Quality Attributes of Ultra-High Temperature-Treated Model Beverages Prepared with Faba Bean Protein Concentrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE) of Faba and Soy Protein Isolates
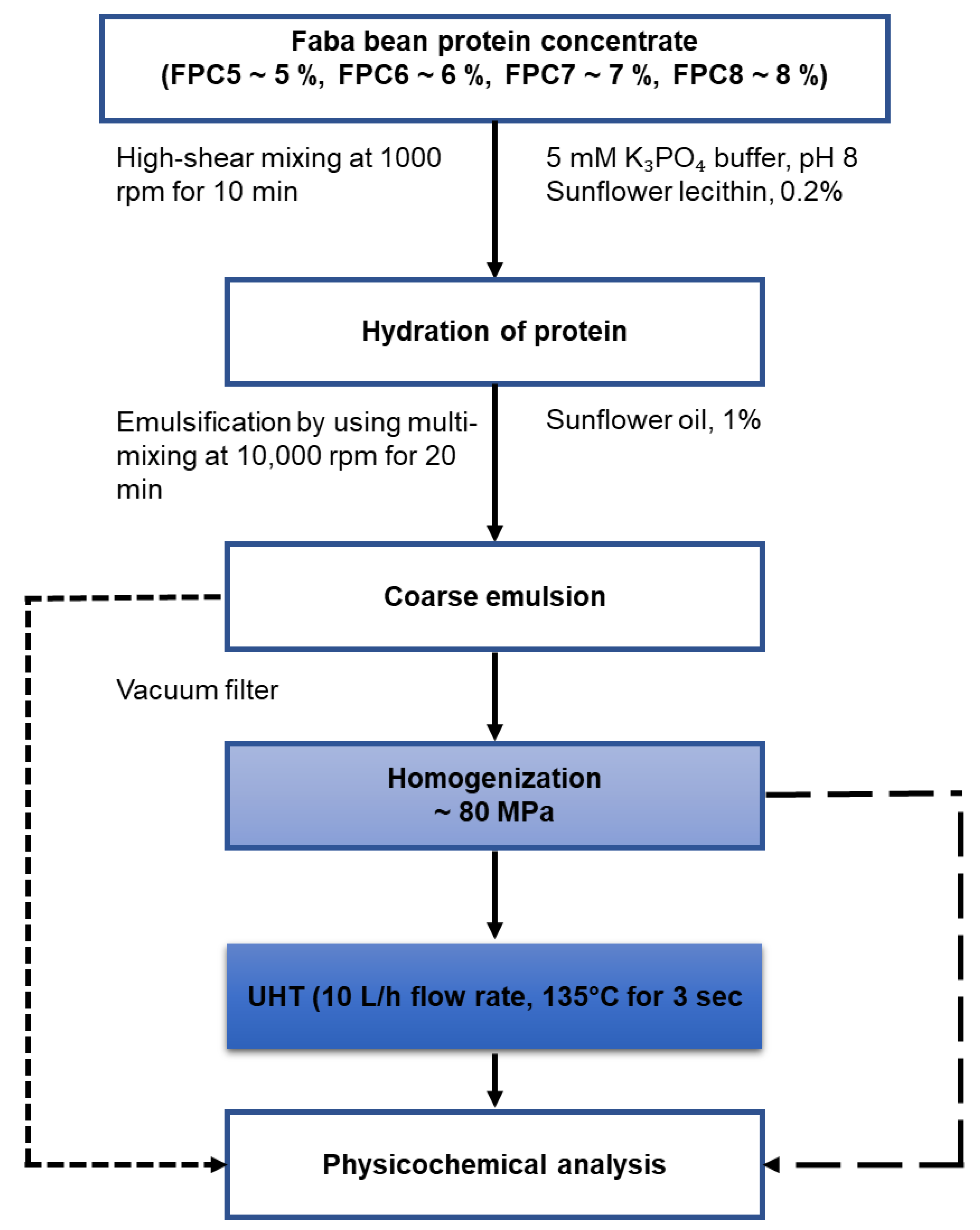
2.3. Preparation of Emulsion
2.3.1. Mixing
2.3.2. Preparation of Homogenised Emulsions
2.3.3. Ultra-High Temperature Processing of the Emulsion
2.4. Physicochemical Properties of the Emulsion
2.4.1. Particle Size Distribution
2.4.2. Flocculation Index (FI) and Coalescence Index (CI)
2.4.3. Confocal Laser Scanning Microscopy
2.4.4. ζ-Potential
2.4.5. Creaming Index
2.4.6. Headspace Gas Chromatography-Mass Spectrometry (GC-MS)
2.5. Statistical Analysis
3. Results and Discussion
3.1. Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE) of Faba and Soy Protein Isolates
3.2. Physicochemical Properties of the Emulsions
3.2.1. Particle Size Distribution
3.2.2. Flocculation Index (FI) and Coalescence Index (CI)
3.2.3. ζ-Potential
3.2.4. Creaming Index
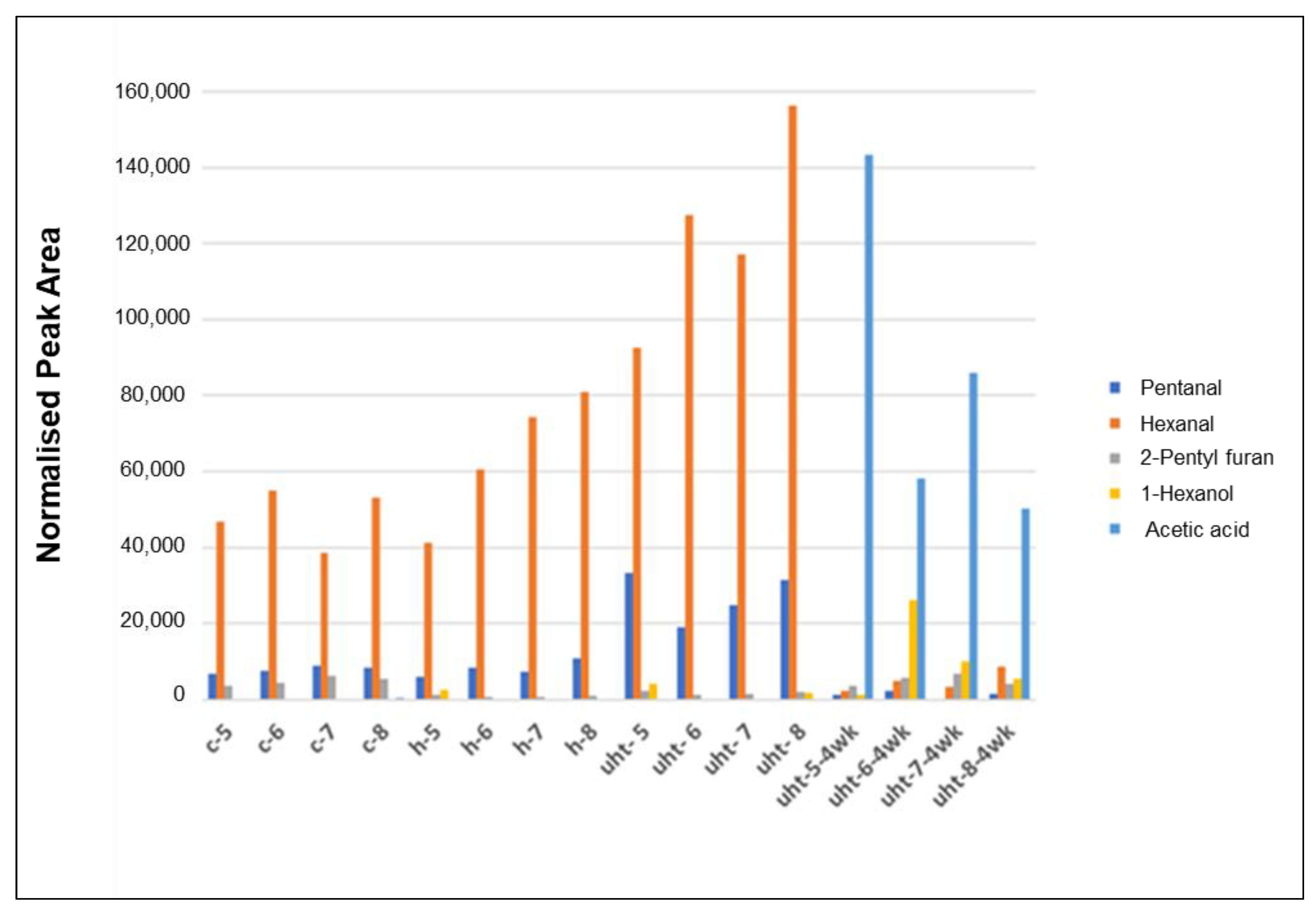
3.2.5. Headspace Gas Chromatography-Mass Spectrometry (GC-MS)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Young, V.R.; Pellett, P.L. Plant proteins in relation to human protein and amino acid nutrition. Am. J. Clin. Nutr. 1994, 59, 1203S–1212S. [Google Scholar] [CrossRef]
- McClements, D.J.; Newman, E.; McClements, I.F. Plant-based Milks: A Review of the Science Underpinning Their Design, Fabrication, and Performance. Compr. Rev. Food Sci. Food Saf. 2019, 18, 2047–2067. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.A.; Tan, M.; Øiseth, S.; Buckow, R. An Emerging Segment of Functional Legume-Based Beverages: A Review. Food Rev. Int. 2020, 1–39. [Google Scholar] [CrossRef]
- Li-Chan, E.C.Y.; Lacroix, I.M.E. 1—Properties of proteins in food systems: An introduction. In Proteins in Food Processing, 2nd ed.; Yada, R.Y., Ed.; Woodhead Publishing: Cambridge, UK, 2018; pp. 1–25. [Google Scholar]
