Packaging of Fresh Sliced Mushrooms with Essential Oils Vapours: A New Technology for Maintaining Quality and Extending Shelf Life
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Packaging Treatments with Vapour Essential Oils
2.3. Gas Composition of Packages throughout Storage
2.4. Physicochemical Analyses
2.5. Sensory Analyses
2.6. Microbial Analyses
2.7. Enzymatic Activity
2.7.1. Polyphenoloxidase (PPO)
2.7.2. Phenyl Ammonia Lyase (PAL)
2.8. Total Phenolic Content
2.9. Total Antioxidant Capacity
2.10. Statistical Analyses
3. Results and Discussion
3.1. Gas Composition
3.2. pH
3.3. Texture
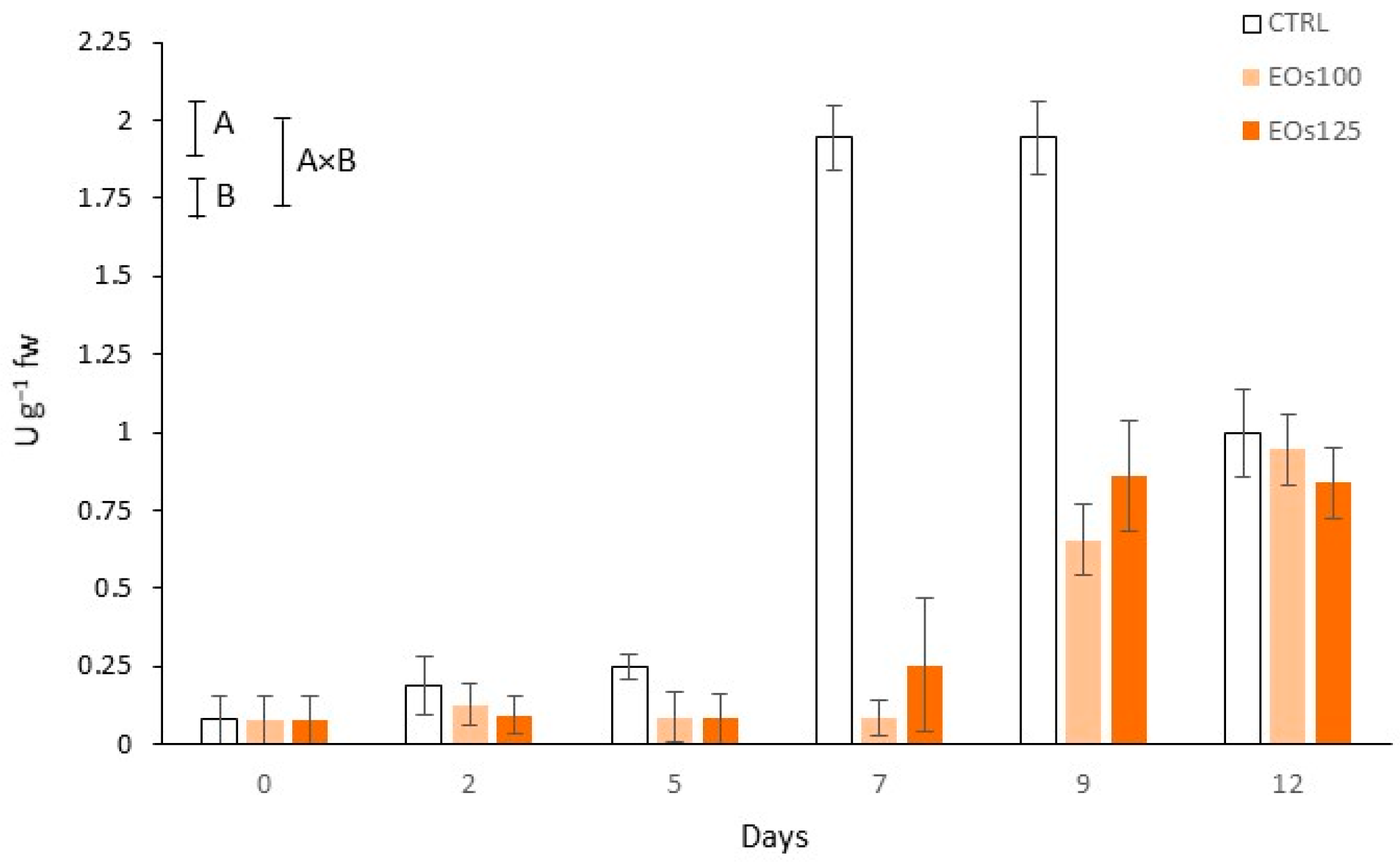
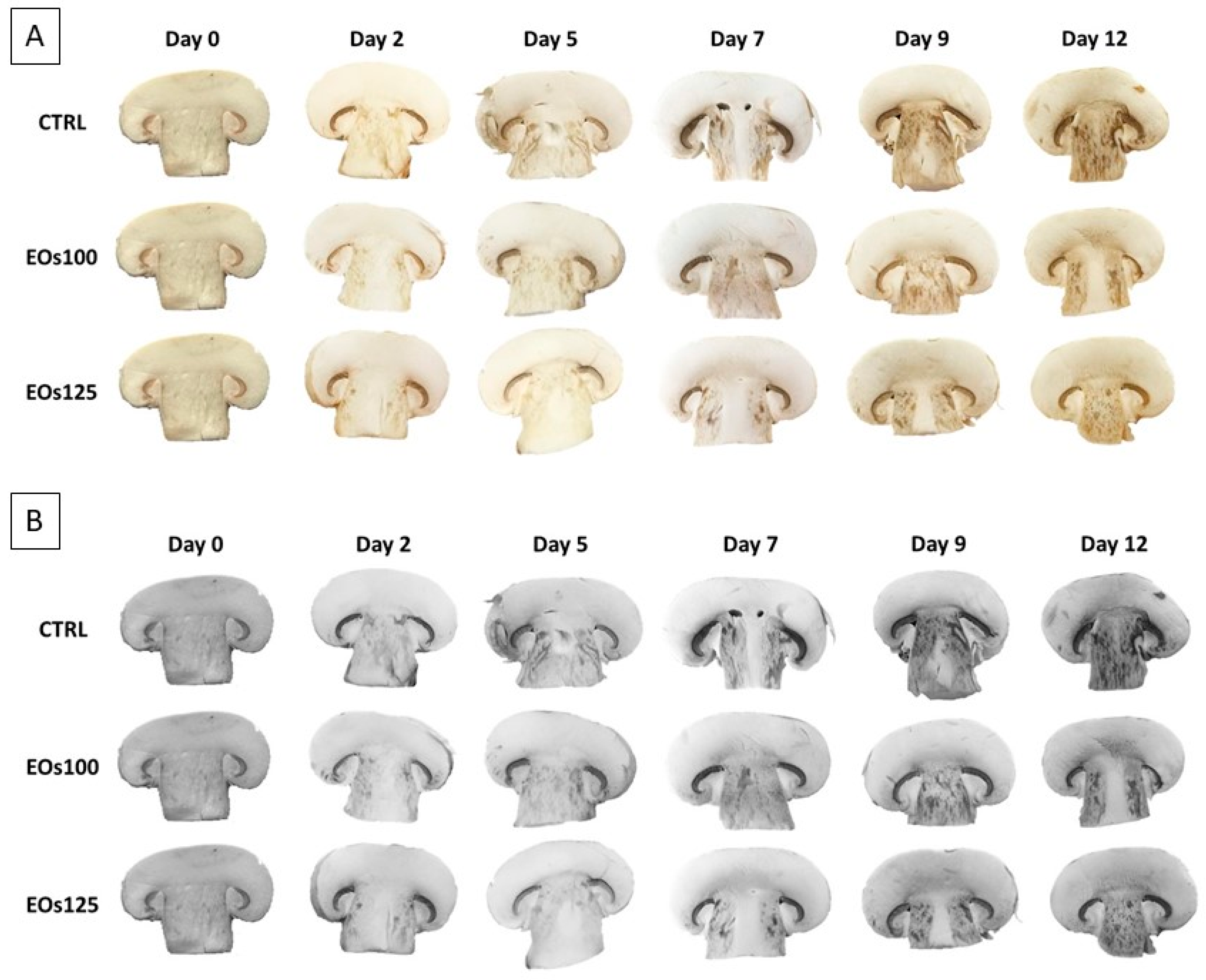
3.4. Colour and Polyphenol Oxidase Activity
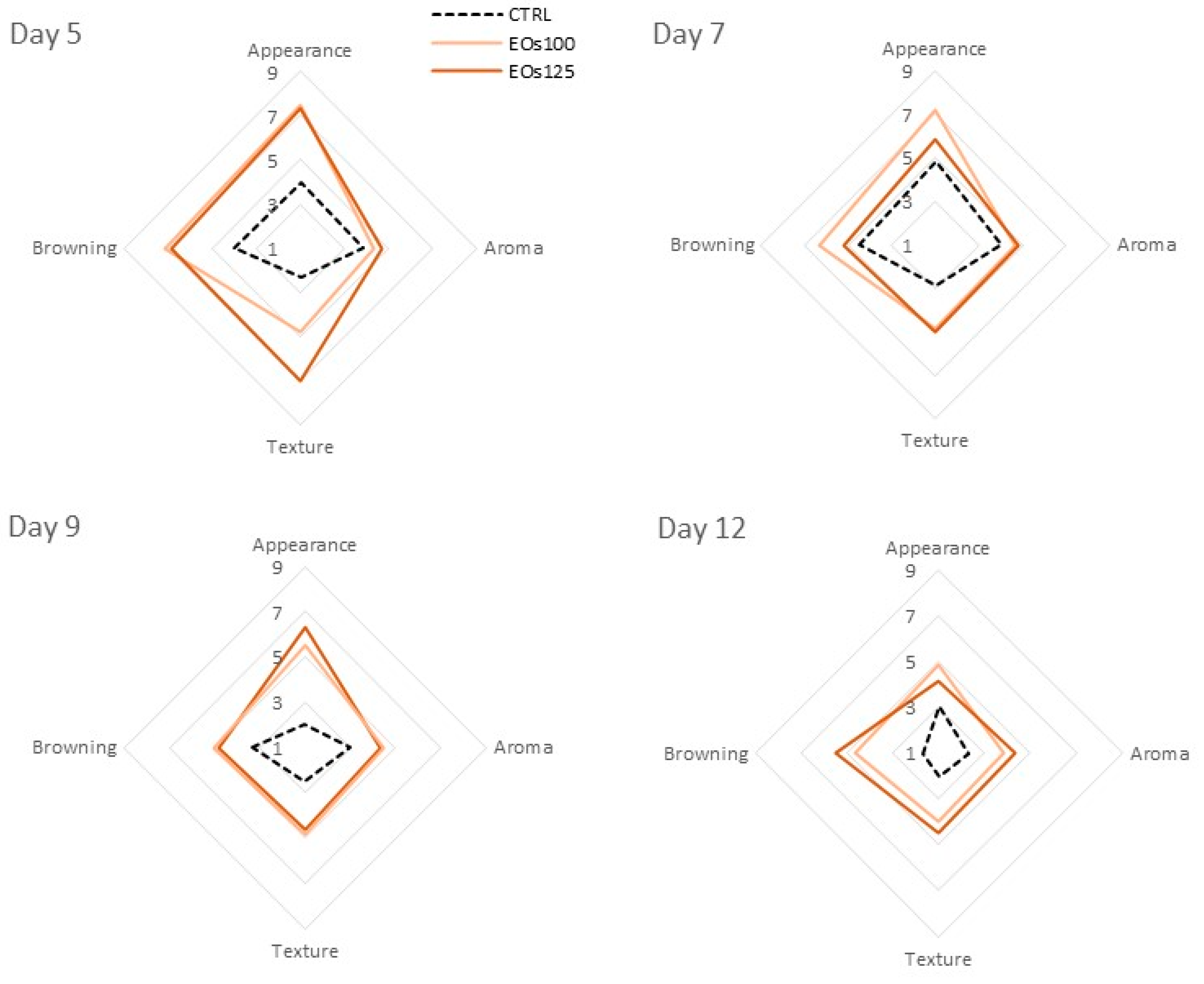
3.5. Sensory Analyses
3.6. Microbial Quality
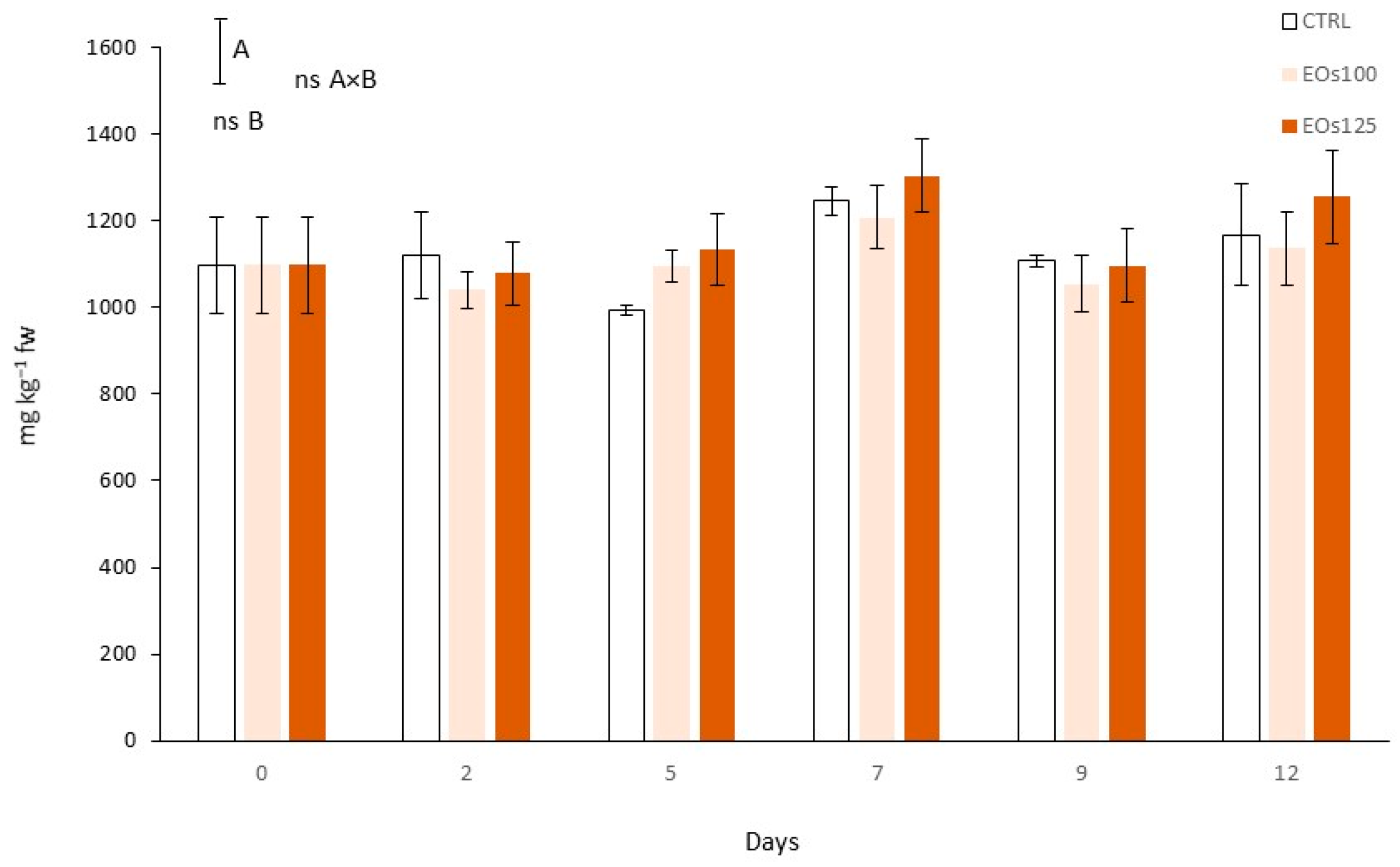
3.7. Phenolic Compounds and Phenyl Ammonia Lyase Activity
3.8. Total Antioxidant Capacity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Royse, D.J. A global perspective on the high five: Agaricus, pleurotus, lentinula, auricularia & flammulina. In Proceedings of the 8th International Conference on Mushroom Biology and Mushroom Products (ICMBMP8), New Delhi, India, 19–22 November 2014; Volume 1, pp. 1–6. [Google Scholar]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data (accessed on 17 October 2018).
