Effect of Adding Resistant Maltodextrin to Pasteurized Orange Juice on Bioactive Compounds and Their Bioaccessibility
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Sample Preparation and Pasteurization
2.3. Analytical Determinations
2.3.1. Brix, Acidity, and pH
2.3.2. Total Phenols (TP)
2.3.3. Total Carotenoids (TC)
2.3.4. Ascorbic Acid (AA) and Vitamin C
2.3.5. Antioxidant Capacity (AC)
2.4. In Vitro Digestion
2.5. Statistical Analysis
3. Results and Discussion
3.1. Effect of RMD on TSS, Acidity, pH, and Bioactive Compounds of Pasteurized Orange Juice
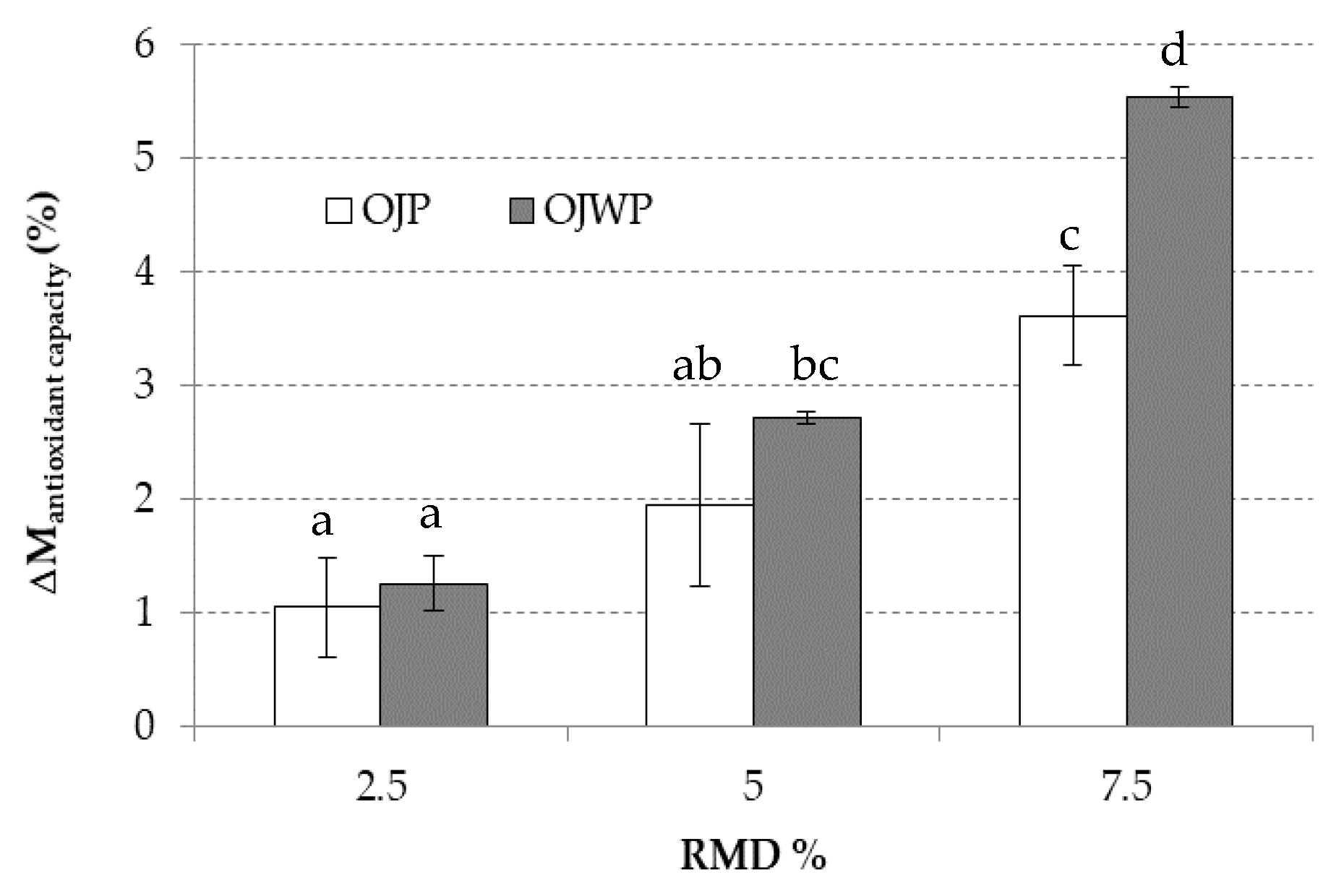
3.2. Effect of RMD on In Vitro Digestibility and Bioactive Compounds Bioaccessibility of Pasteurized Orange Juice
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, R.H. Dietary bioactive compounds and their health implications. J. Food Sci. 2013, 78, A18–A25. [Google Scholar] [CrossRef]
- Allegra, V.; Zarbà, C.; La Via, G.; Zarbà, A.S. Why the new orange juice consumption model favors global trade and growth in orange production. Br. Food J. 2019, 121, 1954–1968. [Google Scholar] [CrossRef]
- Priyadarshini, A.; Priyadarshini, A. Market dimensions of the fruit juice industry. In Fruit Juices: Extraction, Composition, Quality and Analysis, 1st ed.; Rajauria, G., Tiwari, B.K., Eds.; Elsevier: London, UK, 2018; pp. 15–32. [Google Scholar]
- Day, L.; Seymour, R.B.; Pitts, K.F.; Konczak, I.; Lundin, L. Incorporation of functional ingredients into foods. Trends Food Sci. Technol. 2009, 20, 388–395. [Google Scholar] [CrossRef]
- Granato, D.; Barba, F.J.; Bursać Kovačević, D.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional foods: Product development, technological trends, efficacy testing, and safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef]
- Butel, M.J. Probiotics, gut microbiota and health. Med. Mal. Infect. 2014, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Probert, H.M.; Van Loo, J.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef]
- Corzo, N.; Alonso, J.L.; Azpiroz, F.; Calvo, M.A.; Cirici, M.; Leis, R.; Lombó, F.; Mateos-Aparicio, I.; Plou, F.J.; Ruas-Madiedo, P.; et al. Prebiotics: Concept, properties and beneficial effects. Nutr. Hosp. 2015, 31, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Lockyer, S.; Nugent, A.P. Health effects of resistant starch. Nutr. Bull. 2017, 42, 10–41. [Google Scholar] [CrossRef]
- Ye, Z.; Arumugam, V.; Haugabrooks, E.; Williamson, P.; Hendrich, S. Soluble dietary fiber (Fibersol-2) decreased hunger and increased satiety hormones in humans when ingested with a meal. Nutr. Res. 2015, 35, 393–400. [Google Scholar] [CrossRef]
- Livesey, G.; Tagami, H. Interventions to lower the glycemic response to carbohydrate foods with a low-viscosity fiber (resistant maltodextrin): Meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2009, 89, 114–125. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Oga, H.; Hayashi, N.; Yamada, T.; Tagami, H. Suppressive effect of resistant maltodextrin on postprandial blood triacylglycerol elevation. Eur. J. Nutr. 2007, 46, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Baer, D.J.; Stote, K.S.; Henderson, T.; Paul, D.R.; Okuma, K.; Tagami, H.; Kanahori, S.; Gordon, D.T.; Rumpler, W.V.; Ukhanova, M.; et al. The metabolizable energy of dietary resistant maltodextrin is variable and alters fecal microbiota composition in adult men. J. Nutr. 2014, 144, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Tuorila, H.; Cardello, A.V. Consumer responses to an off-flavor in juice in the presence of specific health claims. Food Qual. Prefer. 2002, 13, 561–569. [Google Scholar] [CrossRef]
- Fonteles Vidal, T.; Rodrigues, S. Prebiotic in fruit juice: Processing challenges, advances, and perspectives. Curr. Opin. Food Sci. 2018, 22, 55–61. [Google Scholar] [CrossRef]
- Neves, M.F.; Trombin, V.G.; Marques, V.N.; Martinez, L.F. Global orange juice market: A 16-year summary and opportunities for creating value. Trop. Plant Pathol. 2020, 45, 166–174. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Plaza, L.; de Ancos, B.; Cano, M.P. Quantitative bioactive compounds assessment and their relative contribution to the antioxidant capacity of commercial orange juices. J. Sci. Food Agric. 2003, 83, 430–439. [Google Scholar] [CrossRef]
- Lima, A.C.D.; Cecatti, C.; Fidélix, M.P.; Adorno, M.A.T.; Sakamoto, I.K.; Cesar, T.B.; Sivieri, K. Effect of Daily Consumption of Orange Juice on the Levels of Blood Glucose, Lipids, and Gut Microbiota Metabolites: Controlled Clinical Trials. J. Med. Food 2019, 22, 202–210. [Google Scholar] [CrossRef]
- Perez-Cacho, P.R.; Rouseff, R. Processing and storage effects on orange juice aroma: A review. J. Agric. Food Chem. 2008, 56, 9785–9796. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Peng, Y.; Zhu, C.; Pan, S. Effect of thermal treatment on carotenoids, flavonoids and ascorbic acid in juice of orange cv. Cara Cara. Food Chem. 2018, 265, 39–48. [Google Scholar] [CrossRef]
- Agcam, E.; Akyıldız, A.; Evrendilek, G.A. Comparison of phenolic compounds of orange juice processed by pulsed electric fields (PEF) and conventional thermal pasteurization. Food Chem. 2014, 143, 354–361. [Google Scholar] [CrossRef]
- Saura-Calixto, F.; Serrano, J.; Goñi, I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem. 2007, 101, 492–501. [Google Scholar] [CrossRef]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability and bioaccessibility of food bioactive compounds; overview and assessment by in vitro methods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2862–2884. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, M.S.; Yusof, S.; Abdul Manap, M.Y.B.; Abd-Aziz, S. Storage stability of clarified banana juice fortified with inulin and oligofructose. J. Food Process. Preserv. 2010, 34, 599–610. [Google Scholar] [CrossRef]
- Davim, S.; Andrade, S.; Oliveira, S.; Pina, A.; Barroca, M.J.; Guiné, R.P.F. Development of fruit jams and juices enriched with fructooligosaccharides. J. Fruit Sci. 2015, 15, 100–116. [Google Scholar] [CrossRef]
- Ghavidel, R.A.; Karimi, M.; Davoodi, M.; Jahanbani, R.; Asl, A.F.A. Effect of fructooligosaccharide fortification on quality characteristic of some fruit juice beverages (apple & orange juice). Int. J. Farm. Allied Sci. 2014, 3, 141–146. [Google Scholar]