- Queirós, R.P.; Saraiva, J.A.; da Silva, J.A.L. Tailoring structure and technological properties of plant proteins using high hydrostatic pressure. Crit. Rev. Food Sci. Nutr. 2018, 58, 1538–1556. [Google Scholar] [CrossRef] [PubMed]
- Damodaran, S. Protein Stabilization of Emulsions and Foams. J. Food Sci. 2005, 70, R54–R66. [Google Scholar] [CrossRef]
- Fernandez-Avila, C.; Trujillo, A.J. Ultra-High Pressure Homogenization improves oxidative stability and interfacial properties of soy protein isolate-stabilized emulsions. Food Chem. 2016, 209, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chelikani, V.; Serventi, L. Evaluation of chickpea as alternative to soy in plant-based beverages, fresh and fermented. LWT 2018, 97, 570–572. [Google Scholar] [CrossRef]
- Chao, D.; Aluko, R.E. Modification of the structural, emulsifying, and foaming properties of an isolated pea protein by thermal pretreatment. CyTA J. Food 2018, 16, 357–366. [Google Scholar] [CrossRef]
- Karaca, A.C.; Low, N.; Nickerson, M. Emulsifying properties of chickpea, faba bean, lentil and pea proteins produced by isoelectric precipitation and salt extraction. Food Res. Int. 2011, 44, 2742–2750. [Google Scholar] [CrossRef]
- Tsoukala, A.; Papalamprou, E.; Makri, E.; Doxastakis, G.; Braudo, E.E. Adsorption at the air–water interface and emulsification properties of grain legume protein derivatives from pea and broad bean. Colloids Surf. B Biointerfaces 2006, 53, 203–208. [Google Scholar] [CrossRef]
- Qamar, S.; Bhandari, B.; Prakash, S. Effect of different homogenisation methods and UHT processing on the stability of pea protein emulsion. Food Res. Int. 2019, 116, 1374–1385. [Google Scholar] [CrossRef] [PubMed]
- Crépon, K.; Marget, P.; Peyronnet, C.; Carrouée, B.; Arese, P.; Duc, G. Nutritional value of faba bean (Vicia faba L.) seeds for feed and food. Field Crop. Res. 2010, 115, 329–339. [Google Scholar] [CrossRef]