- Zhang, K.; Pu, Y.Y.; Sun, D.W. Recent advances in quality preservation of postharvest mushrooms (Agaricus bisporus): A review. Trends Food Sci. Technol. 2018, 78, 72–82. [Google Scholar] [CrossRef]
- Singh, P.; Langowski, H.C.; Wani, A.A.; Saengerlaub, S. Recent advances in extending the shelf life of fresh Agaricus mushrooms: A review. J. Sci. Food Agric. 2010, 90, 1393–1402. [Google Scholar] [CrossRef]
- Suslow, T.V.; Cantwell, M. Mushroom: Recommendations for Maintaining Postharvest Quality. Available online: http://postharvest.ucdavis.edu/Commodity_Resources/Fact_Sheets/Datastores/Vegetables_English/?uid=21&ds=799 (accessed on 25 May 2021).
- Cliffe-Byrnes, V.; O’Beirne, D. Effects of gas atmosphere and temperature on the respiration rates of whole and sliced mushrooms (Agaricus bisporus)—Implications for film permeability in modified atmosphere packages. J. Food Sci. 2007, 72. [Google Scholar] [CrossRef] [PubMed]
- Savoie, J.-M.; Minvielle, N.; Largeteau, M.L. Radical-scavenging properties of extracts from the white button mushroom, Agaricus bisporus. J. Sci. Food Agric. 2008, 88, 970–975. [Google Scholar] [CrossRef]
- Zivanovic, S.; Busher, R.W.; Kim, K.S. Textural changes in mushrooms (Agaricus bisporus) associated with tissue ultrastructure and composition. J. Food Sci. 2000, 65, 1404–1408. [Google Scholar] [CrossRef]
- Simón, A.; Gonzalez-Fandos, E.; Tobar, V. The sensory and microbiological quality of fresh sliced mushroom (Agaricus bisporus L.) packaged in modified atmospheres. Int. J. Food Sci. Technol. 2005, 40, 943–952. [Google Scholar] [CrossRef]
- Simón, A.; González-Fandos, E.; Vázquez, M. Effect of washing with citric acid and packaging in modified atmosphere on the sensory and microbiological quality of sliced mushrooms (Agaricus bisporus L.). Food Control. 2010, 21, 851–856. [Google Scholar] [CrossRef]
- Artés-Hernández, F.; Martínez-Hernández, G.B.; Aguayo, E.; Gómez, P.A.; Artés, F. Fresh-cut fruit and vegetables: Emerging eco-friendly techniques for sanitation and preserving safety. In Postharvest Handling; Kahramanoglu, I., Ed.; InTech: London, UK, 2017; pp. 7–45. [Google Scholar]
- Formica-Oliveira, A.C.; Martínez-Hernández, G.B.; Díaz-López, V.; Artés, F.; Artés-Hernández, F. Use of postharvest UV-B and UV-C radiation treatments to revalorize broccoli byproducts and edible florets. Innov. Food Sci. Emerg. Technol. 2017, 43, 77–83. [Google Scholar] [CrossRef]
- Rao, J.; Chen, B.; McClements, D.J. Improving the efficacy of essential oils as antimicrobials in foods: Mechanisms of action. Annu. Rev. Food Sci. Technol. 2019, 10, 365–387. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ren, L.; Li, M.; Qian, J.; Fan, J.; Du, B. Effects of clove essential oil and eugenol on quality and browning control of fresh-cut lettuce. Food Chem. 2017, 214, 432–439. [Google Scholar] [CrossRef]
- Teng, Y.; Murtaza, A.; Iqbal, A.; Fu, J.; Ali, S.W.; Iqbal, M.A.; Xu, X.; Pan, S.; Hu, W. Eugenol emulsions affect the browning processes, and microbial and chemical qualities of fresh-cut Chinese water chestnut. Food Biosci. 2020, 38, 100716. [Google Scholar] [CrossRef]
- Seo, H.-S.; Beuchat, L.R.; Kim, H.; Ryu, J.-H. Development of an experimental apparatus and protocol for determining antimicrobial activities of gaseous plant essential oils. Int. J. Food Microbiol. 2015, 215, 95–100. [Google Scholar] [CrossRef]
- López-Gómez, A.; Ros-Chumillas, M.; Antolinos, V.; Buendía-Moreno, L.; Navarro-Segura, L.; Sánchez-Martínez, M.J.; Martínez-Hernández, G.B.; Soto-Jover, S. Fresh culinary herbs decontamination with essential oil vapours applied under vacuum conditions. Postharvest Biol. Technol. 2019, 156, 110942. [Google Scholar] [CrossRef]
- Gao, M.; Feng, L.; Jiang, T. Browning inhibition and quality preservation of button mushroom (Agaricus bisporus) by essential oils fumigation treatment. Food Chem. 2014, 149, 107–113. [Google Scholar] [CrossRef]
- Jiang, T.; Luo, Z.; Ying, T. Fumigation with essential oils improves sensory quality and enhanced antioxidant ability of shiitake mushroom (Lentinus edodes). Food Chem. 2015, 172, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Segura, L.; Ros-Chumillas, M.; Martínez-Hernández, G.B.; López-Gómez, A. A new advanced packaging system for extending the shelf life of refrigerated farmed fish fillets. J. Sci. Food Agric. 2020, 100, 4601–4611. [Google Scholar] [CrossRef] [PubMed]
- López-Gómez, A.; López-Cánovas, D. Method for the Surface Decontamination of Packaged Solid Food. WO Patent WO2016146864A1, 22 September 2016. [Google Scholar]
- ASTM. Physical Requirement Guidelines for Sensory Evaluation Laboratories; Eggert, J., Zook, K., Eds.; ASTM International: Philadelphia, PA, USA, 1986; ISBN 0803109245. [Google Scholar]
- ISO. ISO 8589:2007—Sensory Analysis—General Guidance for the Design of Test Rooms. Available online: https://www.iso.org/obp/ui/#iso:std:iso:8589:ed-2:v1:en (accessed on 25 May 2021).
