Validation of Antiobesity Effects of Black Soybean Seed Coat Powder Suitable as a Food Material: Comparisons with Conventional Yellow Soybean Seed Coat Powder
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
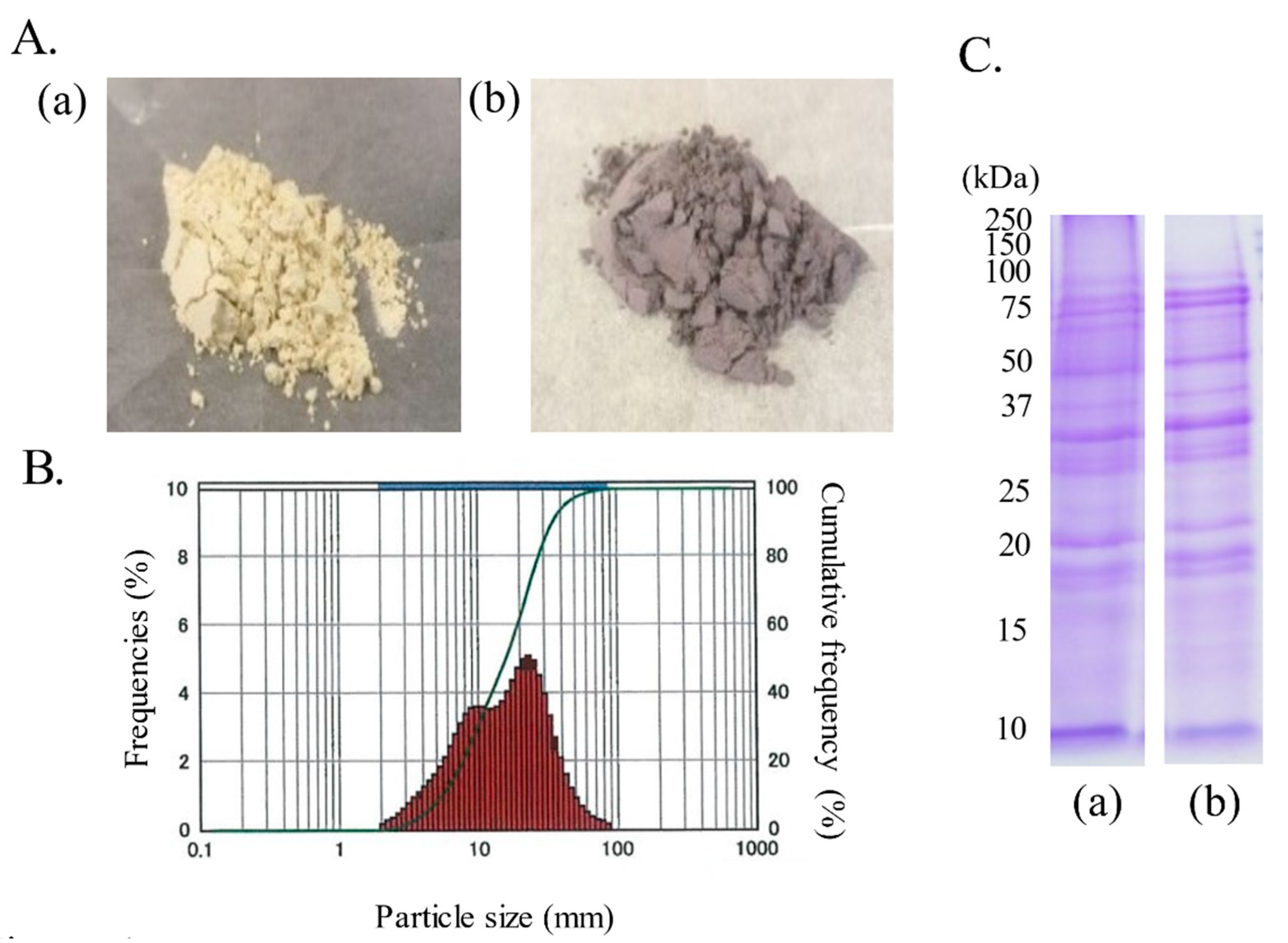
2.2. Preparation of Soybean Seed Coat Powder
2.3. Component Analysis of Fine Soybean Seed Coat Powder
2.4. Animal Experiments
2.5. Analysis of Serum Parameters
2.6. Analysis of Liver Lipids
2.7. SDS-PAGE and Western Blot Analysis
2.8. Statistical Analysis
3. Results
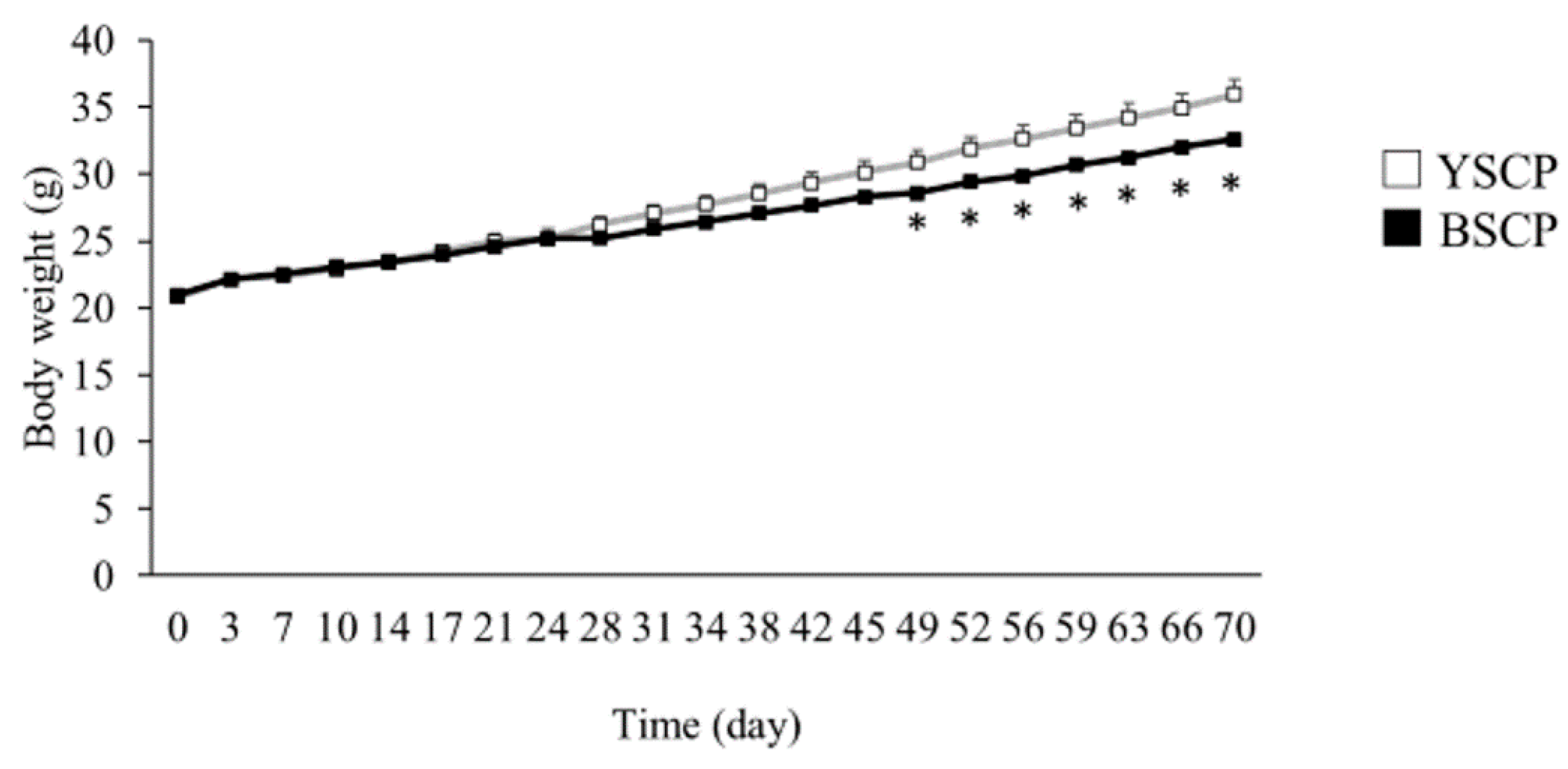
3.1. Average Body Weight Transition, Food Intake, Body Weight Gain, and Feed Efficiency
3.2. Various Fat Weights
3.3. Serum Analysis
3.4. Liver Weights and Lipid Parameters
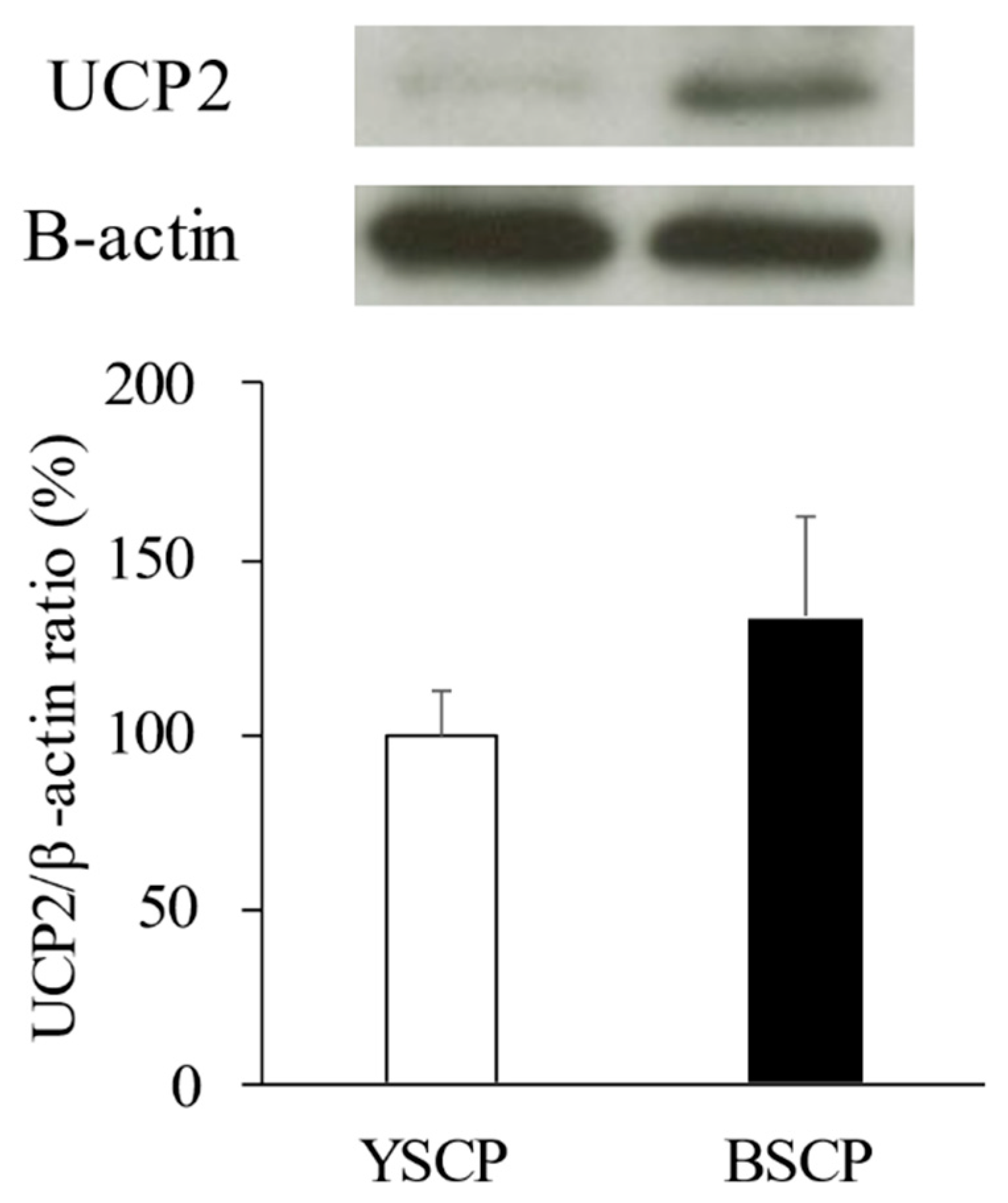
3.5. Western Blot Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwak, J.H.; Lee, J.H.; Ahn, C.W.; Park, S.H.; Shim, S.T.; Song, Y.D.; Han, E.N.; Lee, K.H.; Chae, J.S. Black soy peptide supplementation improves glucose control in subjects with prediabetes and newly diagnosed type 2 diabetes mellitus. J. Med. Food. 2010, 13, 1307–1312. [Google Scholar] [CrossRef]
- Messina, M. Modern applications for an ancient bean: Soybeans and the prevention and treatment of chronic disease. J. Nutr. 1995, 125, 567–569. [Google Scholar] [CrossRef]
- Sirtori, C.R.; Agradi, E.; Conti, F.; Mantero, O.; Gatti, E. Soybean-protein diet in the treatment of type-II hyperlipoproteinaemia. Lancet 1977, 1, 275–277. [Google Scholar] [CrossRef]
- Kito, M.; Moriyama, T.; Kimura, Y.; Kambara, H. Changes in plasma lipid levels in young healthy volunteers by adding an extruder-cooked soy protein to conventional meals. Biosci. Biotechnol. Biochem. 1993, 57, 354–355. [Google Scholar] [CrossRef]
