Characterization of the Fermentation and Sensory Profiles of Novel Yeast-Fermented Acid Whey Beverages
Abstract
1. Introduction
2. Materials and Methods
2.1. Fermentation Characterization
2.1.1. Fermentation Setup
2.1.2. Data Collection and Analysis
2.2. Sensory Study
2.2.1. Sample Preparation
2.2.2. Training Session
2.2.3. Individual Online Questionnaire
2.2.4. Analyses
3. Results and Discussion
3.1. Fermentation Profile
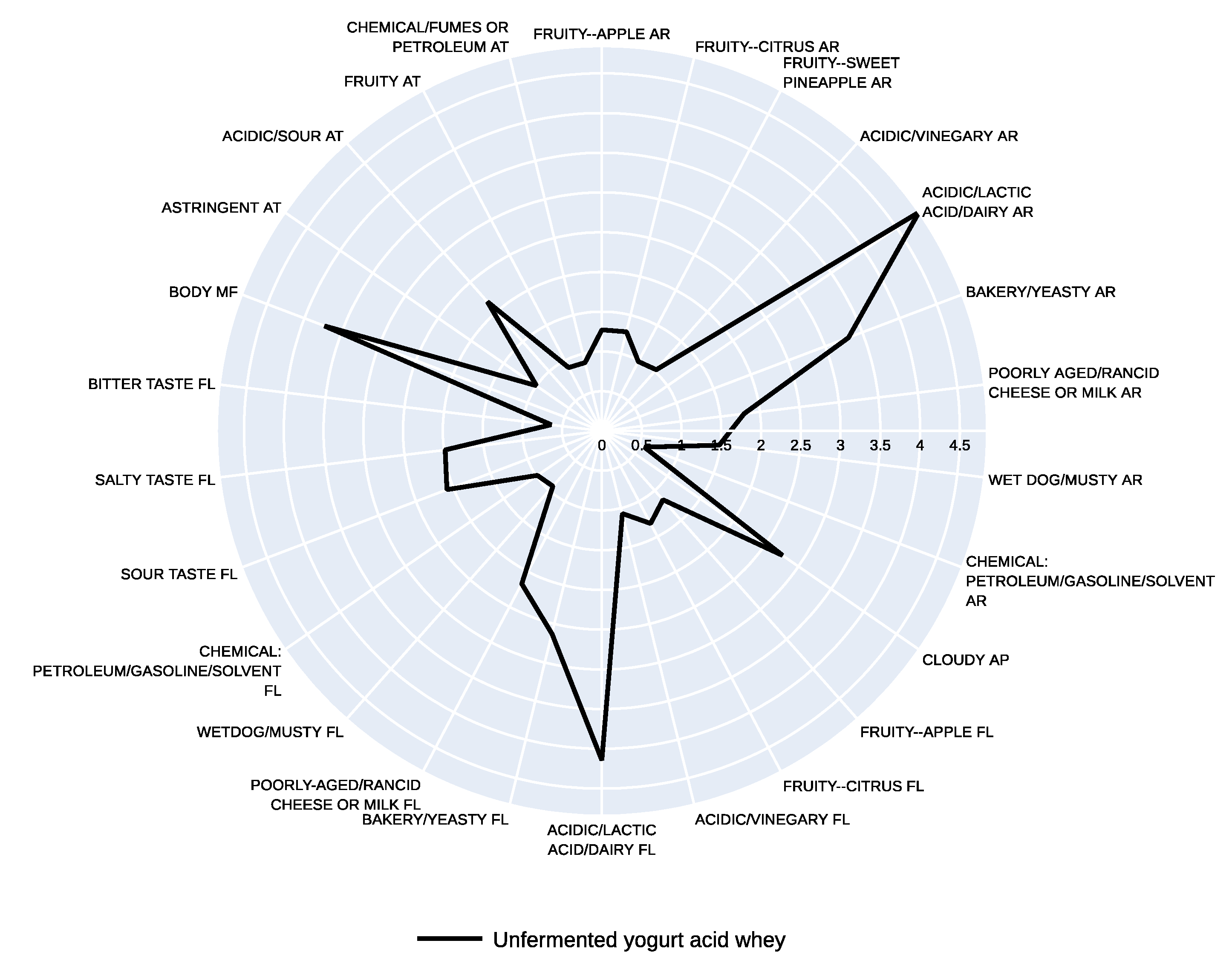
3.2. Sensory Profile
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flinois, J.C.; Dando, R.; Padilla-Zakour, O.I. Effects of replacing buttermilk with yogurt acid whey in ranch dressing. J. Dairy Sci. 2019, 102, 7874–7883. [Google Scholar] [CrossRef]
- El-Abbadi, N.H.; Dao, M.C.; Meydani, S.N. Yogurt: Role in healthy and active aging. Am. J. Clin. Nutr. 2014, 99, 1263s–1270s. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Kleinschmidt, T. Synthesis of galactooligosaccharides using sweet and acid whey as a substrate. Int. Dairy J. 2015, 48, 15–22. [Google Scholar] [CrossRef]
- DEC. Whey Management for Agriculture. Available online: https://www.dec.ny.gov/chemical/94164.html (accessed on 1 April 2021).