- Villaescusa, R.; Gil, M.I. Quality improvement of Pleurotus mushrooms by modified atmosphere packaging and moisture absorbers. Postharvest Biol. Technol. 2003, 28, 169–179. [Google Scholar] [CrossRef]
- Redondo, D.; Venturini, M.E.; Oria, R.; Arias, E. Inhibitory effect of microwaved thinned nectarine extracts on polyphenol oxidase activity. Food Chem. 2016, 197, 603–610. [Google Scholar] [CrossRef]
- Merck Enzymatic Assay of Polyphenol Oxidase. Available online: https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma/General_Information/polyphenol_oxidase.pdf (accessed on 18 November 2020).
- Jacobo-Velázquez, D.A.; Martínez-Hernández, G.B.; Rodríguez, S.; Cao, C.-M.; Cisneros-Zevallos, L. Plants as biofactories: Physiological role of reactive oxygen species on the accumulation of phenolic antioxidants in carrot tissue under wounding and hyperoxia stress. J. Agric. Food Chem. 2011, 59, 6583–6593. [Google Scholar] [CrossRef]
- Antolinos, V.; Sánchez-Martínez, M.J.; Maestre-Valero, J.F.; López-Gómez, A.; Martínez-Hernández, G.B. Effects of Irrigation with Desalinated Seawater and Hydroponic System on Tomato Quality. Water 2020, 12, 518. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Kader, A.A. Postharvest Technology of Horticultural Crops, 3rd ed.; Kader, A.A., Ed.; University of California, Agriculture and Natural Resources: Richmond, CA, USA, 2002. [Google Scholar]
- González-Fandos, E.; Jiménez, A.S.; Pardo, V.T. Quality and shelf life of packaged fresh sliced mushrooms stored at two different temperatures. Agric. Food Sci. 2006, 15, 414–422. [Google Scholar] [CrossRef]
- Barros, L.; Pereira, C.; Ferreira, I.C.F.R. Optimized analysis of organic acids in edible mushrooms from Portugal by ultra fast liquid chromatography and photodiode array detection. Food Anal. Methods 2013, 6, 309–316. [Google Scholar] [CrossRef]
- Ruiz-Herrera, J.; Ortiz-Castellanos, L. Cell wall glucans of fungi. A review. Cell Surf. 2019, 5, 100022. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasaprasad, S.; Burton, K.S.; Wood, D.A. Cloning and characterisation of a chitin synthase gene cDNA from the cultivated mushroom Agaricus bisporus and its expression during morphogenesis. FEMS Microbiol. Lett. 2000, 189, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, H.; Du, Y.; Keyhani, N.O.; Xia, Y.; Jin, K. Members of chitin synthase family in Metarhizium acridum differentially affect fungal growth, stress tolerances, cell wall integrity and virulence. PLoS Pathog. 2019, 15, e1007964. [Google Scholar] [CrossRef]