- Multari, S.; Stewart, D.; Russell, W.R. Potential of Fava Bean as Future Protein Supply to Partially Replace Meat Intake in the Human Diet. Compr. Rev. Food Sci. Food Saf. 2015, 14, 511–522. [Google Scholar] [CrossRef]
- Kimura, A.; Fukuda, T.; Zhang, M.; Motoyama, S.; Maruyama, N.; Utsumi, S. Comparison of Physicochemical Properties of 7S and 11S Globulins from Pea, Fava Bean, Cowpea, and French Bean with Those of Soybean—French Bean 7S Globulin Exhibits Excellent Properties. J. Agric. Food Chem. 2008, 56, 10273–10279. [Google Scholar] [CrossRef] [PubMed]
- Warsame, A.O.; Michael, N.; O’Sullivan, D.M.; Tosi, P. Identification and quantification of major faba bean seed proteins. J. Agric. Food Chem. 2020, 68, 8535–8544. [Google Scholar] [CrossRef]
- Felix, M.; Cermeño, M.; FitzGerald, R.J. Assessment of the microstructural characteristics and the in vitro bioactive properties of sunflower oil-based emulsions stabilized by fava bean (vicia faba) protein. Food Hydrocoll. 2019, 97, 105220. [Google Scholar] [CrossRef]
- Raikos, V.; Neacsu, M.; Russell, W.; Duthie, G. Comparative study of the functional properties of lupin, green pea, fava bean, hemp, and buckwheat flours as affected by pH. Food Sci. Nutr. 2014, 2, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bhattarai, M.; Mikkonen, K.S.; Heinonen, M. Effects of Enzymatic Hydrolysis of Fava Bean Protein Isolate by Alcalase on the Physical and Oxidative Stability of Oil-in-Water Emulsions. J. Agric. Food Chem. 2019, 67, 6625–6632. [Google Scholar] [CrossRef]
- Vogelsang-O’Dwyer, M.; Sahin, A.W.; Zannini, E.; Arendt, E.A. Physicochemical and nutritional properties of high protein emulsion-type lupin-based model milk alternatives: Effect of protein source and homogenisation pressure. J. Sci. Food Agric. 2021. [Google Scholar] [CrossRef]