- Carvalho, F.; Prazeres, A.R.; Rivas, J. Cheese whey wastewater: Characterization and treatment. Sci. Total Environ. 2013, 445, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Menchik, P.; Zuber, T.; Zuber, A.; Moraru, C.I. Short communication: Composition of coproduct streams from dairy processing: Acid whey and milk permeate. J. Dairy Sci. 2019, 102, 3978–3984. [Google Scholar] [CrossRef]
- Turner, T.L.; Kim, E.; Hwang, C.; Zhang, G.C.; Liu, J.J.; Jin, Y.S. Short communication: Conversion of lactose and whey into lactic acid by engineered yeast. J. Dairy Sci. 2017, 100, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Mano, J.; Liu, N.; Hammond, J.H.; Currie, D.H.; Stephanopoulos, G. Engineering Yarrowia lipolytica for the utilization of acid whey. Metab. Eng. 2020, 57, 43–50. [Google Scholar] [CrossRef]
- Alcoholic Beverage Drinking Occasions—US—September 2019; Mintel: London, UK, 2019.
- Beer: Incl Impact of COVID-19—US—November 2020; Mintel: London, UK, 2020.
- Seo, S.; Ahn, H.K.; Jeong, J.; Moon, J. Consumers’ Attitude toward Sustainable Food Products: Ingredients vs. Packaging. Sustainability 2016, 8, 1073. [Google Scholar] [CrossRef]
- Su, C.H.; Tsai, C.H.; Chen, M.H.; Lv, W.Q. US Sustainable Food Market Generation Z Consumer Segments. Sustainability 2019, 11, 3607. [Google Scholar] [CrossRef]
- Parapouli, M.; Vasileiadis, A.; Afendra, A.S.; Hatziloukas, E. Saccharomyces cerevisiae and its industrial applications. Aims Microbiol. 2020, 6, 1–31. [Google Scholar] [CrossRef]
- Goncalves, M.; Pontes, A.; Almeida, P.; Barbosa, R.; Serra, M.; Libkind, D.; Hutzler, M.; Goncalves, P.; Sampaio, J.P. Distinct Domestication Trajectories in Top-Fermenting Beer Yeasts and Wine Yeasts. Curr. Biol. 2016, 26, 2750–2761. [Google Scholar] [CrossRef]
- Oleary, V.S.; Green, R.; Sullivan, B.C.; Holsinger, V.H. Alcohol Production by Selected Yeast Strains in Lactase-Hydrolyzed Acid Whey. Biotechnol. Bioeng. 1977, 19, 1019–1035. [Google Scholar] [CrossRef]
- Fonseca, G.G.; Heinzle, E.; Wittmann, C.; Gombert, A.K. The yeast Kluyveromyces marxianus and its biotechnological potential. Appl. Microbiol. Biotechnol. 2008, 79, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Dragone, G.; Mussatto, S.I.; Oliveira, J.M.; Teixeira, J.A. Characterisation of volatile compounds in an alcoholic beverage produced by whey fermentation. Food Chem. 2009, 112, 929–935. [Google Scholar] [CrossRef]
- Wedral, D.; Shewfelt, R.; Frank, J. The challenge of Brettanomyces in wine. LWT Food Sci. Technol. 2010, 43, 1474–1479. [Google Scholar] [CrossRef]
- Steensels, J.; Daenen, L.; Malcorps, P.; Derdelinckx, G.; Verachtert, H.; Verstrepen, K.J. Brettanomyces yeasts—From spoilage organisms to valuable contributors to industrial fermentations. Int. J Food Microbiol. 2015, 206, 24–38. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Fell, J.W. The Yeasts: A Taxonomic Study, 4th ed.; Elsevier: Amsterdam, The Netherlands; New York, NY, USA, 2000; pp. 174–176. [Google Scholar]
- Marcus, J.F.; DeMarsh, T.A.; Alcaine, S.D. Upcycling of Whey Permeate through Yeast- and Mold-Driven Fermentations under Anoxic and Oxic Conditions. Fermentation 2021, 7, 16. [Google Scholar] [CrossRef]
- Grba, S.; Stehlik-Tomas, V.; Stanzer, D.; Vahčić, N.; Škrlin, A. Selection of yeast strain Kluyveromyces marxianus for alcohol and biomass production on whey. Chem. Biochem. Eng. Q. 2002, 16, 13–16. [Google Scholar]
- OYL-091 HORNINDAL KVEIK. Available online: https://omegayeast.com/yeast/norwegian-kveik/hornindal-kveik (accessed on 2 May 2021).
- Lawton, M.R.; deRiancho, D.L.; Alcaine, S.D. Lactose utilization by Brettanomyces claussenii expands potential for valorization of dairy by-products to functional beverages through fermentation. Curr. Opin. Food Sci. 2021. [Google Scholar] [CrossRef]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food: Principles and Practices, 2nd ed.; Springer: New York, NY, USA, 2010; pp. 382–388. [Google Scholar]
- Sensory Lexicon: Unabridged Definition and References; World Coffee Research: College Station, TX, USA, 2017.
