Impact of Extraction Method on the Detection of Quality Biomarkers in Normal vs. DFD Meat
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Muscle Sample Collection
2.3. Meat Quality Trait Measurements
2.4. Muscle Extraction Methods
2.4.1. Sarcoplasmic Protein Extraction
- TES buffer (TES): 10 mM Tris (pH 7.6), 1 mM EDTA (pH 8.0), 0.25 M sucrose, and 0.6% protease inhibitor cocktail (P8340, Sigma-Aldrich Co., St. Louis, MO, USA) [18].
- Sodium buffer (Na): 50 mM sodium phosphate buffer (pH 7.5) and 0.6% protease inhibitor cocktail (P8340, Sigma-Aldrich Co., St. Louis, MO, USA) [19].
- Sodium with Triton buffer (Na + T): 50 mM sodium phosphate buffer (pH 7.5), 0.1% Triton X-100, and 0.6% protease inhibitor cocktail (P8340, Sigma-Aldrich Co., St. Louis, MO, USA) [20].
- Potassium with Triton buffer (K + T): 10 mM potassium phosphate buffer (pH 7.4), 50 mM NaCl, 0.1% Triton X-100, and 0.6% protease inhibitor cocktail (P8340, Sigma-Aldrich Co., St. Louis, MO, USA) [21].
- (a)
- 1000× g, 6 min at 4 °C;
- (b)
- 20,000× g, 20 min at 4 °C.
2.4.2. Myofibrillar Protein Extraction
- The denaturing extraction was performed on the sample residue after the extraction of sarcoplasmic proteins with the TES buffer and 20 min centrifugation at 20,000× g and 4 °C, as proposed by Bjarnadottir et al. [22]. The resulting pellet was homogenized into 4 mL of lysis buffer (10 mM Tris-HCl (pH 7.6), 7 M urea, 2 M thiourea, 2% CHAPS, and 10 mM DTT) with the polytron 2 × 15 s at 20,000 rpm. Subsequently, this solution was stirred for 1 h in a Multi Reax stirrer (Heidolph Instruments, Schwabach, Germany) and was centrifuged at 20,000 rpm for 20 min at 4 °C. The supernatant containing the myofibrillar proteins was collected and filtered through a nylon filter (5 mm), aliquoted, and stored at −80 °C.
- The non-denaturing myofibrillar extraction was based on the method reported by Hashimoto et al. [23], with the following modifications: 0.5 g of muscle samples were homogenized in 4 mL of non-denaturing extraction buffer (30 mM of sodium phosphate buffer (pH 7)) and 0.6% protease inhibitor cocktail (Sigma-Aldrich Co., St. Louis, MO, USA) using a Polytron PT1200 E (Kinematica Inc., Luzern, Switzerland) two times for 15 s at maximum speed. The homogenates obtained were centrifuged at 8000× g for 20 min at 4 °C. The recovered pellet was resuspended in 4 mL of KCl phosphate buffer ((pH 7.5); 0.45 M KCl, 15.6 mM Na2PO4, and 3.5 mM KH2PO4) and vortexed. Subsequently, this solution was stirred for 30 min in a Multi Reax stirrer (Heidolph Instruments, Schwabach, Germany). The mixture was centrifuged twice at 5000× g for 15 min at 4 °C. After the centrifugation, the supernatant containing the myofibrillar proteins was recovered, aliquoted, and stored at −80 °C.
