Modelling the Effects of Roselle Extract, Potato Peel Flour, and Beef Fat on the Sensory Properties and Heterocyclic Amines Formation of Beef Patties Studied by Using Response Surface Methodology †
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Reagents
2.3. Experimental Design
2.4. Beef Patties Preparation
2.5. Sensory Analysis
2.6. Extraction of Heterocyclic Amines
2.7. Identification and Quantification of Heterocyclic Amines by HPLC-DAD
2.8. Statistical Analysis
3. Results and Discussion
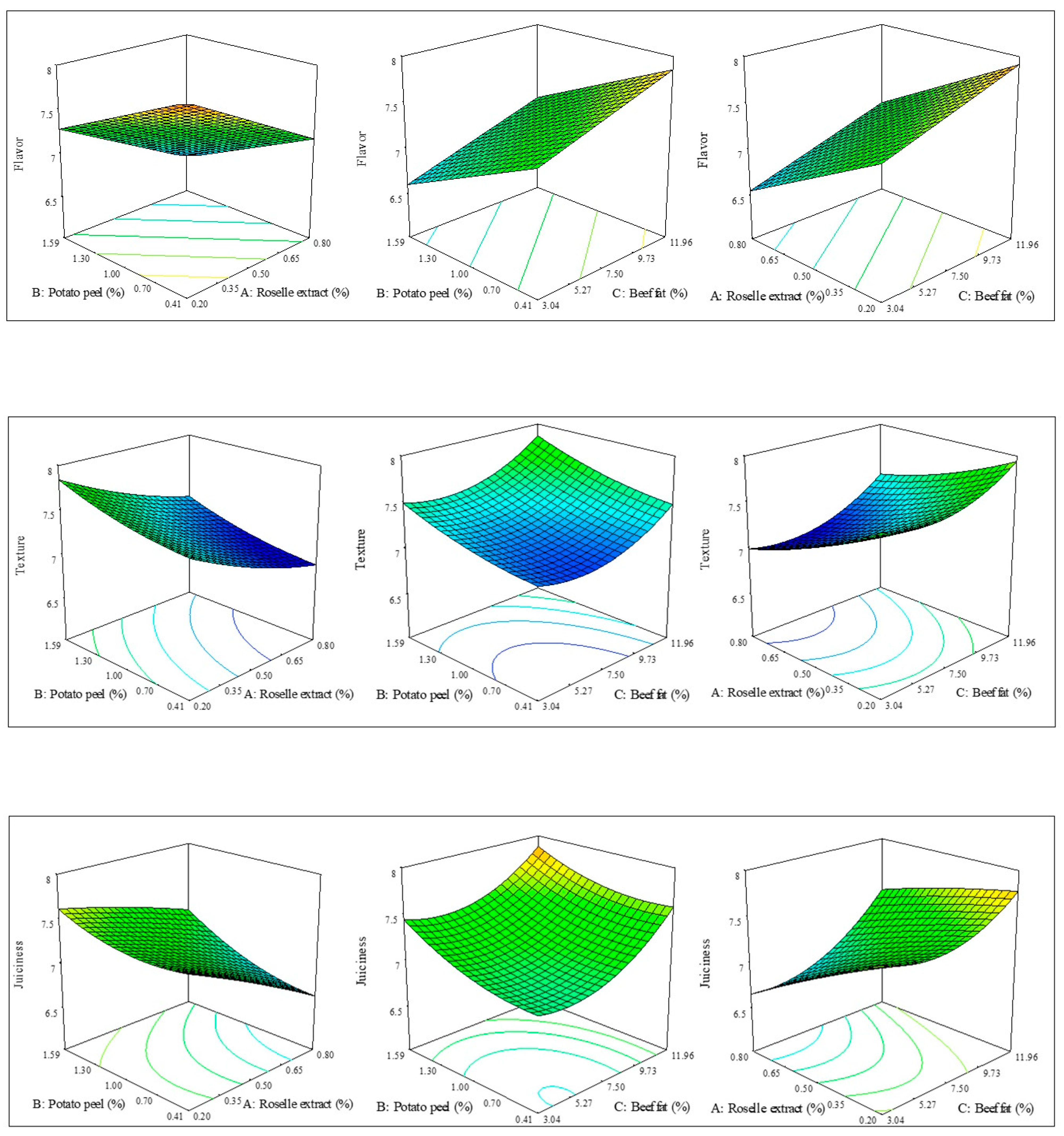
3.1. Sensory Analysis
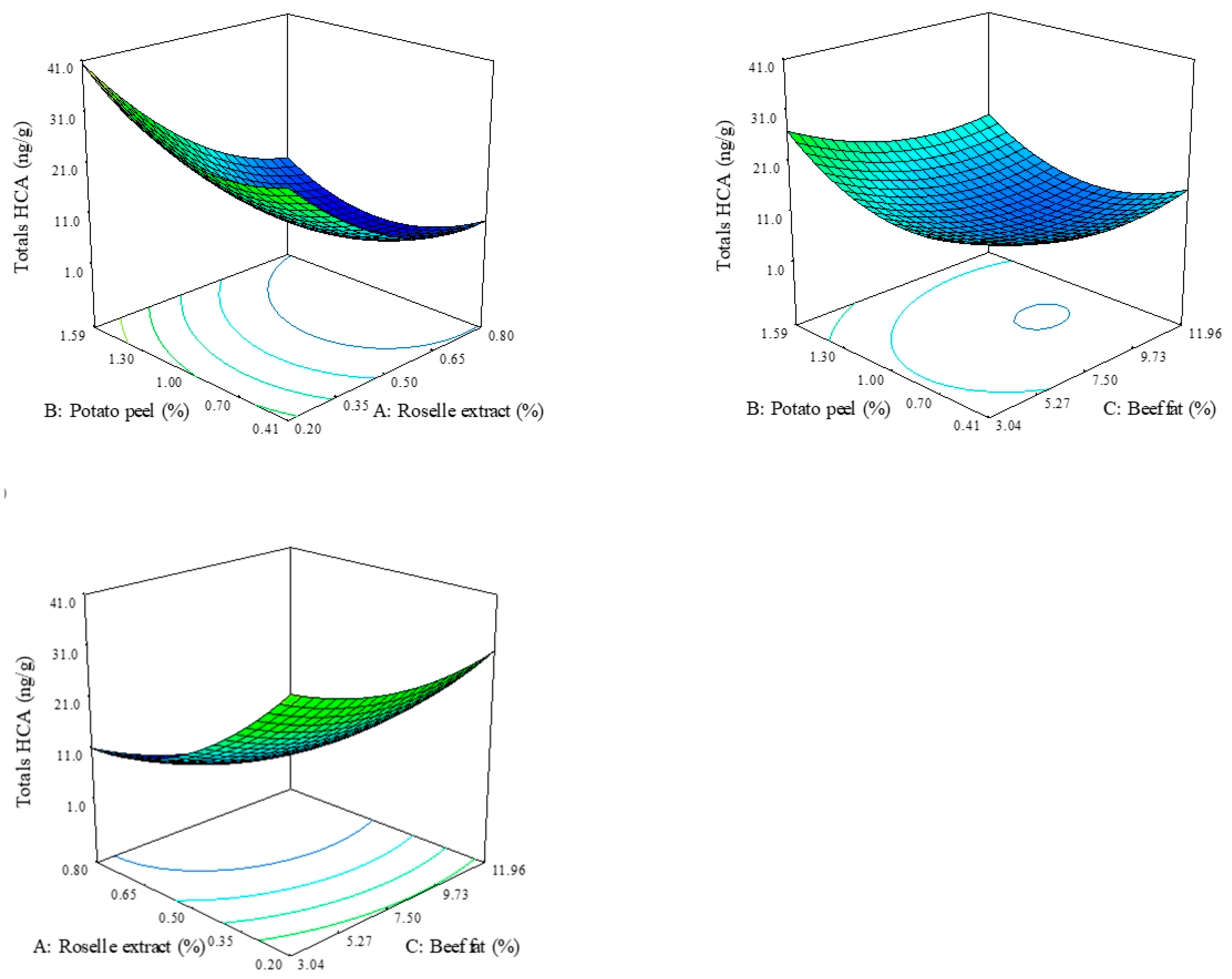
3.2. Heterocyclic Amine Analysis
3.3. Optimization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferro, A.; Costa, A.R.; Morais, S.; Bertuccio, P.; Rota, M.; Pelucchi, C.; Hu, J.; Johnson, K.C.; Zhang, Z.; Palli, D.; et al. Fruits and vegetables intake and gastric cancer risk: A pooled analysis within the Stomach cancer Pooling Project. Int. J. Cancer 2020, 147, 3090–3101. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.E.; Skurtveit, S.; Clausen, T. Many correlates of poor quality of life among substance users entering treatment are not addiction-specific. Health Qual. Life Outcomes 2016, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, F.; Chen, D.; Zhang, C. Red and processed meat consumption and esophageal cancer risk: A systematic review and meta-analysis. Clin. Transl. Oncol. 2020, 22, 532–545. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.S.; Arroyave, W.D.; Lunn, R.M.; Park, Y.-M.M.; Boyd, W.A.; Sandler, D.P. A Prospective Analysis of Red and Processed Meat Consumption and Risk of Colorectal Cancer in Women. Cancer Epidemiol. Biomark. Prev. 2019, 29, 141–150. [Google Scholar] [CrossRef] [PubMed]
- IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2015; pp. 1–53. [Google Scholar]
- World Cancer Research Fund. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective; World Cancer Research Fund: London, UK, 2018. [Google Scholar]
- Huang, Y.; Cao, D.; Chen, Z.; Chen, B.; Li, J.; Guo, J.; Dong, Q.; Liu, L.; Wei, Q. Red and processed meat consumption and cancer outcomes: Umbrella review. Food Chem. 2021, 356, 129697. [Google Scholar] [CrossRef] [PubMed]
- Adeyeye, S.A.O. Heterocyclic amines and polycyclic aromatic hydrocarbons in cooked meat products: A review. Polycycl. Aromat. Compd. 2020, 40, 1557–1567. [Google Scholar] [CrossRef]