- Renuka, B.; Kulkarni, S.G.; Vijayanand, P.; Prapulla, S.G. Fructooligosaccharide fortification of selected fruit juice beverages: Effect on the quality characteristics. LWT Food Sci. Technol. 2009, 42, 1031–1033. [Google Scholar] [CrossRef]
- Arilla, E.; Igual, M.; Martínez-Monzó, J.; Codoñer-Franch, P.; García-Segovia, P. Impact of Resistant Maltodextrin Addition on the Physico-Chemical Properties in Pasteurized Orange Juice. Foods 2020, 9, 1832. [Google Scholar] [CrossRef] [PubMed]
- Igual, M.; García-Martínez, E.; Camacho, M.M.; Martínez-Navarrete, N. Stability of micronutrients and phytochemicals of grapefruit jam as affected by the obtention process. Food Sci. Technol. Int. 2016, 22, 203–212. [Google Scholar] [CrossRef]
- Olives Barba, A.I.; Cámara Hurtado, M.; Sánchez Mata, M.C.; Fernández Ruiz, V.; López Saenz de Tejada, M. Aplication of UV-vis detection-HPLC method for a rapid determination of lycopene and β-carotene in vegetables. Food Chem. 2006, 95, 328–336. [Google Scholar] [CrossRef]
- Xu, G.; Liu, D.; Chen, J.; Ye, X.; Ma, Y.; Shi, J. Juice components and antioxidant capacity of citrus varieties cultivated in China. Food Chem. 2008, 106, 545–551. [Google Scholar] [CrossRef]
- Igual, M.; Cebadera, L.; Cámara, R.M.; Agudelo, C.; Martínez-Navarrete, N.; Cámara, M. Novel ingredients based on grapefruit freeze-dried formulations: Nutritional and bioactive value. Foods 2019, 8, 506. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriére, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Uribe-Wandurraga, Z.N.; Igual, M.; García-Segovia, P.; Martínez-Monzó, J. In vitro bioaccessibility of minerals from microalgae-enriched cookies. Food Funct. 2020, 11, 2186. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Microalgae biomass as an alternative ingredient in cookies: Sensory, physical, and chemical properties, antioxidant activity and in vitro digestibility. Algal Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- Khouzam, R.B.; Pohl, P.; Lobinski, R. Bioaccessibility of essential elements from white cheese, bread, fruit, and vegetables. Talanta 2011, 86, 425–428. [Google Scholar] [CrossRef]
- Wibowo, S.; Grauwet, T.; Santiago, J.S.; Tomic, J.; Vervoort, L.; Hendrickx, M.; Van Loey, A. Quality changes of pasteurized orange juice during storage: A kinetic study of specific parameters and their relation to colour instability. Food Chem. 2015, 187, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Mennah-Govela, Y.A.; Bornhorst, G.M. Fresh-squeezed orange juice properties before and during in vitro digestion as influenced by orange variety and processing method. J. Food Sci. 2017, 82, 2438–2447. [Google Scholar] [CrossRef]
- Pimentel, T.C.; Madrona, G.S.; Prudencio, S.H. Probiotic clarified apple juice with oligofructose or sucralose as sugar substitutes: Sensory profile and acceptability. LWT Food Sci. Technol. 2015, 62, 838–846. [Google Scholar] [CrossRef]
- Priya, B.N. A role of prebiotics in food and health: A review. J. Crit. Rev. 2020, 7, 782–785. [Google Scholar]