- Rahmati, N.F.; Koocheki, A.; Varidi, M.; Kadkhodaee, R. Thermodynamic compatibility and interactions between Speckled Sugar bean protein and xanthan gum for production of multilayer O/W emulsion. J. Food Sci. Technol. 2018, 55, 1143–1153. [Google Scholar] [CrossRef]
- Oliete, B.; Potin, F.; Cases, E.; Saurel, R. Microfluidization as Homogenization Technique in Pea Globulin-Based Emulsions. Food Bioprocess. Technol. 2019, 12, 877–882. [Google Scholar] [CrossRef]
- Felix, M.; Cermeño, M.; Romero, A.; FitzGerald, R.J. Characterisation of the bioactive properties and microstructure of chickpea protein-based oil in water emulsions. Food Res. Int. 2019, 121, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Z.; Dai, C.; Wang, Y.; Chen, W.; Ju, X.; Yuan, J.; He, R. Physical stability and microstructure of rapeseed protein isolate/gum Arabic stabilized emulsions at alkaline pH. Food Hydrocoll. 2019, 88, 50–57. [Google Scholar] [CrossRef]
- Lu, G.W.; Gao, P. CHAPTER 3—Emulsions and Microemulsions for Topical and Transdermal Drug Delivery. In Handbook of Non-Invasive Drug Delivery Systems; Kulkarni, V.S., Ed.; William Andrew Publishing: Boston, MA, USA, 2010; pp. 59–94. [Google Scholar]
- Liu, N.; Chen, Q.; Li, G.; Zhu, Z.; Yi, J.; Li, C.; Chen, X.; Wang, Y. Properties and Stability of Perilla Seed Protein-Stabilized Oil-in-Water Emulsions: Influence of Protein Concentration, pH, NaCl Concentration and Thermal Treatment. Molecules 2018, 23, 1533. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Chen, W.; Xu, X.; Zhang, J.; Singh, T.K.; Liu, S.; Zhang, D.; Tian, L.; White, A.; Shrestha, P. Engineering trienoic fatty acids into cottonseed oil improves low-temperature seed germination, plant photosynthesis and cotton fibre quality. Plant. Cell Physiol. 