2.5. Protein Extractability
2.6. Oxidative Stress
2.7. Sarcoplasmic and Myofibrillar Subproteome Analysis
2.8. Stress Protein: Hsp70
2.9. Statistical Analysis
3. Results and Discussion
3.1. Meat Quality Traits
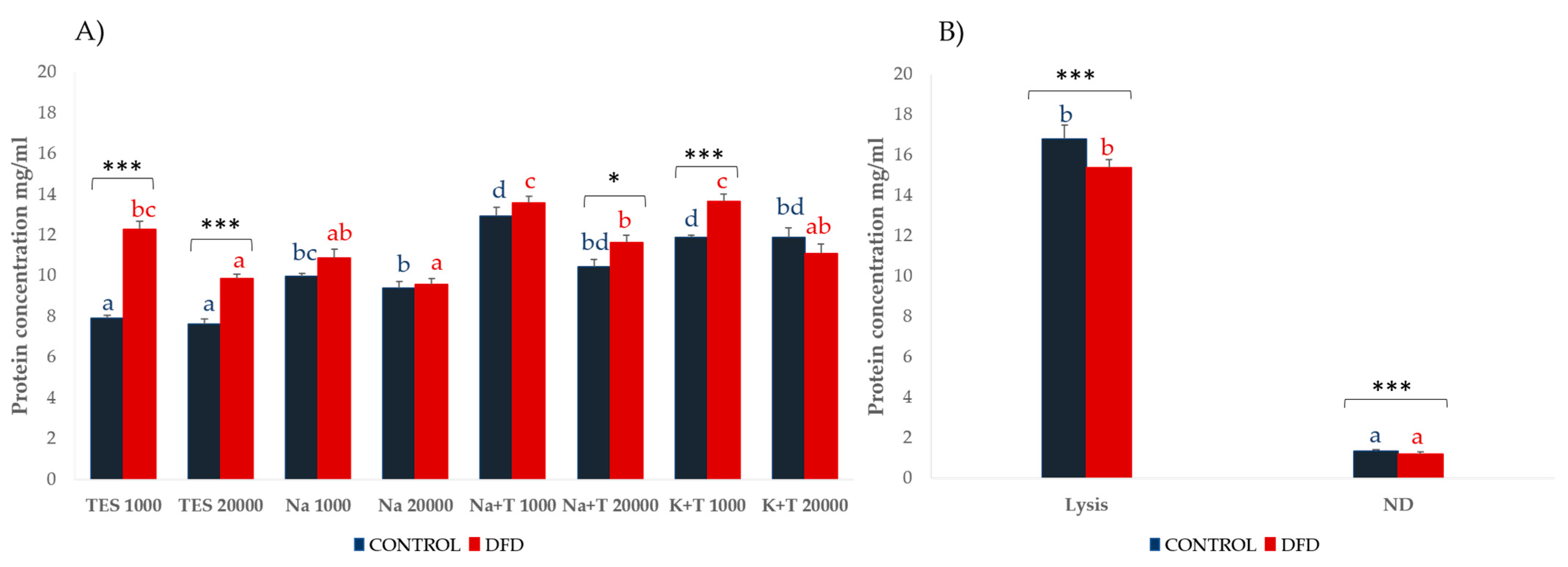
3.2. Protein Extractability
3.3. Oxidative Stress
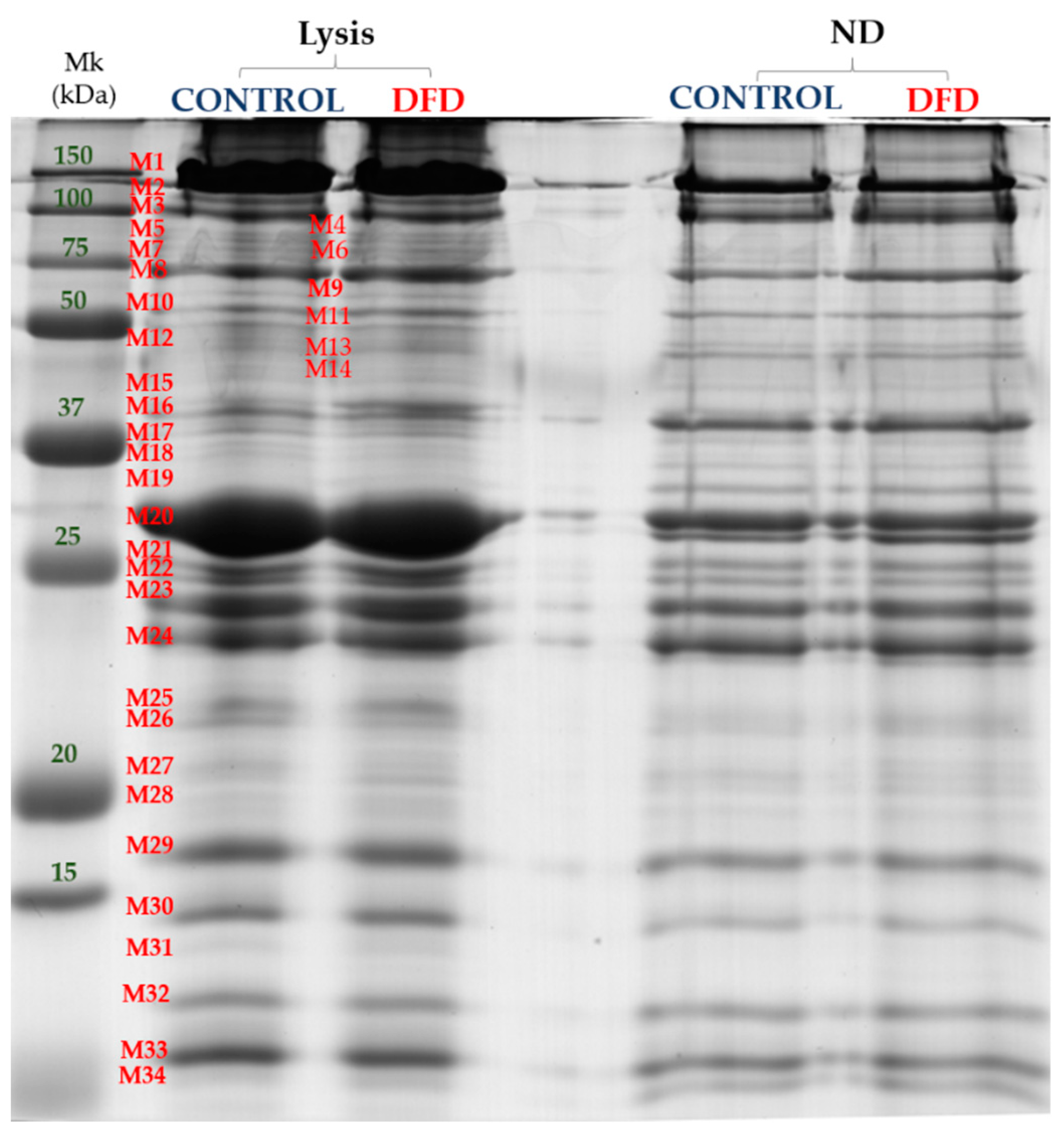
3.4. Sarcoplasmic and Myofibrillar Subproteome
3.5. Stress Protein: Hsp70
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Picard, B.; Gagaoua, M. Meta-proteomics for the discovery of protein biomarkers of beef tenderness: An overview of integrated studies. Food Res. Int. 2020, 127, 108739. [Google Scholar] [CrossRef] [PubMed]
- Lieber, R.L.; Friden, J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve 2000, 23, 1647–1666. [Google Scholar] [CrossRef]
- Eady, M.; Samuel, D.; Bowker, B. Effect of pH and postmortem aging on protein extraction from broiler breast muscle. Poult. Sci. 2014, 93, 1825–1833. [Google Scholar] [CrossRef]
- Au, Y. The muscle ultrastructure: A structural perspective of the sarcomere. Cell. Mol. Life Sci. 2004, 61, 3016–3033. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zou, Y.; Han, M.; Pan, L.; Xing, T.; Xu, X.; Zhou, G. Solubilization of myosin in a solution of low ionic strength L-histidine: Significance of the imidazole ring. Food Chem. 2016, 196, 42–49. [Google Scholar] [CrossRef]
- Anderson, M.J.; Lonergan, S.M.; Huff-Lonergan, E. Myosin light chain 1 release from myofibrillar fraction during postmortem aging is a potential indicator of proteolysis and tenderness of beef. Meat Sci. 2012, 90, 345–351. [Google Scholar] [CrossRef]
- Bowker, B.C.; Fahrenholz, T.M.; Paroczay, E.W.; Eastridge, J.S.; Solomon, M.B. Effect of hydrodynamic pressure processing and ageing on the tenderness and myofibrillar proteins of beef strip loins. J. Muscle Foods 2008, 19, 74–97. [Google Scholar] [CrossRef]
- Lan, Y.H.; Novakofski, J.; Carr, T.R.; McKeith, F.K. Assay and storage conditions affect yield of salt soluble protein from muscle. J. Food Sci. 1993, 58, 963–967. [Google Scholar] [CrossRef]
- Munasinghe, D.M.; Sakai, T. Sodium chloride as a preferred protein extractant for pork lean meat. Meat Sci. 2004, 67, 697–703. [Google Scholar] [CrossRef]
- Thomas, R.; Gadekar, Y.P.; Kandeepan, G.; George, S.K.; Kataria, M. Effect of extraction conditions and postmortem ageing period on yield and salt soluble proteins from buffalo (Bubalus bubalis) lean meat. Am. J. Food Technol. 2007, 2, 313–317. [Google Scholar] [CrossRef]