- Turesky, R.J.; Vouros, P. Formation and analysis of heterocyclic aromatic amine–DNA adducts in vitro and in vivo. J. Chromatogr. B 2004, 802, 155–166. [Google Scholar] [CrossRef]
- Inami, K.; Nagao, M.; Ishikawa, S.; Mochizuki, M. Mutagenicity of Heterocyclic Amines by Biomimetic Chemical Models for Cytochrome P450 in Ames Assay. Genes Environ. 2010, 32, 7–13. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, H.; Zhou, X.; Chen, X.; Hu, N.; Zhang, Y.; Wang, S. 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine Induced Colon Injury by Disrupting the Intestinal Bacterial Composition and Lipid Metabolic Pathways in Rats. J. Agric. Food Chem. 2021, 69, 437–446. [Google Scholar] [CrossRef]
- Hirose, M.; Nishikawa, A.; Shibutani, M.; Imai, T.; Shirai, T. Chemoprevention of heterocyclic amine-induced mammary carcinogenesis in rats. Environ. Mol. Mutagen. 2002, 39, 271–278. [Google Scholar] [CrossRef]
- Lightfoot, T.; Coxhead, J.; Cupid, B.; Nicholson, S.; Garner, R. Analysis of DNA adducts by accelerator mass spectrometry in human breast tissue after administration of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and benzo[a]pyrene. Mutat. Res. Toxicol. Environ. Mutagen. 2000, 472, 119–127. [Google Scholar] [CrossRef]
- Soladoye, O.P.; Shand, P.; Dugan, M.E.R.; Gariépy, C.; Aalhus, J.L.; Estévez, M.; Juárez, M. Influence of cooking methods and storage time on lipid and protein oxidation and heterocyclic aromatic amines production in bacon. Food Res. Int. 2017, 99, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Gibis, M.; Weiss, J. Inhibitory effect of marinades with hibiscus extract on formation of heterocyclic aromatic amines and sensory quality of fried beef patties. Meat Sci. 2010, 85, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Gibis, M.; Weiss, J. Antioxidant capacity and inhibitory effect of grape seed and rosemary extract in marinades on the formation of heterocyclic amines in fried beef patties. Food Chem. 2012, 134, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Kuhnle, G.K.; Cheng, Q. Vegetable oil as fat replacer inhibits formation of heterocyclic amines and polycyclic aromatic hydrocarbons in reduced fat pork patties. Food Control. 2017, 81, 113–125. [Google Scholar] [CrossRef]
