 a | 0.048 ± 0.006 b | MS |
| (E)-2-decenal | n.d. | 0.574 ± 0.007 | MS, RI, Std |
| (2E,4E)-2,4-decanedienal | n.d. | 0.086 ± 0.002 | MS, RI, Std |
| 1-nonanol | n.d. | 0.185 ± 0.003 | MS, RI, Std |
| (E,E)-2,4-dodecadienal | n.d. | 0.087 ± 0.001 | MS, RI, Std |
| terpinyl acetate | 0.316 ± 0.06 a | 0.38 ± 0.006 a | MS, RI, Std |
| α-terpineol | 0.262 ± 0.008 a | 0.352 ± 0.007 b | MS, RI, Std |
| piperitone | 0.052 ± 0.002 a | 0.055 ± 0.002 a | MS, RI, Std |
| carvone | 0.54 ± 0.007 a | 1.06 ± 0.049 b | MS, RI, Std |
| neryl acetate | 0.078 ± 0.001 a | 0.15 ± 0.003 b | MS, RI, Std |
| 6-ethenyltetrahydro-2,2,6-trimethyl-2H-pyran-3-ol | 0.086 ± 0.002 a | 0.236 ± 0.003 b | MS |
| (Z)-p-1,8-menadien-2-ol | 0.082 ± 0.007 a | 0.264 ± 0.006 b | MS |
| (E)-carveol | 0.146 ± 0.003 a | 0.046 ± 0.001 b | MS, RI, Std |
| 2-(4-methylphenyl)propan-2-ol | 0.022 ± 0.002 | n.d. | MS |
| (Z)-carveol | 0.082 ± 0.001 a | 0.041 ± 0.001 b | MS, RI, Std |
| 2-acetylpyrrole | 0.026 ± 0.001 a | 0.065 ± 0.002 b | MS, RI, Std |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Sun, B.; Ren, F.; Chen, H.; Zhang, N.; Zhang, Y.; Zhang, H. Effects of Storage Conditions on the Flavor Stability of Fried Pepper (Zanthoxylum bungeanum) Oil. Foods 2021, 10, 1292. https://doi.org/10.3390/foods10061292
Sun J, Sun B, Ren F, Chen H, Zhang N, Zhang Y, Zhang H. Effects of Storage Conditions on the Flavor Stability of Fried Pepper (Zanthoxylum bungeanum) Oil. Foods. 2021; 10(6):1292. https://doi.org/10.3390/foods10061292
Chicago/Turabian StyleSun, Jie, Baoguo Sun, Fazheng Ren, Haitao Chen, Ning Zhang, Yuyu Zhang, and Huiying Zhang. 2021. "Effects of Storage Conditions on the Flavor Stability of Fried Pepper (Zanthoxylum bungeanum) Oil" Foods 10, no. 6: 1292. https://doi.org/10.3390/foods10061292
APA StyleSun, J., Sun, B., Ren, F., Chen, H., Zhang, N., Zhang, Y., & Zhang, H. (2021). Effects of Storage Conditions on the Flavor Stability of Fried Pepper (Zanthoxylum bungeanum) Oil. Foods, 10(6), 1292. https://doi.org/10.3390/foods10061292