- Surjadinata, B.B.; Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. UVA, UVB and UVC light enhances the biosynthesis of phenolic antioxidants in fresh-cut carrot through a synergistic effect with wounding. Molecules 2017, 22, 668. [Google Scholar] [CrossRef]
- Muszyńska, B.; Piotrowska, J.; Krakowska, A.; Gruba, A.; Kała, K.; Sułkowska-Ziaja, K.; Kryczyk, A.; Opoka, W. Study of physiologically active components in different parts of fruiting bodies of varieties of Agaricus bisporus (white mushroom). Eur. Food Res. Technol. 2017, 243, 2135–2145. [Google Scholar] [CrossRef]
- Lei, J.; Li, B.; Zhang, N.; Yan, R.; Guan, W.; Brennan, C.S.; Gao, H.; Peng, B. Effects of UV-C treatment on browning and the expression of polyphenol oxidase (PPO) genes in different tissues of Agaricus bisporus during cold storage. Postharvest Biol. Technol. 2018, 139, 99–105. [Google Scholar] [CrossRef]
- López-Briones, G.; Varoquaux, P.; Bureau, G.; Pascat, B. Modified atmosphere packaging of common mushroom. Int. J. Food Sci. Technol. 2007, 28, 57–68. [Google Scholar] [CrossRef]
- Echegoyen, Y.; Nerín, C. Performance of an active paper based on cinnamon essential oil in mushrooms quality. Food Chem. 2015, 170, 30–36. [Google Scholar] [CrossRef]
- Mahajan, P.V.; Rodrigues, F.A.S.; Motel, A.; Leonhard, A. Development of a moisture absorber for packaging of fresh mushrooms (Agaricus bisporous). Postharvest Biol. Technol. 2008, 48, 408–414. [Google Scholar] [CrossRef]
- Du, X.; Sissons, J.; Shanks, M.; Plotto, A. Aroma and flavor profile of raw and roasted Agaricus bisporus mushrooms using a panel trained with aroma chemicals. LWT 2021, 138, 110596. [Google Scholar] [CrossRef]
- Formica-Oliveira, A.C.; Martínez-Hernández, G.B.; Aguayo, E.; Gómez, P.A.; Artés, F.; Artés-Hernández, F. UV-C and hyperoxia abiotic stresses to improve healthiness of carrots: Study of combined effects. J. Food Sci. Technol. 2016, 53, 3465–3476. [Google Scholar] [CrossRef] [PubMed]