- Ospina, J.C.E.; Sierra, A.C.; Ochoa, O.; Pérez-Álvarez, J.A.; Fernández-López, J. Substitution of saturated fat in processed meat products: A review. Crit. Rev. Food Sci. Nutr. 2012, 52, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Fleshner, N.; Bagnell, P.S.; Klotz, L.; Venkateswaran, V. Dietary fat and prostate cancer. J. Urol. 2004, 171, S19–S24. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Ma, S.; Wang, S.; Sun, G. Meta-Analysis of Saturated Fatty Acid Intake and Breast Cancer Risk. Medcine 2015, 94, e2391. [Google Scholar] [CrossRef]
- Qiu, W.; Lu, H.; Qi, Y.; Wang, X. Dietary fat intake and ovarian cancer risk: a meta-analysis of epidemiological studies. Oncotarget 2016, 7, 37390–37406. [Google Scholar] [CrossRef]
- Kumar, S.S.; Manoj, P.; Shetty, N.P.; Giridhar, P. Effect of different drying methods on chlorophyll, ascorbic acid and antioxidant compounds retention of leaves of Hibiscus sabdariffa L. J. Sci. Food Agric. 2015, 95, 1812–1820. [Google Scholar] [CrossRef]
- Perez-Baez, A.J.; Camou, J.P.; Valenzuela-Melendres, M.; Lucas-Gonzalez, R.; Viuda-Martos, M. Assessment of Chemical, Physico-Chemical and Sensorial Properties of Frankfurter-Type Sausages Added with Roselle (Hibiscus sabdariffa L.), Extracts. Proceedings 2020, 70, 7690. [Google Scholar] [CrossRef]
- Sampaio, S.L.; Petropoulos, S.A.; Alexopoulos, A.; Heleno, S.A.; Santos-Buelga, C.; Barros, L.; Ferreira, I.C. Potato peels as sources of functional compounds for the food industry: A review. Trends Food Sci. Technol. 2020, 103, 118–129. [Google Scholar] [CrossRef]
- Sepelev, I.; Galoburda, R. Industrial potato peel waste application in food production: A review. Livest. Res. Rural. Dev. 2015, 1, 130–136. [Google Scholar]
- Franco, D.; Pateiro, M.; Rodríguez-Amado, I.; López-Pedrouso, M.; Zapata, C.; Vázquez, J.A.; Lorenzo, J.M. Antioxidant ability of potato (Solanum tuberosum) peel extracts to inhibit soybean oil oxidation. Eur. J. Lipid Sci. Technol. 2016, 118, 1891–1902. [Google Scholar] [CrossRef]
- Colle, M.; Richard, R.P.; Smith, D.M.; Colle, M.J.; Loucks, W.I.; Gray, S.J.; Reynolds, Z.D.; Sutton, H.A.; Nasados, J.A.; Doumit, M.E. Dry potato extracts improve water holding capacity, shelf life, and sensory characteristics of fresh and precooked beef patties. Meat Sci. 2019, 149, 156–162. [Google Scholar] [CrossRef]