2020, 61, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Torkamani, A.E.; Juliano, P.; Ajlouni, S.; Singh, T.K. Impact of ultrasound treatment on lipid oxidation of Cheddar cheese whey. Ultrason. Sonochemistry 2014, 21, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhang, C.; Kong, X.; Hua, Y. Oxidative modification of soy protein by peroxyl radicals. Food Chem. 2009, 116, 295–301. [Google Scholar] [CrossRef]
- Hosseini, A.; Jafari, S.M.; Mirzaei, H.; Asghari, A.; Akhavan, S. Application of image processing to assess emulsion stability and emulsification properties of Arabic gum. Carbohydr. Polym. 2015, 126, 1–8. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, R.; Tian, S.; Gai, J. A study on subunit groups of soybean protein extracts under SDS-PAGE. J. Am. Oil Chem. Soc. 2007, 84, 793–801. [Google Scholar] [CrossRef]
- Ahmadian-Kouchaksaraei, Z.; Varidi, M.; Varidi, M.J.; Pourazarang, H. Influence of processing conditions on the physicochemical and sensory properties of sesame milk: A novel nutritional beverage. LWT Food Sci. Technol. 2014, 57, 299–305. [Google Scholar] [CrossRef]
- O’Sullivan, J.; Murray, B.; Flynn, C.; Norton, I. The effect of ultrasound treatment on the structural, physical and emulsifying properties of animal and vegetable proteins. Food Hydrocoll. 2016, 53, 141–154. [Google Scholar] [CrossRef]
- Boode, K.; Walstra, P.; de Groot-Mostert, A.E.A. Partial coalescence in oil-in-water emulsions 2. Influence of the properties of the fat. Colloids Surf. A Physicochem. Eng. Asp. 1993, 81, 139–151. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Bibette, J.; Calderon, F.L.; Poulin, P. Emulsions: Basic principles. Rep. Prog. Phys. 1999, 62, 969. [Google Scholar] [CrossRef]
- El-Jaby, U.; Cunningham, M.; McKenna, T.F.L. Comparison of emulsification devices for the production of miniemulsions. Ind. Eng. Chem. Res. 2009, 48, 10147–10151. [Google Scholar] [CrossRef]
- Hubbard, A.T. Encyclopedia of Surface and Colloid Science; CRC Press: New York, NY, USA, 2002; Volume 1. [Google Scholar]