- Cisneros-Zevallos, L. The use of controlled postharvest abiotic stresses as a tool for enhancing the nutraceutical content and adding-value of fresh fruits and vegetables. J. Food Sci. 2003, 68, 1560–1565. [Google Scholar] [CrossRef]
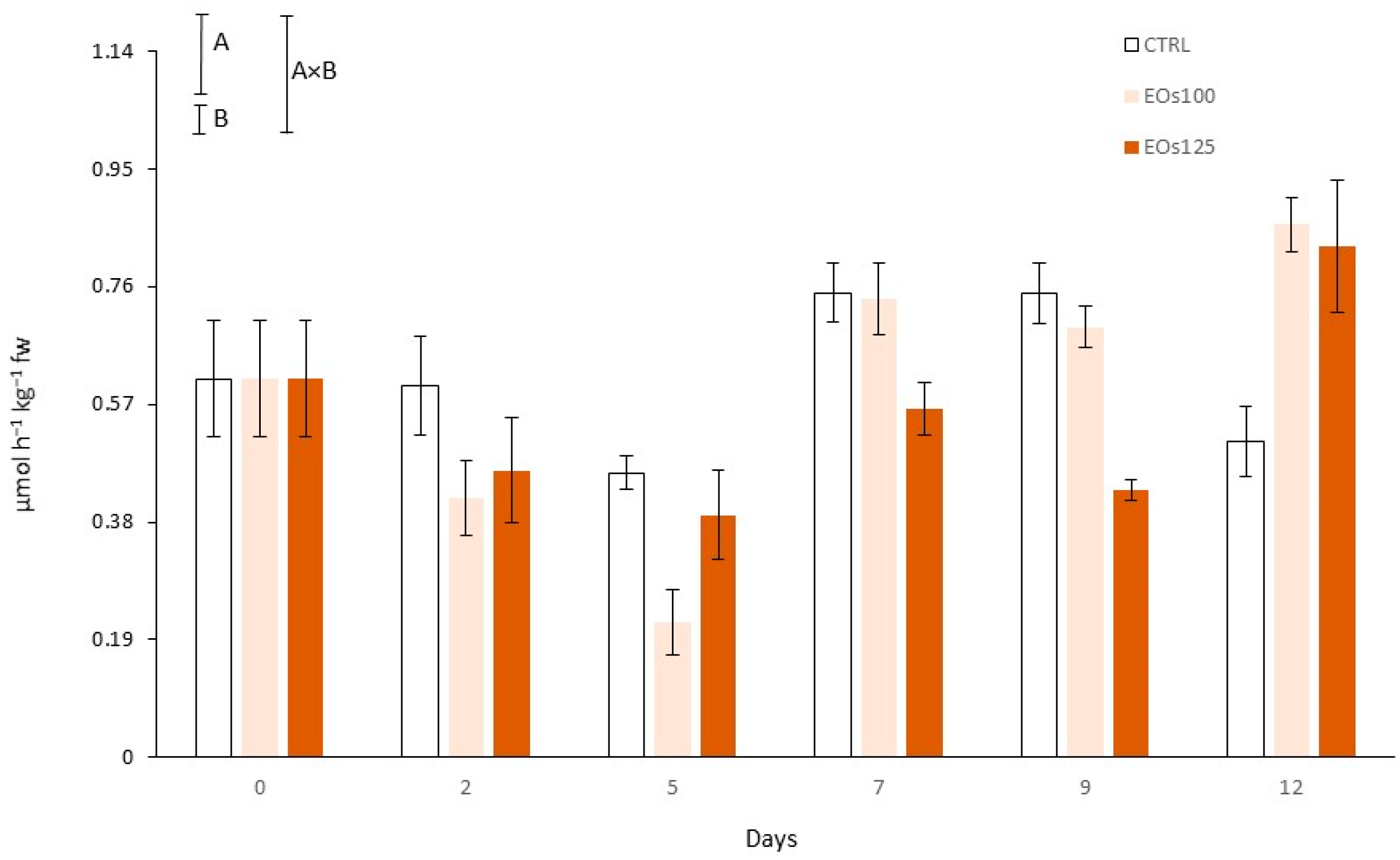
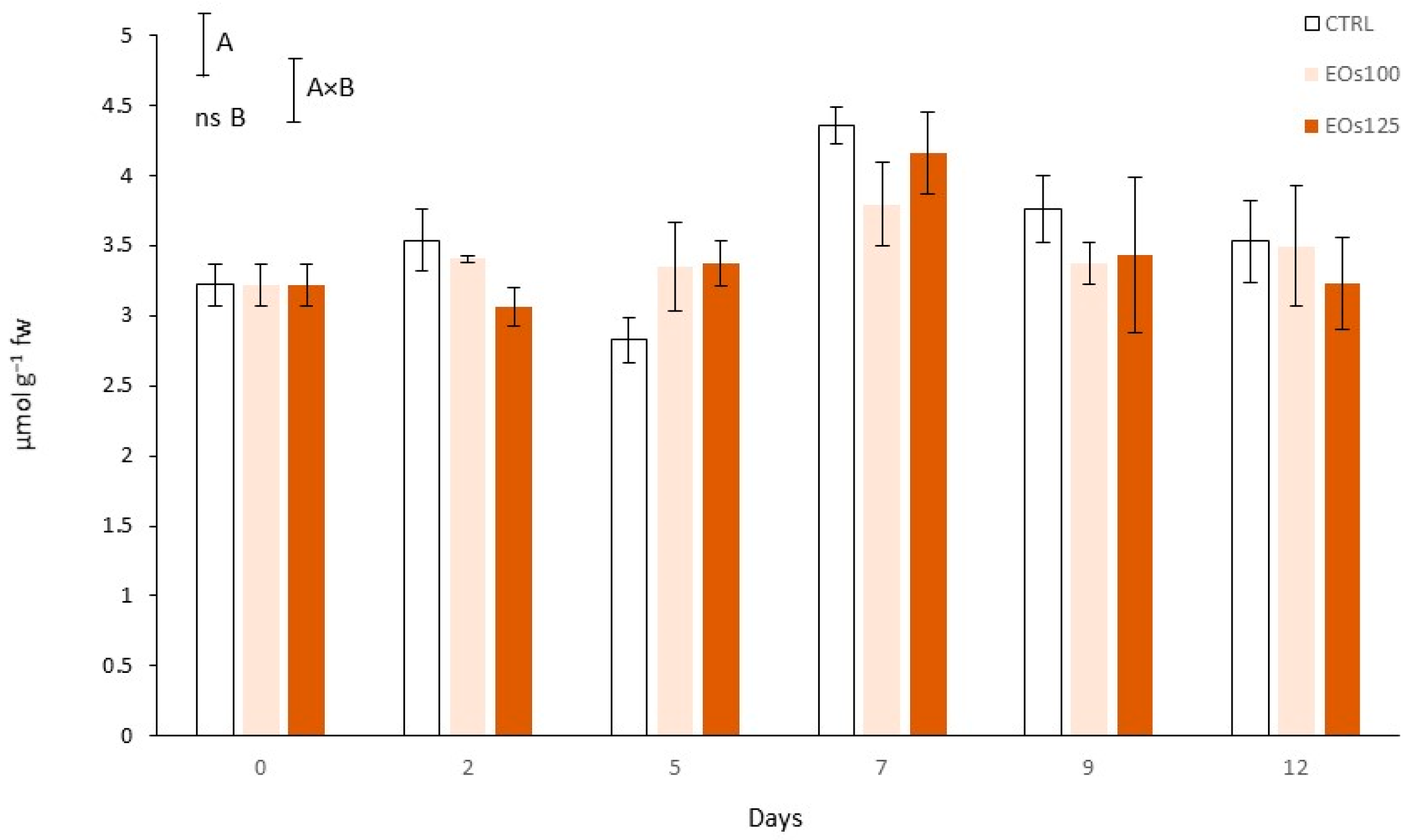
| Vapour EOs MAP | Storage Time (Days) | pH | L* (Cap) | L* (Stipe) | Shear Force (N) |
|---|---|---|---|---|---|
| Processing day | 6.75 ± 0.05 | 92.1 ± 0.8 | 89.2 ± 0.6 | 366.2 ± 65.0 | |
| CTRL | 2 | 7.20 ± 0.12 | 89.6 ± 1.3 | 88.4 ± 1.7 | 707.2 ± 75.8 |
| 5 | 6.96 ± 0.20 | 89.2 ± 1.0 | 85.2 ± 1.7 | 598.3 ± 52.8 | |
| 7 | 6.95 ± 0.01 | 86.4 ± 3.0 | 81.0 ± 2.9 | 621.7 ± 76.6 | |
| 9 | 7.38 ± 0.14 | 83.6 ± 2.5 | 76.9 ± 1.9 | 548.3 ± 61.5 | |
| 12 | 7.41 ± 0.02 | 79.9 ± 2.0 | 75.0 ± 3.8 | 526.8 ± 76.9 | |
| EOs100 | 2 | 7.15 ± 0.02 | 87.9 ± 1.9 | 88.9 ± 1.5 | 784.9 ± 73.2 |
| 5 | 6.94 ± 0.14 | 88.1 ± 4.3 | 85.0 ± 1.4 | 707.2 ± 56.7 | |
| 7 | 7.29 ± 0.09 | 84.2 ± 2.2 | 80.8 ± 3.7 | 803.1 ± 127.8 | |
| 9 | 7.47 ± 0.01 | 80.3 ± 1.9 | 76.6 ± 2.4 | 643.4 ± 61.2 | |
| 12 | 7.51 ± 0.01 | 79.5 ± 2.4 | 75.5 ± 5.0 | 752.5 ± 133.7 | |
| EOs125 | 2 | 7.13 ± 0.04 | 88.7 ± 1.4 | 87.0 ± 2.3 | 811.8 ± 85.9 |
| 5 | 7.03 ± 0.28 | 87.6 ± 1.7 | 83.5 ± 2.3 | 786.6 ± 52.0 | |
| 7 | 7.39 ± 0.06 | 84.3 ± 1.5 | 79.6 ± 2.9 | 930.9 ± 142.1 | |
| 9 | 7.09 ± 0.04 | 81.0 ± 1.1 | 75.7 ± 4.2 | 835.0 ± 118.5 | |
| 12 | 7.23 ± 0.04 | 78.2 ± 1.4 | 74.5 ± 2.4 | 726.3 ± 182.1 | |
| Treatment (A) | (0.07) * | (1.1) ‡ | (1.4) ‡ | (109.1) ‡ | |
| Storage time (B) | (0.18) ‡ | (1.6) ‡ | (1.9) ‡ | (154.3) ‡ | |
| A × B | (0.31) ‡ | ns | ns | (267.3) ‡ |
| Vapour EOs MAP | Storage Time (Days) | Pseudomonas | Mesophilic | Psychrophilic | Enterobacteria |
|---|---|---|---|---|---|
| Processing day | 2.29 ± 0.03 | 4.87 ± 0.07 | 3.24 ± 0.95 | 1.95 ± 0.15 | |
| CTRL | 2 | 4.73 ± 0.06 | 6.67 ± 0.11 | 5.32 ± 0.07 | 3.96 ± 0.41 |
| 5 | 5.84 ± 0.01 | 7.56 ± 0.04 | 7.45 ± 0.01 | 5.74 ± 0.24 | |
| 7 | 4.92 ± 0.11 | 7.81 ± 0.21 | 7.95 ± 0.17 | 6.06 ± 0.14 | |
| 9 | 5.72 ± 0.11 | 8.20 ± 0.06 | 8.21 ± 0.15 | 6.82 ± 0.24 | |
| 12 | 5.79 ± 0.54 | 9.00 ± 0.12 | 9.15 ± 0.13 | 7.41 ± 0.13 | |
| EOs100 | 2 | 4.32 ± 0.02 | 6.19 ± 0.02 | 4.90 ± 0.04 | 4.26 ± 0.21 |