- Persson, E.; Sjöholm, I.; Nyman, M.; Skog, K. Addition of various carbohydrates to beef burgers affects the formation of heterocyclic amines during frying. J. Agric. Food Chem. 2004, 52, 7561–7566. [Google Scholar] [CrossRef]
- Harris, P.J.; Triggs, C.M.; Roberton, A.M.; Watson, M.E.; Ferguson, L.R. The adsorption of heterocyclic aromatic amines by model dietary fibres with contrasting compositions. Chem.Biol. Interact. 1996, 100, 13–25. [Google Scholar] [CrossRef]
- Perez-Baez, A.J.; Camou, J.P.; Valenzuela-Melendres, M.; Gonzalez-Aguilar, G.; Viuda-Martos, M.; Sebranek, J.G.; Tortoledo-Ortiz, O. Effects and interactions of roselle (Hibiscus sabdariffa L.), potato peel flour, and beef fat on quality characteristics of beef patties studied by response surface methodology. J. Food Process. Preserv. 2020, 44, e14659. [Google Scholar] [CrossRef]
- Messner, C.; Murkovic, M. Evaluation of a new model system for studying the formation of heterocyclic amines. J. Chromatogr. B 2004, 802, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Haskaraca, G.; Demirok, E.; Kolsarıcı, N.; Öz, F.; Özsaraç, N. Effect of green tea extract and microwave pre-cooking on the formation of heterocyclic aromatic amines in fried chicken meat products. Food Res. Int. 2014, 63, 373–381. [Google Scholar] [CrossRef]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food: Principles and Practices; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Marconato, A.M.; Hartmann, G.L.; Santos, M.M.R.; Amaral, L.A.d.; Souza, G.H.O.D.; Santos, E.F.D.; Novello, D. Sweet potato peel flour in hamburger: Effect on physicochemical, technological and sensorial characteristics. Braz. J. Food Technol. 2020, 23, e2019115. [Google Scholar] [CrossRef]
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Phenolic content and antioxidant activities of selected potato varieties and their processing by-products. J. Funct. Foods 2013, 5, 590–600. [Google Scholar] [CrossRef]
- Jung, E.; Joo, N. Roselle (Hibiscus sabdariffa L.) and soybean oil effects on quality characteristics of pork patties studied by response surface methodology. Meat Sci. 2013, 94, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Singham, P.; Birwal, P.; Yadav, B. Importance of objective and subjective measurement of food quality and their inter-relationship. Int. J. Food Process. Technol. 2015, 6, 1000448. [Google Scholar]