- Aday, S.; Karagul Yuceer, Y. Physicochemical and Sensory Properties of Mihalic Cheese. Int. J. Food Prop. 2014, 17, 2207–2227. [Google Scholar] [CrossRef]
- Gallardo-Escamilla, F.J.; Kelly, A.L.; Delahunty, C.M. Influence of starter culture on flavor and headspace volatile profiles of fermented whey and whey produced from fermented milk. J. Dairy Sci. 2005, 88, 3745–3753. [Google Scholar] [CrossRef]
- IOC Be Fruits™; Lallemand Australia: Edwardstown, Australia, 2016.
- Callejon, R.M.; Clavijo, A.; Ortigueira, P.; Troncoso, A.M.; Paneque, P.; Morales, M.L. Volatile and sensory profile of organic red wines produced by different selected autochthonous and commercial Saccharomyces cerevisiae strains. Anal. Chim. Acta 2010, 660, 68–75. [Google Scholar] [CrossRef]
- Patrignani, F.; Chinnici, F.; Serrazanetti, D.I.; Vemocchi, P.; Ndagijimana, M.; Riponi, C.; Lanciottii, R. Production of Volatile and Sulfur Compounds by 10 Saccharomyces cerevisiae Strains Inoculated in Trebbiano Must. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Ochando, T.; Mouret, J.R.; Humbert-Goffard, A.; Aguera, E.; Sablayrolles, J.M.; Farines, V. Comprehensive study of the dynamic interaction between SO2 and acetaldehyde during alcoholic fermentation. Food Res. Int. 2020, 136. [Google Scholar] [CrossRef]
- Beshkova, D.; Simova, E.; Frengova, G.; Simov, Z. Production of flavour compounds by yogurt starter cultures. J. Ind. Microbiol. Biotechnol. 1998, 20, 180–186. [Google Scholar] [CrossRef]