- Singla, V.; Chakkaravarthi, S. Applications of prebiotics in food industry: A review. Food Sci. Technol. Int. 2017, 23, 649–667. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Apak, R.; Capanoglu, E.; Shahidi, F. Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications, 1st ed.; John Wiley & Sons Ltd.: Chichester, UK, 2018. [Google Scholar]
- Sádecká, J.; Polovka, M.; Kolek, E.; Belajová, E.; Tobolková, B.; Daško, Ľ.; Durec, J. Orange juice with pulp: Impact of pasteurization and storage on flavour, polyphenols, ascorbic acid and antioxidant activity. J. Food Nutr. Res. 2014, 53, 371–388. [Google Scholar]
- Iglesias-Carres, L.; Mas-Capdevila, A.; Bravo, F.I.; Aragonès, G.; Muguerza, B.; Arola-Arnal, A. Optimization of a polyphenol extraction method for sweet orange pulp (Citrus sinensis L.) to identify phenolic compounds consumed from sweet oranges. PLoS ONE 2019, 14, e0211267. [Google Scholar] [CrossRef] [PubMed]
- De Ancos, B.; Cilla, A.; Barberá, R.; Sánchez-Moreno, C.; Cano, M.P. Influence of orange cultivar and mandarin post-harvest storage on polyphenols, ascorbic acid and antioxidant activity during gastrointestinal digestion. Food Chem. 2017, 225, 114–124. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Cilla, A.; Barberá, R.; Zacarías, L. Carotenoid bioaccessibility in pulp and fresh juice from carotenoid-rich sweet oranges and mandarins. Food Funct. 2015, 6, 1950–1959. [Google Scholar] [CrossRef]
- Al-Juhaimi, F.Y. Citrus fruits by-products as sources of bioactive compounds with antioxidant potential. Pak. J. Bot. 2014, 46, 1459–1462. [Google Scholar]
- Stinco, C.M.; Sentandreu, E.; Mapelli-Brahm, P.; Navarro, J.L.; Vicario, I.M.; Meléndez-Martínez, A.J. Influence of high pressure homogenization and pasteurization on the in vitro bioaccessibility of carotenoids and flavonoids in orange juice. Food Chem. 2020, 331, 127259. [Google Scholar] [CrossRef]
- Velázquez-Estrada, R.M.; Hernández-Herrero, M.M.; Rüfer, C.E.; Guamis-López, B.; Roig-Sagués, A.X. Influence of ultra high pressure homogenization processing on bioactive compounds and antioxidant activity of orange juice. Innov. Food Sci. Emerg. Technol. 2013, 18, 89–94. [Google Scholar] [CrossRef]
- Aschoff, J.K.; Kaufmann, S.; Kalkan, O.; Neidhart, S.; Carle, R.; Schweiggert, R.M. In vitro bioaccessibility of carotenoids, flavonoids, and Vitamin C from differently processed oranges and orange juices [Citrus sinensis (L.) Osbeck]. J. Agric. Food Chem. 2015, 63, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, M.; Cueva, C.; Muñoz-González, I.; Jiménez-Girón, A.; Sánchez-Patán, F.; Santos-Buelga, C.; Moreno-Arribas, M.V.; Bartolomé, B. Studies on Modulation of Gut Microbiota by Wine Polyphenols: From Isolated Cultures to Omic Approaches. Antioxidants 2015, 4, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly)phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J.; Vicario, I.M.; Heredia, F.J. Review: Analysis of carotenoids in orange juice. J. Food Compos. Anal. 2007, 20, 638–649. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Britton, G.; Vicario, I.M.; Heredia, F.J. The complex carotenoid pattern of orange juices from concentrate. Food Chem. 2008, 109, 546–553. [Google Scholar] [CrossRef]