- Moriyama, T.; Kishimoto, K.; Nagai, K.; Urade, R.; Ogawa, T.; Utsumi, S.; Maruyama, N.; Maebuchi, M. Soybean β-conglycinin diet suppresses serum triglyceride levels in normal and genetically obese mice by induction of β-oxidation, downregulation of fatty acid synthase, and inhibition of triglyceride absorption. Biosci. Biotechnol. Biochem. 2004, 68, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wen, X.Y.; Kan, J.; Jin, C.H. Structural characterization of two water-soluble polysaccharides from black soybean (Glycine max (L.) Merr.). J. Agric. Food Chem. 2015, 63, 225–234. [Google Scholar] [CrossRef]
- Takahashi, R.; Ohmori, R.; Kiyose, C.; Momiyama, Y.; Ohsuzu, F.; Kondo, K. Antioxidant activities of black and yellow soybeans against low density lipoprotein oxidation. J. Agric. Food Chem. 2005, 53, 4578–4582. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chang, S.K. Antioxidant capacity of seed coat, dehulled bean, and whole black soybeans in relation to their distributions of total phenolics, phenolic acids, anthocyanins, and isoflavones. J. Agric. Food Chem. 2008, 56, 8365–8373. [Google Scholar] [CrossRef]
- Todd, J.J.; Vodkin, L.O. Pigmented soybean (Glycine max) seed coats accumulate proanthocyanidins during development. Plant. Physiol. 1993, 102, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.G.; Beak, I.Y.; Kang, S.T.; Han, W.Y.; Shin, D.C.; Moon, H.P.; Kang, K.H. Isolation and determination of anthocyanins in seed coats of black soybean (Glycine max (L.) Merr.). J. Agric. Food Chem. 2001, 49, 5848–5851. [Google Scholar] [CrossRef]
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the pharmacological effects of Vitis vinifera (grape) and its bioactive compounds. Phytother Res. 2009, 23, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Syed, D.N.; Afaq, F.; Sarfaraz, S.; Khan, N.; Kedlaya, R.; Setaluri, V.; Mukhtar, H. Delphinidin inhibits cell proliferation and invasion via modulation of Met receptor phosphorylation. Toxicol. Appl. Pharmacol. 