- Basso, R.F.; Alcarde, A.R.; Portugal, C.B. Could non-Saccharomyces yeasts contribute on innovative brewing fermentations? Food Res. Int. 2016, 86, 112–120. [Google Scholar] [CrossRef]
- Crauwels, S.; Zhu, B.; Steensels, J.; Busschaert, P.; De Samblanx, G.; Marchal, K.; Willems, K.A.; Verstrepen, K.J.; Lievens, B. Assessing Genetic Diversity among Brettanomyces Yeasts by DNA Fingerprinting and Whole-Genome Sequencing. Appl. Environ. Microbiol. 2014, 80, 4398–4413. [Google Scholar] [CrossRef] [PubMed]
- Moktaduzzaman, M.; Galafassi, S.; Capusoni, C.; Vigentini, I.; Ling, Z.H.; Piskur, J.; Compagno, C. Galactose utilization sheds new light on sugar metabolism in the sequenced strain Dekkera bruxellensis CBS 2499. Fems Yeast Res. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Chen, J.; Jensen, P.R.; Solem, C. Sweet As Sugar-Efficient Conversion of Lactose into Sweet Sugars Using a Novel Whole-Cell Catalyst. J. Agric. Food Chem. 2019, 67, 6257–6262. [Google Scholar] [CrossRef]
- Fabre, C.E.; Duviau, V.J.; Blanc, P.J.; Goma, G. Identification of Volatile Flavor Compounds Obtained in Culture, of Kluyveromyces-Marxianus. Biotechnol. Lett. 1995, 17, 1207–1212. [Google Scholar] [CrossRef]
- İşleten Hoşoğlu, M. Study of Increasing the Production of Volatile Flavor Compounds by the Yeast Kluyveromyces marxianus through Optimization of Carbon and Nitrogen Sources. Food Health 2018, 4, 112–123. [Google Scholar] [CrossRef]