- Santos, J.; Calero, N.; Trujillo-Cayado, L.A.; Garcia, M.C.; Muñoz, J. Assessing differences between Ostwald ripening and coalescence by rheology, laser diffraction and multiple light scattering. Colloids Surf. B Biointerfaces 2017, 159, 405–411. [Google Scholar] [CrossRef]
- Ralla, T.; Salminen, H.; Braun, K.; Edelmann, M.; Dawid, C.; Hofmann, T.; Weiss, J. Investigations into the Structure-Function Relationship of the Naturally-Derived Surfactant Glycyrrhizin: Emulsion Stability. Food Biophys. 2020, 15, 288–296. [Google Scholar] [CrossRef]
- Dickinson, E. Flocculation of protein-stabilized oil-in-water emulsions. Colloids Surf. B Biointerfaces 2010, 81, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Keerati-u-rai, M.; Corredig, M. Heat-induced changes in oil-in-water emulsions stabilized with soy protein isolate. Food Hydrocoll. 2009, 23, 2141–2148. [Google Scholar] [CrossRef]
- Pinto, I.; Buss, A. ζ Potential as a Measure of Asphalt Emulsion Stability. Energy Fuels 2020, 34, 2143–2151. [Google Scholar] [CrossRef]
- Delahaije, R.J.B.M.; Wierenga, P.A.; van Nieuwenhuijzen, N.H.; Giuseppin, M.L.F.; Gruppen, H. Protein Concentration and Protein-Exposed Hydrophobicity as Dominant Parameters Determining the Flocculation of Protein-Stabilized Oil-in-Water Emulsions. Langmuir 2013, 29, 11567–11574. [Google Scholar] [CrossRef] [PubMed]
- Liang, A.; Tang, C.H. pH-dependent emulsifying properties of pea [Pisum sativum (L.)] proteins. Food Hydrocoll. 2013, 33, 309–319. [Google Scholar] [CrossRef]
- Tangsuphoom, N.; Coupland, J.N. Effect of heating and homogenization on the stability of coconut milk emulsions. J. Food Sci. 2005, 70, e466–e470. [Google Scholar] [CrossRef]
- Welch, R.W.; Wynne Griffiths, D. Variation in the oil content and fatty acid composition of field beans (Vicia faba) and peas (Pisum spp.). J. Sci. Food Agric. 1984, 35, 1282–1289. [Google Scholar] [CrossRef]