- Newton, K.G.; Gill, C.O. The microbiology of DFD fresh meats: A review. Meat Sci. 1981, 5, 223–232. [Google Scholar] [CrossRef]
- Grayson, A.L.; Shackelford, S.D.; King, D.A.; McKeith, R.O.; Miller, R.K.; Wheeler, T.L. Effect of degree of dark cutting on tenderness and sensory attributes of beef. J. Anim. Sci. 2016, 94, 2583–2591. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Hopkins, D.L.; Bruce, H.; Li, D.; Baldi, G.; Bekhit, A.E. Causes and contributing factors to “dark cutting” meat: Current trends and future directions: A review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 400–430. [Google Scholar] [CrossRef]
- Adzitey, F.; Nurul, H. Pale soft exudative (PSE) and dark firm dry (DFD) meats: Causes and measures to reduce these incidences—A mini review. Int. Food Res. J. 2011, 18, 11–20. [Google Scholar]
- AMSA. Meat Color Measurement Guidelines; American Meat Science Association: Champaign, IL, USA, 2012. [Google Scholar]
- Honikel, K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef]
- Díaz, F.; Díaz-Luis, A.; Sierra, V.; Diñeiro, Y.; González, P.; García-Torres, S.; Tejerina, D.; Romero-Fernández, M.P.; de Vaca, M.C.; Coto-Montes, A.; et al. What functional proteomic and biochemical analysis tell us about animal stress in beef? J. Proteom. 2020, 218, 103722. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Veiseth-Kent, E.; Grove, H.; Kuziora, P.; Aass, L.; Hildrum, K.I.; Hollung, K. Peroxiredoxin-6—A potential protein marker for meat tenderness in bovine longissimus thoracis muscle. J. Anim. Sci. 2009, 87, 2391–2399. [Google Scholar] [CrossRef]
- Coto-Montes, A.; Caballero, B.; Sierra, V.; Vega-Naredo, I.; Tomás-Zapico, C.; Hardeland, R.; Tolivia, D.; Ureña, F.; Rodríguez-Colunga, M.J. Actividad de los principales enzimas antioxidantes durante el periodo de Oreo de culones de la raza Asturiana de los Valles. ITEA 2004, 100, 43–45. [Google Scholar]
- Potes, Y.; de Luxán-Delgado, B.; Rodriguez-González, S.; Guimarães, M.R.M.; Solano, J.J.; Fernández-Fernández, M.; Coto-Montes, A. Overweight in elderly people induces impaired autophagy in skeletal muscle. Free Radic. Biol. Med. 2017, 110, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Gonzalez, A.; Potes, Y.; Illan-Rodriguez, D.; Vega-Naredo, I.; Sierra, V.; Caballero, B.; Fabrega, E.; Velarde, A.; Dalmau, A.; Olivan, M.; et al. Effect of animal mixing as a stressor on biomarkers of autophagy and oxidative stress during pig muscle maturation. Animal 2015, 9, 1188–1194. [Google Scholar] [CrossRef]