- Preiss, R.; Tyrawa, C.; Krogerus, K.; Garshol, L.M.; van der Merwe, G. Traditional Norwegian Kveik Are a Genetically Distinct Group of Domesticated Saccharomyces cerevisiae Brewing Yeasts. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
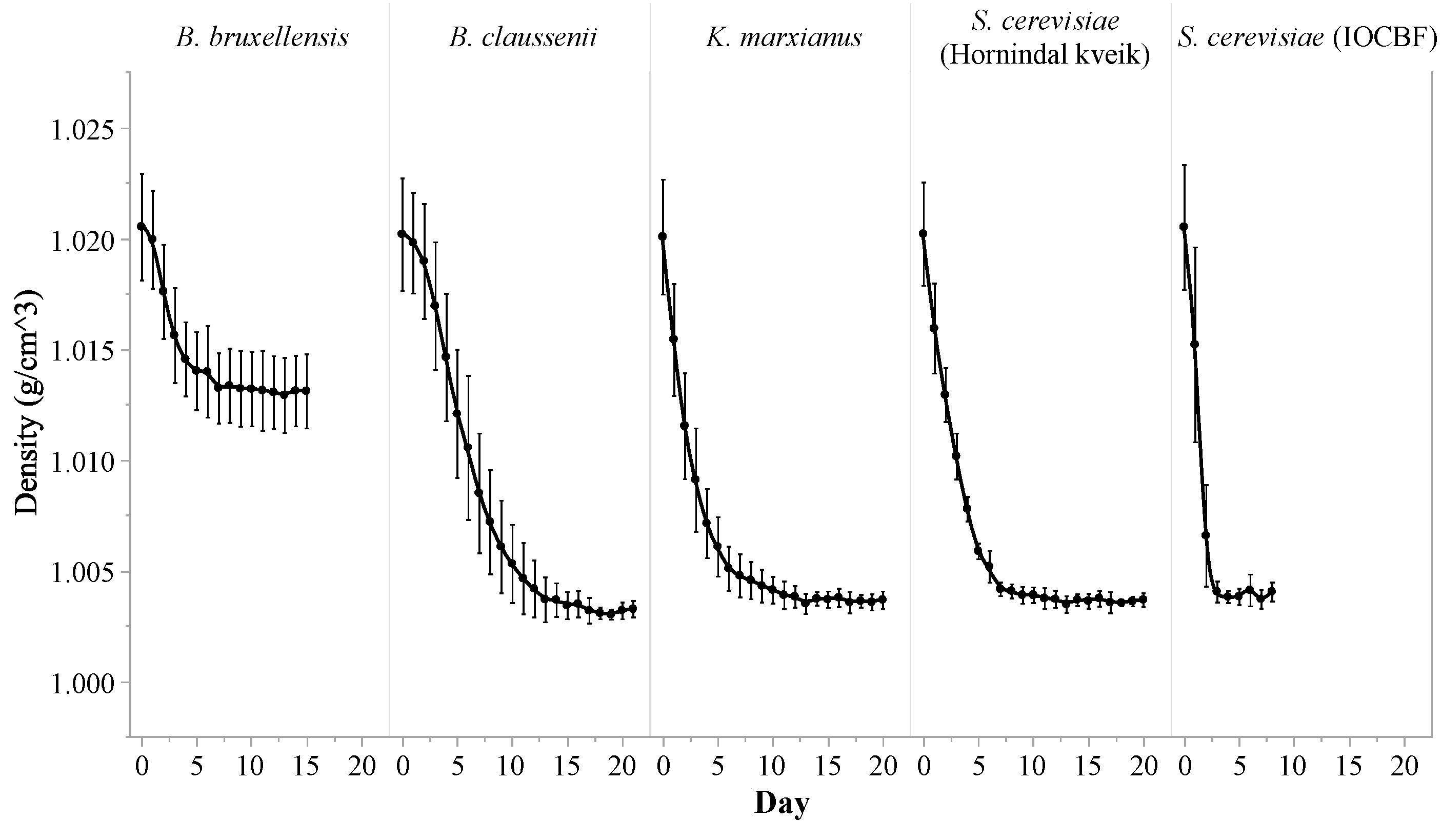
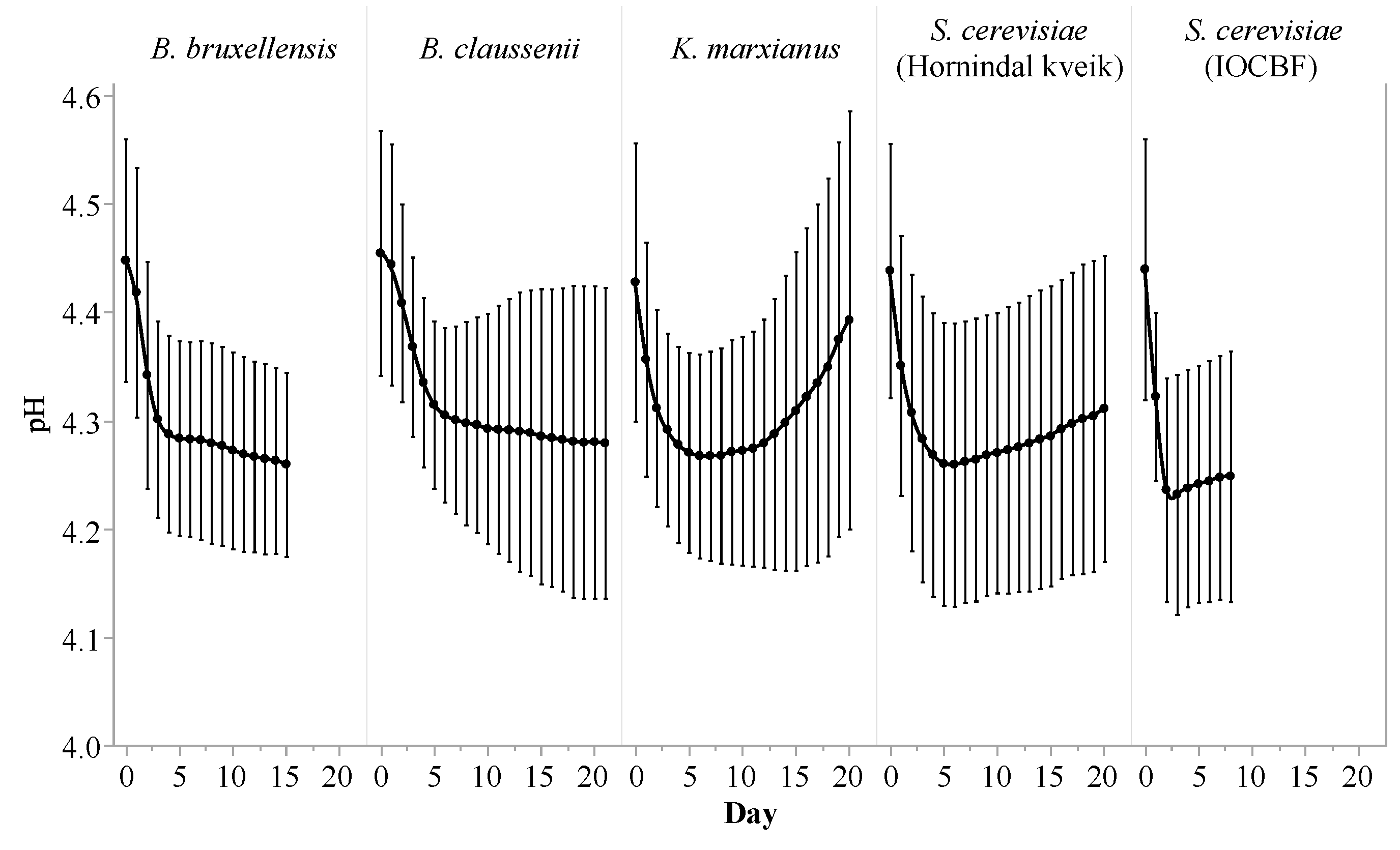
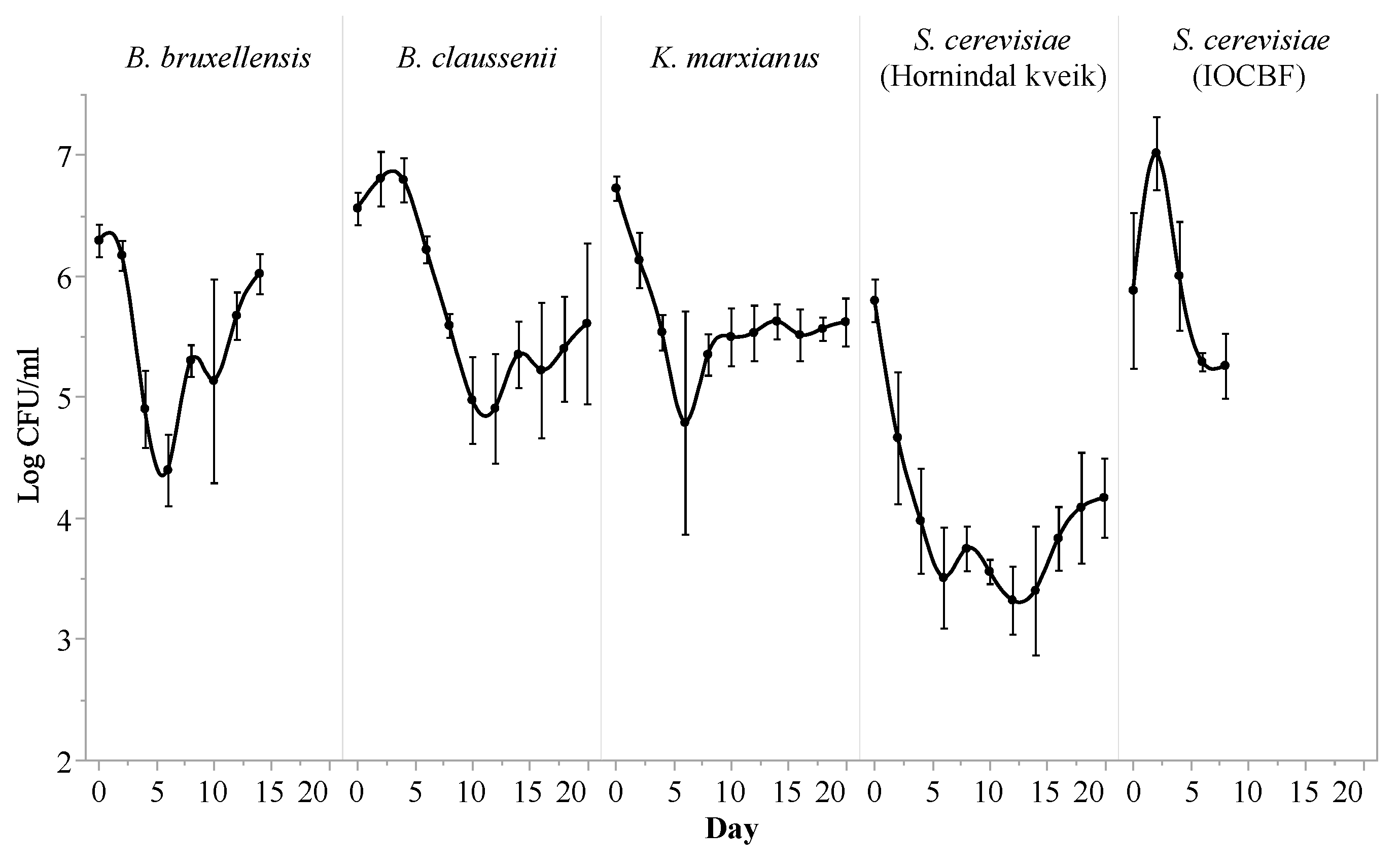
| Citric Acid (g/L) | Lactic Acid (g/L) | Acetic Acid (g/L) | Ethanol (%) | Lactose (g/L) | Galactose (g/L) | Glucose (g/L) | |
|---|---|---|---|---|---|---|---|
| Beginning of fermentation | |||||||
| K. marxianus | 2.00 ± 0.28 | 6.60 ± 0.55 | 1.18 ± 0.34 | 0.15 ± 0.08 ** | 32.85 ± 6.65 ** | 3.13 ± 0.13 ** | 1.06 ± 1.83 |
| S.cerevisiae (Hornindal kveik) | 2.00 ± 0.27 | 6.48 ± 0.42 | 1.04 ± 0.37 | 0.14 ± 0.08 ** | 4.13 ± 3.33 | 19.12 ± 3.43 ** | 14.31 ± 1.55 ** |
| B. bruxellensis | 2.02 ± 0.27 | 6.52 ± 0.37 | 1.07 ± 0.26 | 0.12 ± 0.10 ** | 4.01 ± 3.11 | 19.06 ± 2.84 | 14.73 ± 1.67 ** |
| B. claussenii | 2.01 ± 0.27 | 6.58 ± 0.48 | 1.14 ± 0.42 | 0.12 ± 0.10 ** | 33.26 ± 6.51 ** | 4.22 ± 1.61 ** | nd |
| S.cerevisiae (IOCBF) | 2.02 ± 0.28 | 6.58 ± 0.55 | 1.18 ± 0.65 | 0.13 ± 0.11 ** | 3.70 ± 3.12 | 19.38 ± 3.35 ** | 14.91 ± 2.09 ** |
| End of fermentation | |||||||
| K. marxianus | 1.95 ± 0.25 | 5.97 ± 0.84 | 1.78 ± 0.60 | 2.33 ± 0.42 ** | nd ** | nd ** | nd |
| S.cerevisiae (Hornindal kveik) | 1.94 ± 0.21 | 6.01 ± 0.41 | 0.53 ± 0.24 | 2.28 ± 0.30 ** | nd | nd ** | nd ** |
| B. bruxellensis | 1.99 ± 0.31 | 6.30 ± 0.37 | 1.26 ± 0.12 | 1.07 ± 0.04 ** | nd | 20.28 ± 4.91 | nd ** |