- Oz, F.; Cakmak, I.H. The effects of conjugated linoleic acid usage in meatball production on the formation of heterocyclic aromatic amines. LWT—Food Sci. Technol. 2016, 65, 1031–1037. [Google Scholar] [CrossRef]
- Vitaglione, P.; Fogliano, V. Use of antioxidants to minimize the human health risk associated to mutagenic/carcinogenic heterocyclic amines in food. J. Chromatogr. B 2004, 802, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Borrás-Linares, I.; Fernández-Arroyo, S.; Arráez-Roman, D.; Palmeros-Suárez, P.; Del Val-Díaz, R.; Andrade-Gonzáles, I.; Fernández-Gutiérrez, A.; Gómez-Leyva, J.F.; Segura-Carretero, A. Characterization of phenolic compounds, anthocyanidin, antioxidant and antimicrobial activity of 25 varieties of Mexican Roselle (Hibiscus sabdariffa). Ind. Crop. Prod. 2015, 69, 385–394. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Yang, K.-M.; Chiang, P.-Y. Roselle anthocyanins: Antioxidant properties and stability to heat and pH. Molecules 2018, 23, 1357. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Nhut, P.; Binh, M.L.T.; Thuan, M.; Van, N.T.T.; Lam, T.D.; Nguyen, P.T.N. Effects of extraction conditions on antioxidant activities of Roselle (Hibiscus sabdariffa L.) extracts. Mater. Sci. Forum 2020, 977, 201–206. [Google Scholar] [CrossRef]
- Murkovic, M.; Steinberger, D.; Pfannhauser, W. Antioxidant spices reduce the formation of heterocyclic amines in fried meat. Z. Lebensm. Forsch. A 1998, 207, 477–480. [Google Scholar] [CrossRef]
- Zeng, M.; Li, Y.; He, Z.; Qin, F.; Tao, G.; Zhang, S.; Gao, Y.; Chen, J. Discrimination and investigation of inhibitory patterns of flavonoids and phenolic acids on heterocyclic amine formation in chemical model systems by UPLC-MS profiling and chemometrics. Eur. Food Res. Technol. 2016, 242, 313–319. [Google Scholar] [CrossRef]
- Alvarez, V.H.; Cahyadi, J.; Xu, D.; Saldaña, M.D. Optimization of phytochemicals production from potato peel using subcritical water: Experimental and dynamic modeling. J. Supercrit. Fluids 2014, 90, 8–17. [Google Scholar] [CrossRef]
- Hwang, D.K.; Ngadi, M. Kinetics of heterocyclic amines formation in meat emulsion at different fat contents. LWT—Food Sci. Technol. 2002, 35, 600–606. [Google Scholar] [CrossRef]