2008, 231, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Shiga, K.; Ohshima, K.; Kawakishi, S.; Osawa, T. Inhibition of lipid peroxidation and the active oxygen radical scavenging effect of anthocyanin pigments isolated from Phaseolus vulgaris L. Biochem. Pharmacol. 1996, 52, 1033–1039. [Google Scholar] [CrossRef]
- Lo, C.W.; Huang, H.P.; Lin, H.M.; Chien, C.T.; Wang, C.J. Effect of hibiscus anthocyanins-rich extract induces apoptosis of proliferating smooth muscle cell via activation of P38 MAPK and p53 pathway. Mol. Nutr. Food Res. 2007, 51, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Ito, C.; Oki, T.; Yoshida, T.; Nanba, F.; Yamada, K.; Toda, T. Characterisation of proanthocyanidins from black soybeans: Isolation and characterisation of proanthocyanidin oligomers from black soybean seed coats. Food Chem. 2013, 141, 2507–2512. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D.; Bagchi, M.; Stohs, S.J.; Das, D.K.; Ray, S.D.; Kuszynski, C.A.; Joshi, S.S.; Pruess, H.G. Free radicals and grape seed proanthocyanidin extract: Importance in human health and disease prevention. Toxicology 2000, 148, 187–197. [Google Scholar] [CrossRef]
- Fu, C.; Yang, D.; Peh, W.Y.E.; Lai, S.; Feng, X.; Yang, H. Structure and antioxidant activities of proanthocyanidins from elephant apple (Dillenia indica Linn.). J. Food Sci. 2015, 80, C2191–C2199. [Google Scholar] [CrossRef]
- Bordiga, M.; Travaglia, F.; Giuffrida, D.; Mangraviti, D.; Rigano, F.; Mondello, L.; Arlorio, M.; Coisson, J.D. Characterization of peel and pulp proanthocyanidins and carotenoids during ripening in persimmon “Kaki Tipo” cv, cultivated in Italy. Food Res. Int. 2019, 120, 800–809. [Google Scholar] [CrossRef]