- Bjarnadóttir, S.G.; Hollung, K.; Faergestad, E.M.; Veiseth-Kent, E. Proteome changes in bovine longissimus thoracis muscle during the first 48 h postmortem: Shifts in energy status and myofibrillar stability. J. Agric. Food Chem. 2010, 58, 7408–7414. [Google Scholar] [CrossRef]
- Hashimoto, K.; Watabe, S.; Kono, M.; Shiro, K. Muscle protein composition of sardine and mackerel. Bull. Jpn. Soc. Sci. Fish 1979, 45, 1435–1441. [Google Scholar] [CrossRef]
- Chen, X.; Tume, R.K.; Xu, X.; Zhou, G. Solubilization of myofibrillar proteins in water or low ionic strength media: Classic techniques, basic principles and novel functionalities. Crit. Rev. Food Sci. Nutr. 2015, 57, 3260–3280. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Lubinsky, S.; Bewley, G.C. Genetics of catalase in Drosophila melanogaster: Rates of synthesis and degradation of the enzyme in flies aneuploid and euploid for the structural gene. Genetics 1979, 91, 723–742. [Google Scholar] [CrossRef] [PubMed]
- Joseph, P.; Suman, S.P.; Rentfrow, G.; Li, S.; Beach, C.M. Proteomics of muscle-specific beef color stability. J. Agric. Food Chem. 2012, 60, 3196–3203. [Google Scholar] [CrossRef]
- Picard, B.; Gagaoua, M. Chapter 11—proteomic investigations of beef tenderness. Proteom. Food Sci. 2017, 177–197. [Google Scholar] [CrossRef]
- Huang, C.; Hou, C.; Ijaz, M.; Yan, T.; Li, X.; Li, Y.; Zhang, D. Proteomics discovery of protein biomarkers linked to meat quality traits in post-mortem muscles: Current trends and future prospects: A review. Trends Food Sci. Tech. 2020, 105, 416–432. [Google Scholar] [CrossRef]
- García-Torres, S.; de Vaca, M.C.; Tejerina, D.; Romero-Fernández, M.P.; Ortiz, A.; Díaz, F.; Sierra, V.; González, P.; Franco, D.; Zapata, C.; et al. ¿Es el pH un criterio suficiente para la clasificación de carne DFD en vacuno? In Proceedings of the I Congreso Iberoamericano de Marcas de Calidad de Carne y de Productos Cárnicos, Bragança, Portugal, 24–25 October 2019. [Google Scholar]
- Poleti, M.; Moncau, C.; Silva-Vignato, B.; Fernandes-Rosa, A.; Lobo, A.; Cataldi, T.; Negrão, J.; Silva, S.; Eler, J.; Balieiro, J. Label-free quantitative proteomic analysis reveals muscle contraction and metabolism proteins linked to ultimate pH in bovine skeletal muscle. Meat Sci. 2018, 145, 209–219. [Google Scholar] [CrossRef]