- Szterk, A.; Jesionkowska, K. Influence of the cold storage time of raw beef meat and grilling parameters on sensory quality and content of heterocyclic aromatic amines. LWT—Food Sci. Technol. 2015, 61, 299–308. [Google Scholar] [CrossRef]
| Run a | Coded Values | Experimental Values | ||||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | RE | PP | BF | |
| 1 | −1 | −1 | −1 | 0.20 | 0.40 | 3.04 |
| 2 | 1 | −1 | −1 | 0.80 | 0.40 | 3.04 |
| 3 | −1 | 1 | −1 | 0.20 | 1.60 | 3.04 |
| 4 | −1 | −1 | 1 | 0.20 | 0.40 | 11.96 |
| 5 | 1 | 1 | −1 | 0.80 | 1.60 | 3. 04 |
| 6 | 1 | −1 | 1 | 0.80 | 0.40 | 11.96 |
| 7 | −1 | 1 | 1 | 0.20 | 1.60 | 11.96 |
| 8 | 1 | 1 | 1 | 0.80 | 1.60 | 11.96 |
| 9 | −1.68 | 0 | 0 | 0 | 1.00 | 7.50 |
| 10 | 1.68 | 0 | 0 | 1.00 | 1.00 | 7.50 |
| 11 | 0 | −1.68 | 0 | 0.50 | 0 | 7.50 |
| 12 | 0 | 1.68 | 0 | 0.50 | 2.00 | 7.50 |
| 13 | 0 | 0 | −1.68 | 0.50 | 1.00 | 0 |
| 14 | 0 | 0 | 1.68 | 0.50 | 1.00 | 15.00 |
| 15 | 0 | 0 | 0 | 0.50 | 1.00 | 7.50 |
| 16 | 0 | 0 | 0 | 0.50 | 1.00 | 7.50 |
| 17 | 0 | 0 | 0 | 0.50 | 1.00 | 7.50 |
| 18 | 0 | 0 | 0 | 0.50 | 1.00 | 7.50 |
| 19 | 0 | 0 | 0 | 0.50 | 1.00 | 7.50 |
| 20 | 0 | 0 | 0 | 0.50 | 1.00 | 7.50 |
| Run | Sensory Properties | Heterocyclic Amines (ng/g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Flavor | Texture | Juiciness | IQx | IQ | MeIQx | MeIQ | DiMeIQx | PhIP | Totals | |
| 1 | 7.8 ± 0.8 | 7.7 ± 1.2 | 7.4 ± 0.8 | 10.1 ± 5.9 | 5.5 ± 2.6 | 3.9 ± 0.4 | 1.9 ± 0.7 | 1.2 ± 0.1 | 14.1 ± 0.5 | 36.8 ± 5.8 |
| 2 | 6.5 ± 1.5 | 7.0 ± 1.0 | 6.3 ± 1.6 | 0.1 ± 0.0 | 3.0 ± 0.1 | 2.2 ± 0.2 | 0.2 ± 0.0 | 0.7 ± 0.2 | 10.8 ± 0.8 | 17.0 ± 0.9 |
| 3 | 7.4 ± 1.4 | 8.0 ± 0.7 | 7.7 ± 1.5 | 10.6 ± 1.4 | 11.7 ± 0.4 | 3.8 ± 0.1 | 11.5 ± 1.1 | 1.5 ± 0.5 | 12.7 ± 0.4 | 51.8 ± 1.7 |
| 4 | 6.2 ± 1.3 | 7.2 ± 0.9 | 7.1 ± 1.1 | 4.6 ± 0.6 | 6.2 ± 0.2 | 2.0 ± 0.2 | 1.0 ± 0.7 | 0.8 ± 0.1 | 6.9 ± 1.4 | 21.5 ± 0.3 |
| 5 | 8.3 ± 1.0 | 7.8 ± 0.7 | 7.8 ± 1.3 | 2.7 ± 0.0 | 6.7 ± 0.5 | 3.1 ± 0.4 | 6.2 ± 0.1 | 1.7 ± 0.4 | 14.0 ± 1.4 | 34.4 ± 1.1 |
| 6 | 7.3 ± 0.9 | 7.2 ± 0.7 | 7.4 ± 1.1 | 1.6 ± 0.1 | 1.7 ± 0.1 | 0.3 ± 0.1 | 1.4 ± 0.1 | 0.8 ± 0.1 | 4.6 ± 0.8 | 10.5 ± 1.2 |
| 7 | 7.8 ± 0.8 | 8.0 ± 0.8 | 8.1 ± 1.4 | 18.1 ± 0.0 | 7.2 ± 0.2 | 2.4 ± 0.3 | 8.0 ± 0.5 | 1.7 ± 0.2 | 8.3 ± 1.4 | 45.7 ± 1.1 |
| 8 | 7.0 ± 0.8 | 7.5 ± 0.9 | 7.8 ± 1.1 | 2.2 ± 0.3 | 1.5 ± 0.3 | 2.7 ± 0.3 | 1.3 ± 0.3 | 1.3 ± 0.0 | 2.4 ± 0.3 | 11.2 ± 0.9 |