- Ou, K.; Gu, L. Absorption and metabolism of proanthocyanidins. J. Funct. Foods. 2014, 7, 43–53. [Google Scholar] [CrossRef]
- de Vries, J.H.; Hollman, P.C.; Meyboom, S.; Buysman, M.N.; Zock, P.L.; van Staveren, W.A.; Katan, M.B. Plasma concentrations and urinary excretion of the antioxidant flavonols quercetin and kaempferol as biomarkers for dietary intake. Am. J. Clin. Nutr. 1998, 68, 60–65. [Google Scholar] [CrossRef]
- Hollman, P.C.; Katan, M.B. Bioavailability and health effects of dietary flavonols in man. Arch. Toxicol. Suppl. 1998, 20, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Takahata, Y.; Ohnishi-Kameyama, M.; Furuta, S.; Takahashi, M.; Suda, I. Highly polymerized procyanidins in brown soybean seed coat with a high radical-scavenging activity. J. Agric. Food Chem. 2001, 49, 5843–5847. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Hayashi, H. Drying technologies of foods—Their history and future. Dry. Technol. 1989, 7, 315–369. [Google Scholar] [CrossRef]
- Kanamoto, Y.; Yamashita, Y.; Nanba, F.; Yoshida, T.; Tsuda, T.; Fukuda, I.; Nakamura-Tsuruta, S.; Ashida, H. A black soybean seed coat extract prevents obesity and glucose intolerance by up-regulating uncoupling proteins and down-regulating inflammatory cytokines in high-fat diet-fed mice. J. Agric. Food Chem. 2011, 59, 8985–8993. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef]
- Mattu, H.S.; Randeva, H.S. Role of adipokines in cardiovascular disease. J. Endocrinol. 2013, 216, T17–T36. [Google Scholar] [CrossRef]
- Shklyaev, S.; Aslanidi, G.; Tennant, M.; Prima, V.; Kohlbrenner, E.; Kroutov, V.; Campbell-Thompson, M.; Crawford, J.; Shek, E.W.; Scarpace, P.J.; et al. Sustained peripheral expression of transgene adiponectin offsets the development of diet-induced obesity in rats. Proc. Natl. Acad. Sci. USA 2003, 100, 14217–14222. [Google Scholar] [CrossRef]