| B. claussenii | 1.99 ± 0.40 | 6.47 ± 1.09 | 1.16 ± 0.13 | 2.41 ± 0.37 ** | nd ** | nd ** | nd |
| S.cerevisiae (IOCBF) | 2.01 ± 0.28 | 6.11 ± 0.46 | 0.91 ± 0.37 | 2.30 ± 0.40 ** | nd | nd ** | nd ** |
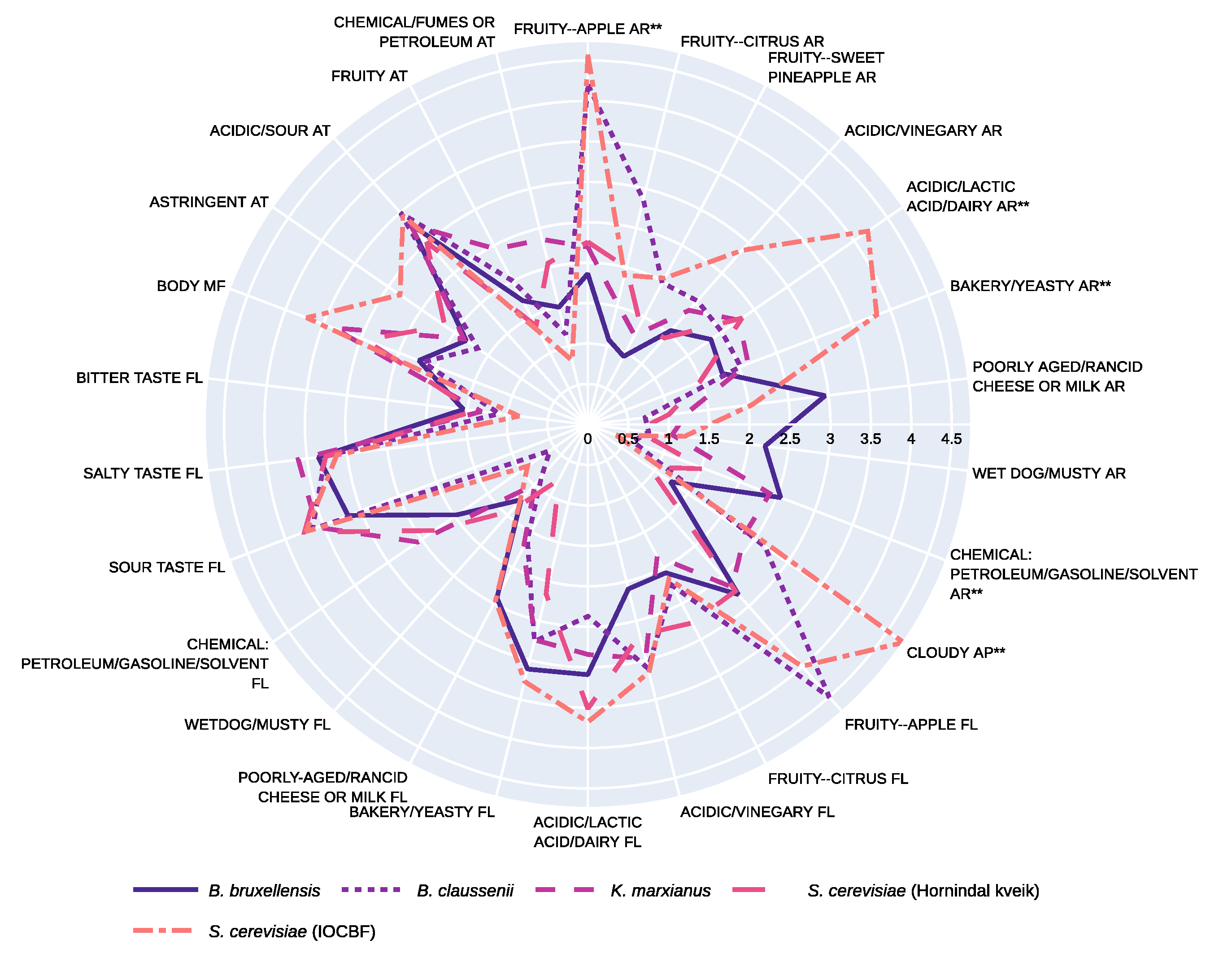
| K. marxianus | S. cerevisiae (Hornindal Kveik) | B. bruxellensis | B. claussenii | S. cerevisiae (IOCBF) | Unfermented YAW | |
|---|---|---|---|---|---|---|
| Aroma | ||||||
| Fruity--apple | 2.19 ± 1.89 ab | 2.26 ± 1.85 ab | 1.86 ± 1.96 b | 4.20 ± 2.53 ab | 4.57 ± 2.62 a | 1.27 ± 1.00 |
| Fruity--citrus | 1.54 ± 0.96 a | 2.00 ± 1.90 a | 1.08 ± 1.24 a | 2.86 ± 2.13 a | 1.90 ± 1.83 a | 1.29 ± 1.50 |
| Fruity--sweet pineapple | 1.24 ± 1.15 a | 1.39 ± 1.82 a | 0.95 ± 1.58 a | 1.98 ± 1.33 a | 2.04 ± 2.37 a | 0.99 ± 1.26 |
| Acidic, vinegary | 1.89 ± 1.31 a | 1.43 ± 1.27 a | 1.55 ± 1.31 a | 2.04 ± 2.00 a | 2.88 ± 2.47 a | 1.03 ± 0.90 |
| Acidic, lactic acid, dairy | 2.27 ± 1.44 ab | 2.31 ± 2.02 ab | 1.85 ± 1.85 b | 2.01 ± 1.38 b | 4.21 ± 2.25 a | 4.84 ± 3.24 |
| Bakery, yeasty | 2.12 ± 2.26 ab | 1.48 ± 1.48 b | 1.78 ± 1.45 ab | 2.02 ± 2.18 ab | 3.82 ± 2.59 a | 3.31 ± 2.73 |
| Poorly aged, Rancid cheese or milk | 1.24 ± 1.39 a | 1.01 ± 2.16 a | 2.96 ± 2.65 a | 0.71 ± 1.07 a | 2.05 ± 2.68 a | 1.80 ± 3.04 |
| Wet dog, musty | 1.05 ± 1.21 a | 0.61 ± 0.90 a | 2.21 ± 2.74 a | 0.76 ± 1.04 a | 1.21± 2.40 a | 1.49 ± 3.09 |
| Chemical: petroleum, gasoline, solvent | 2.41 ± 2.55 ab | 1.55 ± 1.58 ab | 2.55 ± 2.73 a | 0.60 ± 0.85 ab | 0.38 ± 0.57 b | 0.57 ± 1.02 |
| Appearance | ||||||