- Yoshida, H.; Saiki, M.; Yoshida, N.; Tomiyama, Y.; Mizushina, Y. Fatty acid distribution in triacylglycerols and phospholipids of broad beans (Vicia faba). Food Chem. 2009, 112, 924–928. [Google Scholar] [CrossRef]

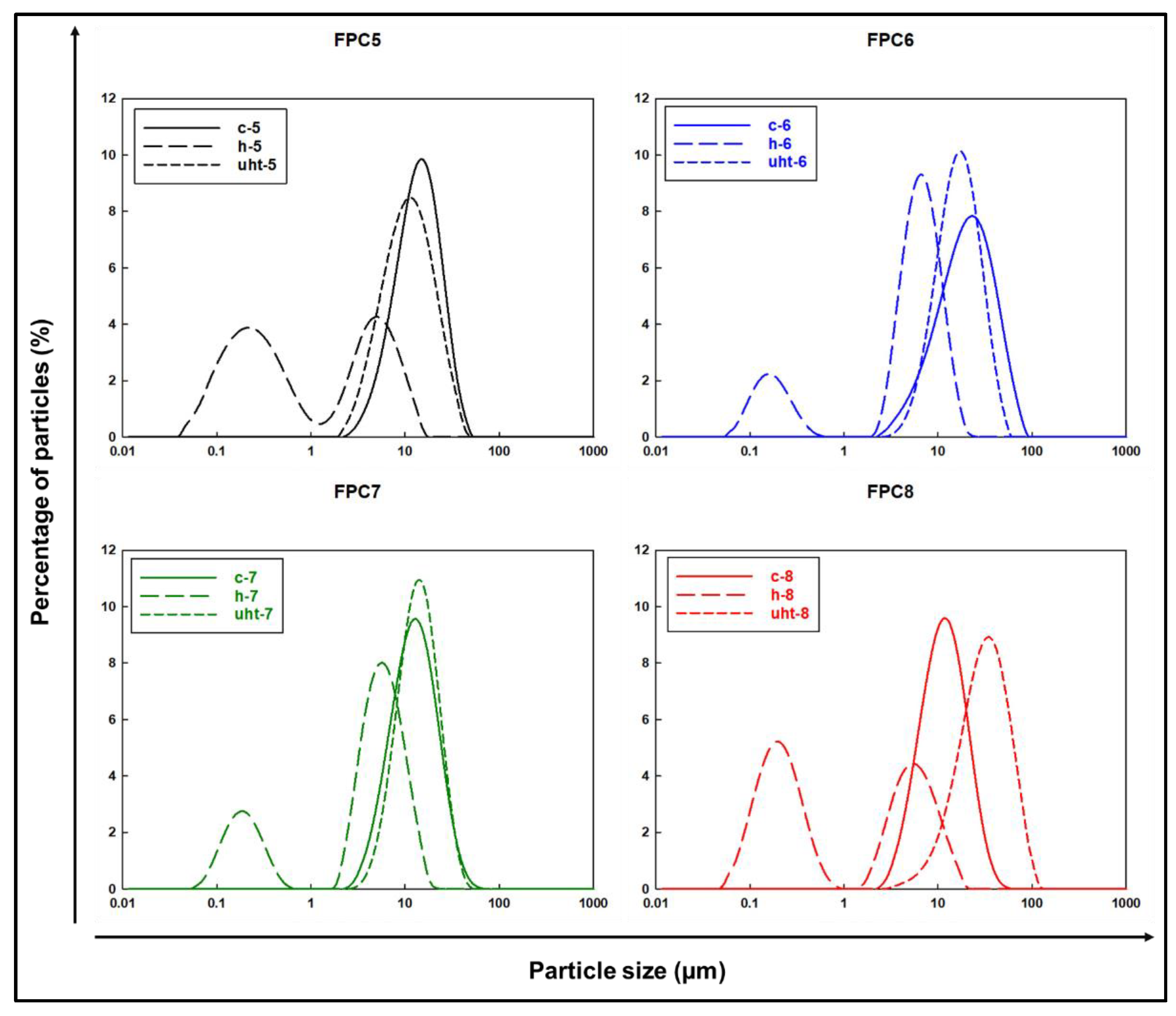
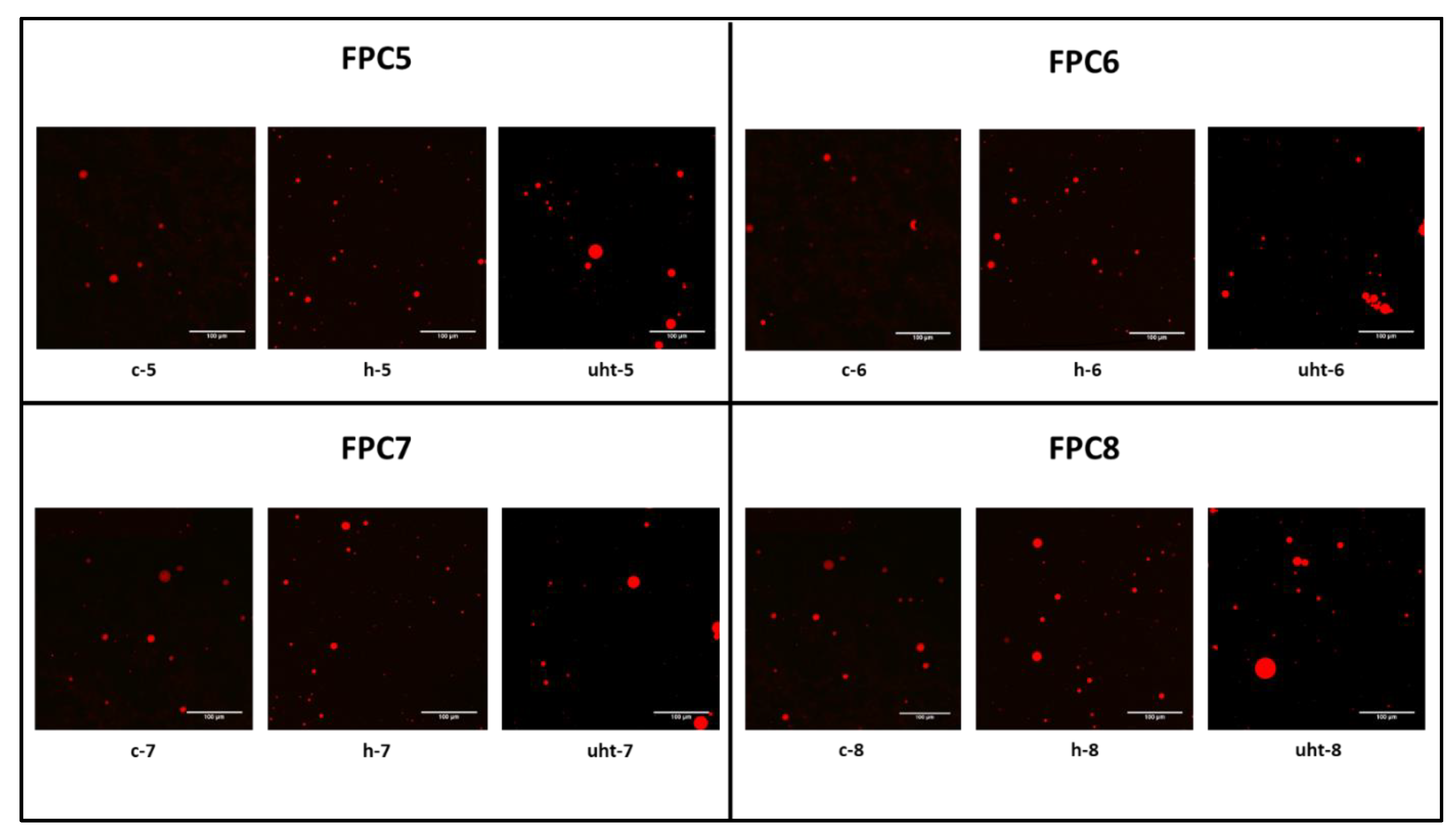

| Treatment | Emulsion Type | Particle Size Distribution | PDI (%) | FI | CI | ζ-Potential (mV) | Creaming Index | |||
|---|---|---|---|---|---|---|---|---|---|---|
| D 4,3–Volume-Weighed Mean (µm) | Uniformity (µm) | Specific Surface Area (µm) | D 3,2–Surface-Weighed Mean (µm) | |||||||
| FPC5 | coarse | 14.39 ± 0.00 c,d | 0.45 ± 0.00 b,c,d | 0.56 ± 0.01 e,f,g | 10.71 ± 0.00 c | 24.38 ± 0.01 c | 88.81 ± 0.35 d | 101.26 ± 5.24 c,d | −27.60 ± 3.25 d | 12.45 ± 0.35 b |
| homogenised | 2.48 ± 0.04 f | 0.23 ± 0.02 d | 21.50 ± 0.14 a | 0.28 ± 0.00 f | 6.88 ± 0.24 e | 4.79 ± 0.19 e | 63.93 ± 0.34 e,f | −24.63 ± 1.50 c,d | 7.00 ± 0.28 g | |
| UHT | 11.83 ± 0.16 c,d | 0.45 ± 0.02 b,c,d | 0.65 ± 0.01 e,f | 9.24 ± 0.20 d | 21.37 ± 0.71 c,d | 105.06 ± 3.66 d | 142.34 ± 9.92 b | −23.10 ± 0.96 b,c | 9.25 ± 021 d,e | |
| FPC6 | coarse | 22.19 ± 2.85 b | 0.57 ± 0.01 a,b | 0.44 ± 0.02 g,h | 13.57 ± 0.60 b | 41.52 ± 5.45 b | 160.82 ± 17.80 c | 101.57 ± 2.24 c,d | −23.10 ± 2.10 b,c | 13.05 ± 0.21 b |
| homogenised | 5.55 ± 1.52 e,f | 0.70 ± 0.20 a | 9.17 ± 0.05 b | 0.50 ± 0.22 f | 10.44 ± 0.13 d,e | 11.10 ± 2.87 e | 47.81 ± 0.49 f | −21.20 ± 0.80 b | 7.20 ± 0.42 g | |
| UHT | 17.25 ± 0.92 b,c | 0.44 ± 0.00 b,c,d | 0.45 ± 0.01 g,h | 13.38 ± 0.29 b | 29.03 ± 1.81 c | 194.98 ± 0.15 a,b | 175.32 ± 4.81 a | −21.47 ± 1.10 b,c | 10.25 ± 0.50 c,d | |
| FPC7 | coarse | 10.69 ± 0.26 d,e | 0.44 ± 0.00 b,c,d | 0.73 ± 0.01 e | 8.26 ± 0.08 d | 22.81 ± 0.33 c | 182.22 ± 12.42 b,c | 110.95 ± 5.30 c | −0.02 ± 0.02 a | 14.55 ± 0.21 a |