- Ahima, R.S. Metabolic actions of adipocyte hormones: Focus on adiponectin. Obesity 2006, 14, 95–155. [Google Scholar] [CrossRef]
- Steppan, C.M.; Bailey, S.T.; Bhat, S.; Brown, E.J.; Banerjee, R.R.; Wright, C.M.; Patel, H.R.; Ahima, R.S.; Lazar, M.A. The hormone resistin links obesity to diabetes. Nature 2001, 409, 307–312. [Google Scholar] [CrossRef]
- Lehrke, M.; Reilly, M.P.; Millington, S.C.; Iqbal, N.; Rader, D.J.; Lazar, M.A. An inflammatory cascade leading to hyperresistinemia in humans. PLoS Med. 2004, 1, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Bezzina, R.; Hinch, E.; Lewandowski, P.A.; Cameron-Smith, D.; Mathai, M.L.; Jois, M.; Sinclair, A.J.; Begg, D.P.; Wark, J.D.; et al. Green tea, black tea, and epigallocatechin modify body composition, improve glucose tolerance, and differentially alter metabolic gene expression in rats fed a high-fat diet. Ann. Nutr. Metab. 2009, 29, 784–793. [Google Scholar] [CrossRef]
- Surwit, R.S.; Wang, S.; Petro, A.E.; Sanchis, D.; Raimbault, S.; Ricquier, D.; Collins, S. Diet-induced changes in uncoupling proteins in obesity-prone and obesity-resistant strains of mice. Proc. Natl. Acad. Sci. USA 1998, 95, 4061–4065. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Okabe, M.; Natsume, M.; Ashida, H. Prevention mechanisms of glucose intolerance and obesity by cacao liquor procyanidin extract in high-fat diet-fed C57BL/6 mice. Arch. Biophys. 2012, 527, 95–104. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMPK: Positive and negative regulation, and its role in whole-body energy homeostasis. Curr. Opin. Cell Biol. 2015, 33, 1–7. [Google Scholar] [CrossRef]
- Hattori, Y.; Nakano, Y.; Hattori, S.; Tomizawa, A.; Inukai, K.; Kasai, K. High molecular weight adiponectin activates AMPK and suppresses cytokine-induced NF-κB activation in vascular endothelial cells. FEBS Lett. 2008, 582, 1719–1724. [Google Scholar] [CrossRef]