- Valente, A.; Sanches-Silva, A.; Albuquerque, T.G.; Costa, H.S. Development of an orange juice in-house reference material and its application to guarantee the quality of vitamin C determination in fruits, juices and fruit pulps. Food Chem. 2014, 154, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Roque, M.J.; Rojas-Graü, M.A.; Elez-Martínez, P.; Martín-Belloso, O. Changes in vitamin C, phenolic, and carotenoid profiles throughout in vitro gastrointestinal digestion of a blended fruit juice. J. Agric. Food Chem. 2013, 1, 1859–1867. [Google Scholar] [CrossRef]
- Carr, A.C.; Rowe, S. The Emerging Role of Vitamin C in the Prevention and Treatment of COVID-19. Nutrients 2020, 12, 3286. [Google Scholar] [CrossRef]
- Alves Filho, E.G.; Silva, L.M.A.; de Brito, E.S.; Wurlitzer, N.J.; Fernandes, F.A.N.; Rabelo, M.C.; Fonteles, T.V.; Rodrigues, S. Evaluation of thermal and non-thermal processing effect on non-prebiotic and prebiotic acerola juices using 1H qNMR and GC–MS coupled to chemometrics. Food Chem. 2018, 265, 23–31. [Google Scholar] [CrossRef]
- Fonteles, T.V.; Alves Filho, E.G.; de Araújo Barroso, M.K.; Linhares, M.F.D.; Rabelo, M.C.; Silva, L.M.A.; de Brito, E.S.; Wurlitzer, N.J.; Rodrigues Pereira, E.P.; Ferreira, B.M.; et al. Protective effect of inulin on thermally treated acerola juice: In vitro bioaccessibility of bioactive compounds. Food Biosci. 2021, 41, 101018. [Google Scholar] [CrossRef]
- Choi, M.H.; Kim, G.H.; Lee, H.S. Effects of ascorbic acid retention on juice color and pigment stability in blood orange (Citrus sinensis) juice during refrigerated storage. Food Res. Int. 2002, 35, 753–759. [Google Scholar] [CrossRef]
- Schalow, S.; Baloufaud, M.; Cottancin, T.; Fischer, J.; Drusch, S. Orange pulp and peel fibres: Pectin-rich by-products from citrus processing for water binding and gelling in foods. Eur. Food Res. Technol. 2018, 244, 235–244. [Google Scholar] [CrossRef]
- Thakur, N.; Raigond, P.; Singh, Y.; Mishra, T.; Singh, B.; Lal, M.K.; Dutt, S. Recent updates on bioaccessibility of phytonutrients. Trends Food Sci. Technol. 2020, 97, 366–380. [Google Scholar] [CrossRef]
- Moser, S.E.; Shin, J.E.; Kasturi, P.; Hamaker, B.R.; Ferruzzi, M.G.; Bordenave, N. Formulation of orange juice with dietary fibers enhances bioaccessibility of orange flavonoids in juice but limits their ability to inhibit in vitro glucose transport. J. Agric. Food Chem. 2020, 68, 9387–9397. [Google Scholar] [CrossRef] [PubMed]
- Gil-Izquierdo, A.; Gil, M.I.; Ferreres, F.; Tomás-Barberán, F.A. In vitro availability of flavonoids and other phenolics in orange juice. J. Agric. Food Chem. 2001, 49, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Tao, Y.; Zeng, M.; Zhang, S.; Tao, G.; Qin, F.; Chen, J. High pressure homogenization processing, thermal treatment and milk matrix affect in vitro bioaccessibility of phenolics in apple, grape and orange juice to different extents. Food Chem. 2016, 200, 107–116. [Google Scholar] [CrossRef]