| homogenised | 4.65 ± 1.79 f | 0.37 ± 0.01 b,c,d | 1.16 ± 0.04 d | 5.18 ± 0.15 e | 9.17 ± 0.41 e | 10.83 ± 3.06 e | 83.41 ± 7.59 d,e | 0.08 ± 0.03 a | 7.95 ± 0.21 f,g | |
| UHT | 14.08 ± 0.29 c,d | 0.39 ± 0.02 b,c,d | 0.53 ± 0.01 f,g | 11.26 ± 0.14 c | 22.89 ± 1.45 c | 212.14 ± 3.91 a,b | 191.81 ± 11.82 a | −0.12 ± 0.07 a | 10.40 ± 0.28 c | |
| FPC8 | coarse | 12.22 ± 0.10 c,d | 0.48 ± 0.00 a,b,c | 0.66 ± 0.00 e,f | 9.11 ± 0.07 d | 20.89 ± 0.18 c,d | 199.74 ± 18.27 a,b | 109.06 ± 1.30 c | 0.01 ± 0.07 a | 14.95 ± 0.21 a |
| homogenised | 2.87 ± 0.01 f | 0.28 ± 0.00 c,d | 4.92 ± 0.01 c | 1.22 ± 0.00 f | 7.84 ± 0.34 e | 16.10 ± 1.27 e | 78.98 ± 0.32 d,e | 0.01 ± 0.20 a | 8.40 ± 0.14 e,f | |
| UHT | 33.27 ± 3.55 a | 0.49 ± 0.02 a,b,c | 0.27 ± 0.01 h | 22.11 ± 0.78 a | 59.49 ± 7.40 a | 226.50 ± 0.26 a | 197.43 ± 7.08 a | 0.24 ± 0.11 a | 15.15 ± 0.21 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawaz, M.A.; Singh, T.K.; Stockmann, R.; Jegasothy, H.; Buckow, R. Quality Attributes of Ultra-High Temperature-Treated Model Beverages Prepared with Faba Bean Protein Concentrates. Foods 2021, 10, 1244. https://doi.org/10.3390/foods10061244
Nawaz MA, Singh TK, Stockmann R, Jegasothy H, Buckow R. Quality Attributes of Ultra-High Temperature-Treated Model Beverages Prepared with Faba Bean Protein Concentrates. Foods. 2021; 10(6):1244. https://doi.org/10.3390/foods10061244
Chicago/Turabian StyleNawaz, Malik Adil, Tanoj Kumar Singh, Regine Stockmann, Hema Jegasothy, and Roman Buckow. 2021. "Quality Attributes of Ultra-High Temperature-Treated Model Beverages Prepared with Faba Bean Protein Concentrates" Foods 10, no. 6: 1244. https://doi.org/10.3390/foods10061244
APA StyleNawaz, M. A., Singh, T. K., Stockmann, R., Jegasothy, H., & Buckow, R. (2021). Quality Attributes of Ultra-High Temperature-Treated Model Beverages Prepared with Faba Bean Protein Concentrates. Foods, 10(6), 1244. https://doi.org/10.3390/foods10061244