| YSCP | BSCP | |
|---|---|---|
| Energy (kcal/100 g) | 253 | 240 |
| Fat (g/100 g) | 3.9 | 2.0 |
| Protein (g/100 g) | 13.5 | 9.7 |
| Carbohydrate (g/100 g) | 74.1 | 78.2 |
| Sugar (g/100 g) | 7.9 | 13.2 |
| Dietary fiber (g/100 g) | 66.2 | 65.0 |
| Ash (g/100 g) | 4.5 | 4.8 |
| Moisture (g/100 g) | 4.0 | 5.3 |
| YSCP | BSCP | |||
|---|---|---|---|---|
| Content (g) | Energy (kcal) | Content (g) | Energy (kcal) | |
| Cornstarch | 18.1 | 72.2 | 17.8 | 71.4 |
| Casein | 10.5 | 37.8 | 10.7 | 38.4 |
| Sucrose | 5.0 | 20.0 | 5.0 | 20.0 |
| Cellulose | 0.3 | 0.7 | 0.4 | 0.8 |
| AIN-93M mineral mix | 1.6 | 0.0 | 1.6 | 0.0 |
| AIN-93M vitamin mix | 0.5 | 0.0 | 0.5 | 0.0 |
| Methionine | 0.1 | 0.0 | 0.1 | 0.0 |
| Choline chloride | 0.1 | 0.0 | 0.1 | 0.0 |
| Soybean powder | 4.0 | 10.1 | 4.0 | 9.6 |
| Lard | 9.8 | 88.4 | 9.9 | 89.1 |
| Water | 50.0 | 0.0 | 49.9 | 0.0 |
| Total | 100.0 | 229.2 | 100.0 | 229.2 |
| YSCP | BSCP | ||
|---|---|---|---|
| Total food intake (g) | 473 ± 6.5 | 543 ± 19.7 | |
| Weight gain (g) | 15.1 ± 0.81 | 11.6 ± 0.56 * | |
| Feed efficiency (%) | 3.20 ± 0.15 | 2.14 ± 0.06 * | |
| Body fat weight (g) | 3.07 ± 0.10 | 2.21 ± 0.09 * | |
| Peritesticular fat | 1.85 ± 0.05 | 1.26 ± 0.03 * | |
| Perirenal fat | 0.66 ± 0.25 | 0.49 ± 0.13 * | |
| Mesenteric fat | 0.57 ± 0.28 | 0.46 ± 0.22 | |
| Body fat percentage (%) | 8.44 ± 0.07 | 6.76 ± 0.07 * | |
| Peritesticular fat | 5.06 ± 0.12 | 3.87 ± 0.07 * | |
| Perirenal fat | 1.80 ± 0.43 | 1.50 ± 0.39 | |
| Mesenteric fat | 1.57 ± 0.03 | 1.39 ± 0.03 * | |
| YSCP | BSCP | |
|---|---|---|
| Glucose (mg/dL) | 308 ± 21 | 318 ± 24 |
| Triglyceride (mg/dL) | 71 ± 6 | 64 ± 11 |
| Total cholesterol (mg/dL) | 135 ± 6 | 132 ± 10 |
| HDL-cholesterol (mg/dL) | 103 ± 3 | 96 ± 5 |