- Hoffmann, J.; Linseisen, J.; Riedl, J.; Wolfram, G. Dietary fiber reduces the antioxidative effect of a carotenoid and α-tocopherol mixture on LDL oxidation ex vivo in humans. Eur. J. Nutr. 1999, 38, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Yonekura, L.; Nagao, A. Intestinal absorption of dietary carotenoids. Mol. Nutr. Food Res. 2007, 51, 107–115. [Google Scholar] [CrossRef]
- Rodríguez-Roque, M.J.; de Ancos, B.; Sánchez-Moreno, C.; Cano, M.P.; Elez-Martínez, P.; Martín-Belloso, O. Impact of food matrix and processing on the in vitro bioaccessibility of vitamin C, phenolic compounds, and hydrophilic antioxidant activity from fruit juice-based beverages. J. Funct. Foods 2015, 14, 33–43. [Google Scholar] [CrossRef]
- Uğur, H.; Çatak, J.; Mızrak, Ö.F.; Cebi, N.; Yaman, M. Determination and evaluation of in vitro bioaccessibility of added vitamin C in commercially available fruit-, vegetable-, and cereal-based baby foods. Food Chem. 2020, 330, 127166. [Google Scholar] [CrossRef]






| Sample | TSS | Acidity (g CA/100 mL) | pH |
|---|---|---|---|
| OJP0 | 11.38 ± 0.03 a | 0.77 ± 0.04 h | 3.68 ± 0.02 a |
| OJP2.5 | 13.58 ± 0.02 c | 0.747 ± 0.002 g | 3.68 ± 0.02 a |
| OJP5 | 15.75 ± 0.04 e | 0.725 ± 0.002 f | 3.69 ± 0.02 a |
| OJP7.5 | 17.99 ± 0.04 g | 0.711 ± 0.002 e | 3.71 ± 0.02 a |
| OJWP0 | 11.47 ± 0.08 b | 0.691 ± 0.002 d | 3.80 ± 0.03 b |
| OJWP2.5 | 13.72 ± 0.03 d | 0.670 ± 0.003 c | 3.90 ± 0.04 b |
| OJWP5 | 15.90 ± 0.05 f | 0.6530 ± 0.0005 b | 3.83 ± 0.02 b |
| OJWP7.5 | 18.09 ± 0.02 h | 0.636 ± 0.002 a | 3.82 ± 0.02 b |
| Compounds | OJP0 | OJWP0 |
|---|---|---|
| TP (mgGAE/100 mL) | 99.8 ± 1.2 a | 88.9 ± 0.6 b |
| TC (mgβ-carotene/100 mL) | 7.13 ± 0.02 a | 6.97 ± 0.02 b |
| AA (mgAA/100 mL) | 5.81 ± 0.13 a | 5.76 ± 0.07 a |
| Vitamin C (mgVitamin C/100 mL) | 6.80 ± 0.04 a | 6.66 ± 0.02 b |
| AC (mgTE/100 mL) | 106.1 ± 0.5 a | 102.3 ± 0.3 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arilla, E.; García-Segovia, P.; Martínez-Monzó, J.; Codoñer-Franch, P.; Igual, M. Effect of Adding Resistant Maltodextrin to Pasteurized Orange Juice on Bioactive Compounds and Their Bioaccessibility. Foods 2021, 10, 1198. https://doi.org/10.3390/foods10061198
Arilla E, García-Segovia P, Martínez-Monzó J, Codoñer-Franch P, Igual M. Effect of Adding Resistant Maltodextrin to Pasteurized Orange Juice on Bioactive Compounds and Their Bioaccessibility. Foods. 2021; 10(6):1198. https://doi.org/10.3390/foods10061198
Chicago/Turabian StyleArilla, Elías, Purificación García-Segovia, Javier Martínez-Monzó, Pilar Codoñer-Franch, and Marta Igual. 2021. "Effect of Adding Resistant Maltodextrin to Pasteurized Orange Juice on Bioactive Compounds and Their Bioaccessibility" Foods 10, no. 6: 1198. https://doi.org/10.3390/foods10061198
APA StyleArilla, E., García-Segovia, P., Martínez-Monzó, J., Codoñer-Franch, P., & Igual, M. (2021). Effect of Adding Resistant Maltodextrin to Pasteurized Orange Juice on Bioactive Compounds and Their Bioaccessibility. Foods, 10(6), 1198. https://doi.org/10.3390/foods10061198