- Della Malva, A.; Albenzio, M.; Santillo, A.; Russo, D.; Figliola, L.; Caroprese, M. Methods for Extraction of Muscle Proteins from Meat and Fish Using Denaturing and Nondenaturing Solutions. J. Food Qual. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Saw, M.M.; Riederer, B.M. Sample preparation for two-dimensional gel electrophoresis. Proteomics 2003, 3, 1408–1417. [Google Scholar] [CrossRef]
- Rabilloud, T. Use of thiourea to increase the solubility of membrane proteins in two-dimensional electrophoresis. Electrophoresis 1998, 19, 758–760. [Google Scholar] [CrossRef]
- Malafaia, C.B.; Guerra, M.L.; Silva, T.D.; Paiva, P.M.; Souza, E.B.; Correia, M.T.; Silva, M.V. Selection of a protein solubilization method suitable for phytopathogenic bacteria: A proteomics approach. Proteome Sci. 2015, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.L.; Lo, S.C.; Hodgkiss, I.J. Proteomic study of a model causative agent of harmful red tide. Prorocentrum triestinum I: Optimization of sample preparation methodologies for analyzing with two-dimensional electrophoresis. Proteomics 2002, 2, 1168–1186. [Google Scholar] [CrossRef]
- Molloy, M.P.; Herbert, B.R.; Walsh, B.J.; Tyler, M.I.; Traini, M.; Sanchez, J.C.; Hochstrasser, D.F.; Williams, K.L.; Gooley, A.A. Extraction of membrane proteins by differential solubilization for separation using two-dimensional gel electrophoresis. Electrophoresis 1998, 19, 837–844. [Google Scholar] [CrossRef]
- Di Luca, A.; Mullen, A.M.; Elia, G.; Davey, G.; Hamill, R.M. Centrifugal drip is an accessible source for protein indicators of pork ageing and water-holding capacity. Meat Sci. 2011, 88, 261–270. [Google Scholar] [CrossRef]
- Yu, J.; Tang, S.; Bao, E.; Zhang, M.; Hao, Q.; Yue, Z. The effect of transportation on the expression of heat shock proteins and meat quality of M. longissimus dorsi in pigs. Meat Sci. 2009, 83, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.; Xu, X.L.; Zhou, G.H.; Wang, P.; Jiang, N.N. The effect of transportation of broilers during summer on the expression of heat shock protein 70, postmortem metabolism and meat quality. J. Anim. Sci. 2015, 93, 62–70. [Google Scholar] [CrossRef]
- Díaz-Luis, A.; Díaz, F.; Diñeiro, Y.; González-Blanco, L.; Arias, E.; Coto-Montes, A.; Oliván, M.; Sierra, V. Nuevos indicadores de carnes (DFD): Estrés oxidativo, autofagia y apoptosis. ITEA 2020, 117, 3–18. [Google Scholar] [CrossRef]