| Cloudy | 2.36 ± 1.08 b | 0.93 ± 0.71 b | 1.24 ± 1.00 b | 2.66 ± 2.50 b | 4.74 ± 2.62 a | 2.76 ± 1.54 |
| Flavor | ||||||
| Fruity--apple | 2.69 ± 2.15 a | 2.75 ± 2.10 a | 2.81 ± 2.01 a | 4.50 ± 1.94 a | 3.99 ± 2.48 a | 1.16 ± 1.03 |
| Fruity--citrus | 1.84 ± 1.81 a | 2.77 ± 2.46 a | 2.07 ± 1.88 a | 2.25 ± 2.14 a | 2.16 ± 2.00 a | 1.31± 1.38 |
| Acidic, vinegary | 2.98 ± 1.71 a | 2.68 ± 1.80 a | 2.09 ± 2.10 a | 3.11 ± 2.97 a | 3.16 ± 2.50 a | 1.07 ± 0.99 |
| Acidic, lactic acid, dairy | 2.84 ± 1.64 a | 3.51 ± 1.52 a | 3.09 ± 1.89 a | 2.37 ± 1.30 a | 3.68 ± 2.23 a | 4.14 ± 2.36 |
| Bakery, yeasty | 2.74 ± 2.05 a | 2.16 ± 1.45 a | 3.11 ± 2.32 a | 2.76 ± 2.00 a | 3.27 ± 2.03 a | 2.63 ± 1.32 |
| Poorly aged, rancid cheese or milk | 1.71 ± 1.79 a | 0.77 ± 0.79 a | 2.42 ± 2.18 a | 1.61 ± 1.95 a | 2.46 ± 2.75 a | 2.17 ± 1.24 |
| Wet dog, musty | 0.99 ± 1.18 a | 1.43 ± 2.33 a | 1.24 ± 2.01 a | 0.86 ± 1.35 a | 1.26 ± 1.98 a | 0.93 ± 1.35 |
| Chemical: petroleum, gasoline, solvent | 2.56 ± 2.88 a | 2.31± 2.45 a | 1.96 ± 2.45 a | 0.57 ± 0.81 a | 0.91 ± 1.79 a | 0.99 ± 2.05 |
| Sour taste | 3.59 ± 1.85 a | 3.75 ± 1.52 a | 3.17 ± 2.08 a | 3.64 ± 1.45 a | 3.73 ± 2.45 a | 2.09 ± 1.64 |
| Salty taste | 3.61 ± 2.07 a | 3.26 ± 1.98 a | 3.36 ± 2.38 a | 3.27 ± 1.79 a | 3.13 ± 2.23 a | 1.99 ± 1.32 |
| Bitter taste | 1.33 ± 1.75 a | 1.41± 1.54 a | 1.55 ± 1.81 a | 1.11± 1.26 a | 0.86 ± 1.04 a | 0.64 ± 1.01 |
| Mouthfeel | ||||||
| Body | 3.37 ± 1.68 a | 2.95 ± 1.46 a | 2.24 ± 1.20 a | 2.18 ± 1.15 a | 3.72 ± 2.00 a | 3.73 ± 2.30 |