| 9 | 7.8 ± 1.1 | 7.9 ± 1.1 | 7.6 ± 1.6 | 9.2 ± 0.3 | 6.9 ± 0.3 | 1.7 ± 0.1 | 8.4 ± 0.2 | 1.4 ± 0.4 | 15.2 ± 0.2 | 42.8 ± 0.0 |
| 10 | 6.9 ± 1.3 | 7.0 ± 1.0 | 6.6 ± 1.2 | 0.2 ± 0.1 | 0.2 ± 0.0 | 0.4 ± 0.3 | 1.0 ± 0.4 | 0.7 ± 0.0 | 1.9 ± 0.3 | 4.3 ± 0.1 |
| 11 | 8.3 ± 1.2 | 7.1 ± 1.1 | 7.2 ± 1.2 | 3.3 ± 0.2 | 2.6 ± 0.3 | 3.8 ± 0.1 | 4.6 ± 0.7 | 1.3 ± 0.4 | 8.8 ± 0.7 | 24.3 ± 1.4 |
| 12 | 6.2 ± 1.5 | 7.9 ± 1.2 | 7.7 ± 1.3 | 13.5 ± 0.8 | 4.2 ± 0.1 | 0.7 ± 0.2 | 5.6 ± 0.2 | 1.0 ± 0.1 | 2.1 ± 0.4 | 27.1 ± 2.9 |
| 13 | 7.0 ± 1.5 | 7.3 ± 0.8 | 7.6 ± 1.0 | 7.7 ± 1.8 | 4.8 ± 1.1 | 2.9 ± 0.4 | 2.5 ± 1.5 | 0.7 ± 0.1 | 4.6 ± 0.5 | 23.1 ± 1.0 |
| 14 | 7.6 ± 0.7 | 8.4 ± 1.0 | 8.0 ± 1.2 | 3.3 ± 0.3 | 3.3 ± 0.6 | 1.3 ± 0.2 | 1.0 ± 0.2 | 1.3 ± 0.3 | 2.3 ± 0.2 | 12.6 ± 1.3 |
| 15 | 7.1 ± 1.7 | 7.3 ± 1.1 | 7.0 ± 1.6 | 0.4 ± 0.1 | 4.5 ± 0.5 | 1.8 ± 0.3 | 0.5 ± 0.0 | 1.1 ± 0.1 | 4.6 ± 0.4 | 12.8 ± 0.7 |
| 16 | 7.1 ± 1.2 | 7.2 ± 1.1 | 7.0 ± 1.3 | 0.4 ± 0.1 | 3.9 ± 0.0 | 0.3 ± 0.1 | 1.0 ± 0.3 | 1.5 ± 0.1 | 5.3 ± 0.3 | 12.4 ± 0.6 |
| 17 | 7.2 ± 1.0 | 7.4 ± 0.7 | 7.2 ± 0.8 | 0.0 ± 0.0 | 2.7 ± 0.1 | 0.5 ± 0.0 | 0.3 ± 0.0 | 1.0 ± 0.3 | 2.5 ± 0.3 | 6.9 ± 0.6 |
| 18 | 6.9 ± 0.9 | 7.3 ± 0.8 | 7.2 ± 0.7 | 0.0 ± 0.0 | 4.6 ± 2.2 | 0.4 ± 0.0 | 0.8 ± 0.1 | 1.4 ± 0.2 | 4.3 ± 0.3 | 11.6 ± 2.6 |
| 19 | 6.9 ± 1.3 | 7.2 ± 0.5 | 7.1 ± 1.2 | 0.0 ± 0.0 | 2.7 ± 0.5 | 0.3 ± 0.0 | 0.9 ± 0.0 | 0.8 ± 0.3 | 5.2 ± 1.0 | 9.7 ± 0.3 |
| 20 | 7.4 ± 1.2 | 7.1 ± 1.2 | 7.2 ± 1.9 | 0.0 ± 0.0 | 2.5 ± 0.1 | 0.3 ± 0.0 | 0.0 ± 0.0 | 0.8 ± 0.2 | 4.3 ± 0.4 | 8.0 ± 0.0 |
| Response | Model | Means ± SD | R2 a | F-Value | Prob > F | Polynomial Equation b |
|---|---|---|---|---|---|---|
| Sensory properties | ||||||
| Flavor | linear | 7.23 ± 0.29 | 0.80 | 21.07 | 0.0001 | 7.23 − 0.42A − 0.36B * + 0.26C |
| Texture | Quadratic | 7.47 ± 0.17 | 0.90 | 9.78 | 0.0007 | 7.24 − 0.30A * + 0.18B + 0.17C * − 0.02AB + 0.03AC + 0.01BC + 0.06A2 * + 0.06B2 + 0.20C2 |
| Juiciness | Quadratic | 7.35 ± 0.15 | 0.95 | 18.81 | 0.0001 | 7.12 − 0.30A * + 0.19B + 0.26C + 0.08AB * + 0.11AC − 0.04BC *– 0.02A2 * + 0.12B2 + 0.24C2 * |
| Heterocyclic amines | ||||||
| IQx | Quadratic | 4.40 ± 2.39 | 0.90 | 9.40 | 0.0008 | 0.14 − 3.52A * + 2.79B * − 0.60C − 1.36AB − 0.12AC + 1.38BC + 1.55A2 * + 2.86B2 * + 1.83C2 * |
| IQ | Linear | 4.32 ± 1.57 | 0.70 | 11.60 | 0.0003 | 4.32 − 2.19A * + 0.90B * − 0.85C |