| 5 | 5.93 ± 0.25 | 7.44 ± 0.17 | 6.72 ± 0.14 | 5.54 ± 0.34 | |
| 7 | 5.71 ± 0.17 | 7.61 ± 0.28 | 7.60 ± 0.56 | 5.70 ± 0.29 | |
| 9 | 4.74 ± 0.49 | 8.10 ± 0.10 | 8.22 ± 0.20 | 6.32 ± 0.28 | |
| 12 | 4.09 ± 0.19 | 8.60 ± 0.09 | 8.91 ± 0.12 | 6.21 ± 0.38 | |
| EOs125 | 2 | 5.31 ± 0.73 | 6.58 ± 0.02 | 5.58 ± 0.20 | 4.99 ± 0.38 |
| 5 | 5.74 ± 0.36 | 7.60 ± 0.14 | 7.57 ± 0.06 | 5.74 ± 0.09 | |
| 7 | 6.29 ± 0.25 | 7.82 ± 0.10 | 7.97 ± 0.18 | 5.67 ± 0.59 | |
| 9 | 5.75 ± 0.11 | 9.38 ± 0.09 | 9.00 ± 0.38 | 7.13 ± 0.59 | |
| 12 | 4.13 ± 0.30 | 8.87 ± 0.09 | 9.13 ± 0.08 | 5.87 ± 0.79 | |
| Treatment (A) | (0.46) ‡ | (0.19) ‡ | (0.42) ‡ | (0.67) ‡ | |
| Storage time (B) | (0.65) ‡ | (0.27) ‡ | (0.59) ‡ | (0.94) ‡ | |
| A × B | (1.12) ‡ | (0.47) ‡ | ns | (0.92) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Gómez, A.; Ros-Chumillas, M.; Navarro-Martínez, A.; Barón, M.; Navarro-Segura, L.; Taboada-Rodríguez, A.; Marín-Iniesta, F.; Martínez-Hernández, G.B. Packaging of Fresh Sliced Mushrooms with Essential Oils Vapours: A New Technology for Maintaining Quality and Extending Shelf Life. Foods 2021, 10, 1196. https://doi.org/10.3390/foods10061196
López-Gómez A, Ros-Chumillas M, Navarro-Martínez A, Barón M, Navarro-Segura L, Taboada-Rodríguez A, Marín-Iniesta F, Martínez-Hernández GB. Packaging of Fresh Sliced Mushrooms with Essential Oils Vapours: A New Technology for Maintaining Quality and Extending Shelf Life. Foods. 2021; 10(6):1196. https://doi.org/10.3390/foods10061196
Chicago/Turabian StyleLópez-Gómez, Antonio, María Ros-Chumillas, Alejandra Navarro-Martínez, Marta Barón, Laura Navarro-Segura, Amaury Taboada-Rodríguez, Fulgencio Marín-Iniesta, and Ginés Benito Martínez-Hernández. 2021. "Packaging of Fresh Sliced Mushrooms with Essential Oils Vapours: A New Technology for Maintaining Quality and Extending Shelf Life" Foods 10, no. 6: 1196. https://doi.org/10.3390/foods10061196
APA StyleLópez-Gómez, A., Ros-Chumillas, M., Navarro-Martínez, A., Barón, M., Navarro-Segura, L., Taboada-Rodríguez, A., Marín-Iniesta, F., & Martínez-Hernández, G. B. (2021). Packaging of Fresh Sliced Mushrooms with Essential Oils Vapours: A New Technology for Maintaining Quality and Extending Shelf Life. Foods, 10(6), 1196. https://doi.org/10.3390/foods10061196