| Aftertaste | ||||||
| Astringent | 1.86 ± 1.59 a | 2.15 ± 2.02 a | 1.83 ± 2.09 a | 1.66 ± 1.46 a | 2.82 ± 2.49 a | 1.00 ± 0.81 |
| Acidic, sour | 3.30 ± 1.39 a | 3.01 ± 0.93 a | 3.31 ± 2.16 a | 3.49 ± 2.05 a | 3.45 ± 1.93 a | 2.17 ± 1.41 |
| Fruity | 2.45 ± 2.26 a | 1.38 ± 1.36 a | 1.73 ± 1.84 a | 2.01 ± 1.38 a | 1.29 ± 1.51 a | 0.90 ± 0.72 |
| Chemical, fumes or petroleum | 2.36 ± 2.75 a | 2.06 ± 2.32 a | 1.49 ± 1.87 a | 1.15 ± 1.19 a | 0.81 ± 1.53 a | 0.89 ± 1.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, S.; DeMarsh, T.A.; deRiancho, D.; Stelick, A.; Alcaine, S.D. Characterization of the Fermentation and Sensory Profiles of Novel Yeast-Fermented Acid Whey Beverages. Foods 2021, 10, 1204. https://doi.org/10.3390/foods10061204
Luo S, DeMarsh TA, deRiancho D, Stelick A, Alcaine SD. Characterization of the Fermentation and Sensory Profiles of Novel Yeast-Fermented Acid Whey Beverages. Foods. 2021; 10(6):1204. https://doi.org/10.3390/foods10061204
Chicago/Turabian StyleLuo, Siyi (Rossie), Timothy A. DeMarsh, Dana deRiancho, Alina Stelick, and Samuel D. Alcaine. 2021. "Characterization of the Fermentation and Sensory Profiles of Novel Yeast-Fermented Acid Whey Beverages" Foods 10, no. 6: 1204. https://doi.org/10.3390/foods10061204
APA StyleLuo, S., DeMarsh, T. A., deRiancho, D., Stelick, A., & Alcaine, S. D. (2021). Characterization of the Fermentation and Sensory Profiles of Novel Yeast-Fermented Acid Whey Beverages. Foods, 10(6), 1204. https://doi.org/10.3390/foods10061204