| Phospholipid (mg/dL) | 169 ± 5 | 164 ± 3 |
| NEFA (mEq/L) | 1.15 ± 0.08 | 1.17 ± 0.10 |
| Adiponectin (µg/mL) | 7.71 ± 0.26 | 8.54 ± 0.26 * |
| Resistin (ng/mL) | 105 ± 19.3 | 62.6 ± 14.5 |
| Leptin (ng/mL) | 12.8 ± 1.08 | 6.67 ± 0.67 * |
| YSCP | BSCP | |
|---|---|---|
| Liver weight (g/100 g bw) | 3.87 ± 0.26 | 3.43 ± 0.05 |
| Liver triglyceride (mg/liver g) | 19.9 ± 5.9 | 17.7 ± 2.2 |
| Liver total cholesterol (mg/liver g) | 0.56 ± 0.15 | 0.56 ± 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moriyasu, Y.; Fukumoto, C.; Wada, M.; Yano, E.; Murase, H.; Mizuno, M.; Zaima, N.; Moriyama, T. Validation of Antiobesity Effects of Black Soybean Seed Coat Powder Suitable as a Food Material: Comparisons with Conventional Yellow Soybean Seed Coat Powder. Foods 2021, 10, 841. https://doi.org/10.3390/foods10040841
Moriyasu Y, Fukumoto C, Wada M, Yano E, Murase H, Mizuno M, Zaima N, Moriyama T. Validation of Antiobesity Effects of Black Soybean Seed Coat Powder Suitable as a Food Material: Comparisons with Conventional Yellow Soybean Seed Coat Powder. Foods. 2021; 10(4):841. https://doi.org/10.3390/foods10040841
Chicago/Turabian StyleMoriyasu, Yuuki, Chiho Fukumoto, Maki Wada, Erika Yano, Hiroshi Murase, Masatoshi Mizuno, Nobuhiro Zaima, and Tatsuya Moriyama. 2021. "Validation of Antiobesity Effects of Black Soybean Seed Coat Powder Suitable as a Food Material: Comparisons with Conventional Yellow Soybean Seed Coat Powder" Foods 10, no. 4: 841. https://doi.org/10.3390/foods10040841
APA StyleMoriyasu, Y., Fukumoto, C., Wada, M., Yano, E., Murase, H., Mizuno, M., Zaima, N., & Moriyama, T. (2021). Validation of Antiobesity Effects of Black Soybean Seed Coat Powder Suitable as a Food Material: Comparisons with Conventional Yellow Soybean Seed Coat Powder. Foods, 10(4), 841. https://doi.org/10.3390/foods10040841