| MeIQx | Quadratic | 1.74 ± 0.94 | 0.73 | 3.07 | 0.048 | 0.56 − 0.61A * − 0.28B − 0.45C + 0.37AB + 0.13AC + 0.25BC + 0.32A2 + 0.73B2 * + 0.69C2 * |
| MeIQ | Quadratic | 2.90 ± 1.22 | 0.93 | 14.38 | 0.0001 | 0.59 − 2.65A * + 1.01B * − 0.03C − 1.34AB * + 0.09AC − 1.09BC * + 1.43A2 * + 1.57B2 * + 0.40C2 |
| DiMeIQx | Linear | 1.14 ± 0.23 | 0.66 | 10.08 | 0.0006 | 1.14 − 0.28A * + 0.03B + 0.18C * |
| PhIP | Quadratic | 6.74 ± 2.31 | 0.86 | 6.59 | 0.003 | 4.26 − 3.43A * − 1.79B * − 1.39C * + 0.11AB − 0.77 AC − 0.34BC + 2.18A2 * + 1.07B2 + 0.37C2 |
| Totals | Quadratic | 21.23 ± 4.16 | 0.95 | 23.33 | 0.0001 | 10.07 − 12.68A * + 2.65B * − 3.15C * –2.64AB − 1.02AC − 0.95BC + 5.87A2 * + 6.62B2 * + 3.85C2 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Báez, A.J.; Valenzuela-Melendres, M.; Camou, J.P.; González-Aguilar, G.; Tortoledo-Ortiz, O.; González-Ríos, H.; Viuda-Martos, M. Modelling the Effects of Roselle Extract, Potato Peel Flour, and Beef Fat on the Sensory Properties and Heterocyclic Amines Formation of Beef Patties Studied by Using Response Surface Methodology. Foods 2021, 10, 1184. https://doi.org/10.3390/foods10061184
Pérez-Báez AJ, Valenzuela-Melendres M, Camou JP, González-Aguilar G, Tortoledo-Ortiz O, González-Ríos H, Viuda-Martos M. Modelling the Effects of Roselle Extract, Potato Peel Flour, and Beef Fat on the Sensory Properties and Heterocyclic Amines Formation of Beef Patties Studied by Using Response Surface Methodology. Foods. 2021; 10(6):1184. https://doi.org/10.3390/foods10061184
Chicago/Turabian StylePérez-Báez, Anna Judith, Martin Valenzuela-Melendres, Juan Pedro Camou, Gustavo González-Aguilar, Orlando Tortoledo-Ortiz, Humberto González-Ríos, and Manuel Viuda-Martos. 2021. "Modelling the Effects of Roselle Extract, Potato Peel Flour, and Beef Fat on the Sensory Properties and Heterocyclic Amines Formation of Beef Patties Studied by Using Response Surface Methodology" Foods 10, no. 6: 1184. https://doi.org/10.3390/foods10061184
APA StylePérez-Báez, A. J., Valenzuela-Melendres, M., Camou, J. P., González-Aguilar, G., Tortoledo-Ortiz, O., González-Ríos, H., & Viuda-Martos, M. (2021). Modelling the Effects of Roselle Extract, Potato Peel Flour, and Beef Fat on the Sensory Properties and Heterocyclic Amines Formation of Beef Patties Studied by Using Response Surface Methodology. Foods, 10(6), 1184. https://doi.org/10.3390/foods10061184