- Larkins, N.T.; Murphy, R.M.; Lamb, G.D. Influences of temperature, oxidative stress, and phosphorylation on binding of heat shock proteins in skeletal muscle fibers. Am. J. Physiol. Cell Physiol. 2012, 303, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, G.; Vissing, K.; Kalhovde, J.M.; Ugelstad, I.; Bayer, M.L.; Kadi, F.; Schjerling, P.; Hallén, J.; Raastad, T. Maximal eccentric exercise induces a rapid accumulation of small heat shock proteins on myofibrils and a delayed HSP70 response in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.; Wang, M.F.; Han, M.Y.; Zhu, X.S.; Xu, X.L.; Zhou, G.H. Expression of heat shock protein 70 in transport-stressed broiler pectoralis major muscle and its relationship with meat quality. Animal 2017, 11, 1599–1607. [Google Scholar] [CrossRef] [PubMed]




| Variable | Time post-mortem | Control (n = 7) | DFD (n = 7) | Sig. |
|---|---|---|---|---|
| pH Drip loss (%) L* | 24 h | 5.48 ± 0.05 | 6.49 ± 0.27 | *** |
| 48 h | 1.19 ± 0.64 | 1.06 ± 0.31 | NS | |
| 48 h | 34.35 ± 2.53 | 27.71 ± 2.34 | *** | |
| a* b* | 48 h | 9.84 ± 2.82 | 5.83 ± 0.97 | ** |
| 48 h | 11.87 ± 2.45 | 6.14 ± 2.38 | *** | |
| Meat toughness (WBSF, kg) | 3 days | 7.15 ± 1.74b | 6.63 ± 2.50 | NS |
| 7 days | 6.02 ± 1.31ab | 5.56 ± 1.85 | NS | |
| 14 days | 4.97 ± 1.01a | 5.33 ±1.35 | NS | |
| Mesophilic (log UFC/kg) | 3 days | 3.73 ± 0.37a | 3.72 ± 1.06a | NS |
| 7 days | 4.31 ± 0.84a | 4.42 ± 1.71a | NS | |
| 14 days | 6.05 ± 0.37b | 7.22 ± 0.64b | *** | |
| Enterobacteriaceae (log UFC/kg) | 3 days | 1.26 ± 1.23 | 1.53 ± 1.32a | NS |
| 7 days | 1.45 ± 1.43 | 2.13 ± 1.64a | NS | |
| 14 days | 3.08 ± 1.59 | 4.91 ± 0.83b | * |
| Sarcoplasmic Bands (MWe 1) | TES 1000 | TES 20,000 | Na 1000 | Na 20,000 | Na + T 1000 | Na + T 20,000 | K + T 1000 | K + T 20,000 | SEM | Sig. |
|---|---|---|---|---|---|---|---|---|---|---|
| S2 (137.9 kDa) | 0.188 | 0.211 | 0.246 | 0.262 | 0.431 | 0.446 | 0.42 | 0.316 | 0.061 | ** |
| S3 (115.8 kDa) | 0.305a | 0.249a | 0.406ab | 0.344a | 0.753bc | 0.911c | 0.602abc | 0.421ab | 0.084 | *** |
| S6 (81.31 kDa) | 0.55a | 0.572a | 1.556b | 1.256b | 1.442b | 1.559b | 1.705b | 1.482b | 0.146 | *** |
| S10 (53.60 kDa) | 0.895a | 0.992ab | 0.998ab | 1.031ab | 1.336b | 1.325b | 1.211ab | 1.197ab | 0.087 | ** |
| S11 (50.70 kDa) | 1.264abc | 1.107a | 1.244abc | 1.223ab | 1.438abcd | 1.68d | 1.603bcd | 1.611cd | 0.086 | *** |
| S12 (45.55 kDa) | 8.244ab | 8.814b | 8.101ab | 7.837ab | 7.536ab | 7.049a | 7.474ab | 6.874a | 0.351 | ** |
| S13 (40.72 kDa) | 10.805b | 10.448ab | 10.159ab | 10.154ab | 9.258a | 9.34ab | 8.513a | 8.98a | 0.427 | ** |
| S14 (37.6 kDa) | 8.775ab | 8.707ab | 9.287b | 9.16ab | 8.524ab | 8.413ab | 8.18a | 8.345ab | 0.242 | * |
| S15 (34.74 kDa) | 10.859bcd | 10.457abc | 11.59d | 11.376cd | 10.74abcd | 9.77a | 10.341abc | 9.857ab | 0.241 | *** |
| S16 (32.14 kDa) | 8.128b | 8.079b | 6.672a | 6.878a | 6.53a | 6.136a | 6.446a | 6.38a | 0.211 | *** |
| S17 (29.74 kDa) | 1.576a | 1.826a | 2.399c | 2.835cd | 2.649cd | 2.677cd | 3.28d | 3.179d | 0.109 | *** |
| S19 (26.68 kDa) | 2.889b | 2.89b | 2.258a | 2.546ab | 2.419ab | 2.585ab | 2.22a | 2.521ab | 0.128 | *** |
| S20 (25.76 kDa) | 4.162b | 4.103b | 3.635ab | 3.661ab | 3.425a | 3.329a | 3.482a | 3.483a | 0.133 | *** |
| Myofibrillar Bands(MWe 1) | Lysis | ND | SEM | Sig. |
|---|---|---|---|---|
| M2 (170.8 kDa) | 1.667 | 2.464 | 0.142 | ** |
| M3 (143.58 kDa) | 3.139 | 5.893 | 0.417 | *** |
| M6 (110.53 kDa) | 0.719 | 1.066 | 0.096 | *** |
| M11 (74.77 kDa) | 0.896 | 0.500 | 0.063 | ** |
| M18 (49.7 kDa) | 0.698 | 1.245 | 0.113 | *** |
| M19 (47.58 kDa) | 0.899 | 1.717 | 0.104 | ** |
| M20 (41.07 kDa) | 14.276 | 8.959 | 1.033 | ** |
| M23 (34.80 kDa) | 5.503 | 4.660 | 0.242 | * |
| M24 (32.76 kDa) | 4.874 | 7.404 | 0.303 | *** |
| M27 (26.31 kDa) | 1.466 | 2.128 | 0.085 | *** |
| M30 (19.46 kDa) | 3.033 | 2.388 | 0.128 | *** |
| M31 (18.40 kDa) | 0.693 | 0.406 | 0.051 | *** |
| M32 (17.09 kDa) | 2.254 | 3.100 | 0.117 | *** |
| M34 (14.94 kDa) | 0.817 | 2.314 | 0.123 | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Blanco, L.; Diñeiro, Y.; Díaz-Luis, A.; Coto-Montes, A.; Oliván, M.; Sierra, V. Impact of Extraction Method on the Detection of Quality Biomarkers in Normal vs. DFD Meat. Foods 2021, 10, 1097. https://doi.org/10.3390/foods10051097
González-Blanco L, Diñeiro Y, Díaz-Luis A, Coto-Montes A, Oliván M, Sierra V. Impact of Extraction Method on the Detection of Quality Biomarkers in Normal vs. DFD Meat. Foods. 2021; 10(5):1097. https://doi.org/10.3390/foods10051097
Chicago/Turabian StyleGonzález-Blanco, Laura, Yolanda Diñeiro, Andrea Díaz-Luis, Ana Coto-Montes, Mamen Oliván, and Verónica Sierra. 2021. "Impact of Extraction Method on the Detection of Quality Biomarkers in Normal vs. DFD Meat" Foods 10, no. 5: 1097. https://doi.org/10.3390/foods10051097
APA StyleGonzález-Blanco, L., Diñeiro, Y., Díaz-Luis, A., Coto-Montes, A., Oliván, M., & Sierra, V. (2021). Impact of Extraction Method on the Detection of Quality Biomarkers in Normal vs. DFD Meat. Foods, 10(5), 1097. https://doi.org/10.3390/foods10051097







