Abstract
This work aims to analyze the chemical and biological evaluation of two extracts obtained by olive mill wastewater (OMW), an olive oil processing byproduct. The exploitation of OMW is becoming an important aspect of development of the sustainable olive oil industry. Here we chemically and biologically evaluated one liquid (L) and one solid (S) extract obtained by liquid–liquid extraction followed by acidic hydrolysis (LLAC). Chemical characterization of the two extracts indicated that S has higher phenol content than L. Hydroxytyrosol and tyrosol were the more abundant phenols in both OMW extracts, with hydroxytyrosol significantly higher in S as compared to L. Both extracts failed to induce cell death when challenged with endothelial cells and vascular smooth muscle cells in cell viability experiments. On the contrary, the higher extract dosages employed significantly affected cell metabolic activity, as indicated by the MTT tests. Their ability to counteract H2O2-induced oxidative stress and cell death was assessed to investigate potential antioxidant activities of the extracts. Fluorescence measurements obtained with the reactive oxygen species (ROS) probe H2DCF-DA indicated strong antioxidant activity of the two OMW extracts in both cell models, as indicated by the inhibition of H2O2-induced ROS generation and the counteraction of the oxidative-induced cell death. Our results indicate LLAC-obtained OMW extracts as a safe and useful source of valuable compounds harboring antioxidant activity.
1. Introduction
The olive oil industry is a large productive sector globally, and three-quarters of world production takes place in Europe. Among different commercial categories, extra virgin olive oil (EVOO) is produced by the sole employment of mechanical processes, and its quality is tightly related to different parameters, such as agronomic practices, the olive cultivar, and the olive oil extraction technology used [1]. The final chemical composition of the EVOO is influenced by different factors, among which the olive variety is the first one to play a primary role across the supply chain. Indeed, the olive cultivars display a large range of genetic variability for several agronomic traits such as the fruit size, oil content, and the degree of adaptation to severe environmental stress [2,3]. Olive drupes possess various health-promoting bioactive substances primarily represented by biophenol secoiridoids (oleuropein, ligstroside) and their hydrolytic derivatives [4]. Although olive fruit is rich in phenolic compounds, only 2% of the entire phenolic content transfers into the oil phase, while the rest goes into the olive mill wastewater (OMW) (approximately 53%), the pomace, the olive oil filtration residue, and olive leaves (approximately 45%) [5]. As a result, the high percentage of phenolic compounds in the olive industry’s byproducts is attracting great interest as a potential source of such phenolic compounds, with special attention oriented to the olive mill, which is the primary potential source of such molecules. The major components in OMW include hydroxytyrosol, tyrosol, oleuropein, ligstroside and their secoiridoids derivatives, and a variety of hydroxycinnamic and hydroxybenzoic acids [6]. Given the potential environmental impact, active molecule extraction from olive oil byproducts should embrace methodologies that employ green technologies, considering their possible exploitation as food antioxidants or nutraceuticals [7]. In fact, phenol recovery from these byproducts should be organized in order to promote their reintroduction into the food chain and coincide with greater valorization and improved olive oil industry waste management. In addition to its main implications for the local and international economy, this could reduce the environmental impact of olive oil manufacturing and contribute to this valuable production chain’s sustainability. However, the quality of OMW phenolic compounds differs according to several factors, including the olive oil production technological process employed, and for this reason, it is essential to evaluate different OMW technological processes to provide promising bioactive compounds. In this regard, OMW-derived products have been tested for certain biological effects and have showed an interesting bioactivity spectrum [8]. For instance, EVOO-containing phenolic compounds have shown In Vivo and In Vitro antioxidant activity, likely due to molecules such as hydroxytyrosol, tyrosol, and secoiridoid derivatives [9,10].
Reactive oxygen species (ROS) are aerobic metabolism products and exert a pivotal role in regulating cellular functions such as proliferation, differentiation, and migration [11]. Under physiological conditions, a series of cellular antioxidant mechanisms maintain vascular ROS levels in homeostatic conditions [12]. However, an aberrant modulation of the above-mentioned mechanisms leads to critical increases in ROS levels, thus promoting different vascular-associated pathological conditions, including cancer and cardiovascular diseases [13]. In this context, endogenous ROS, released by endothelial and vascular smooth muscle cells as a result of pro-inflammatory and pro-atherosclerotic stimuli, can trigger vascular injury and blood vessel restructuring by affecting diverse intracellular signaling pathways [14]. Indeed, ROS-activated molecular machinery can modulate both endothelial cells and vascular smooth muscle cells functions including proliferation, migration, and invasion, leading to vascular pathologies such as hypertension, atherosclerosis, and cancer [15], which may be counteracted by OMW-contained compounds [16].
In this light, the present work aims to investigate whether (i) the considered OMW samples can be a source of valuable antioxidants, and (ii) whether the obtained extracts can protect human vascular cells against oxidative cell death.
2. Materials and Methods
2.1. Chemicals
Unless stated in the text, all the reagents used were from Sigma (Sigma, St. Louis, MO, USA).
2.2. Sample Collection
Ottobratica olives were sampled in November 2019 at a ripening index of 4 according to Guzmán et al. [17]. Oil extraction was performed by means of a three-phase decanter system (Alfa Laval, Monza, Italy) at 25–26 °C and 20 min of malaxation parameters in an olive oil mill located in the Calabria region (Italy). The obtained OMWs were transferred to the Food Technologies laboratory of the Mediterranea University of Reggio Calabria for the experimental project.
2.3. OMW Acquisition and Preparation
The extract was obtained following the method reported by De Marco et al., with some modifications [18]. Two liters of olive oil mill wastewater (OMW) were acidified to pH 2 with HCl and washed three times with hexane (1:1, v:v) in order to remove the lipid fraction. The mixture was vigorously shaken and centrifuged under 3000 rpm for 3 min at 10 °C. The phenolic compounds were extracted by mean of ethyl acetate three times in a separating funnel (1:4 v:v), and then the combined extracts were centrifuged for 5 min at 3000 rpm at 10 °C. The organic phase was separated and filtered through a sintered glass Buchner apparatus. Then the ethyl acetate was evaporated under vacuum using a rotary vacuum evaporator at 25 °C (headspace analysis was performed). Finally, the dry residue was again dissolved in 100 mL of water, filtered using PTFE 0.45 μm (diameter 15 mm) syringe filter, and stored at 4 °C until subsequent analyses. An aliquot of the obtained extract (called L) was freeze-dried in a VirTis lyophilizer (Gardiner, NY, USA) and the obtained sample was called S. For all the experiments, we used both types of phenolic extracts, the freeze-dried (S) and the wet (L) type.
2.4. Determination of Total and Individual Phenolic Content of OMW Polyphenolic Extracts
The total phenol content was determined spectrophotometrically as previously described by Bruno et al. with some modifications [19]. An aliquot portion (0.1 mL) of phenolic extract was placed in a 25 mL volumetric flask and mixed with 20 mL of deionized water and 0.625 mL of the Folin–Ciocalteau reagent. After 3 min, 2.5 mL of saturated solution of Na2CO3 (20%) was added. After that, the mixture was incubated for 12 h at room temperature and in the dark. The sample’s absorbance was measured at 725 nm against a blank and compared with a gallic acid (GA) calibration curve (concentration between 1 and 10 mg L−1). The results were expressed as mg of GA g−1 of phenolic extract.
Identification and quantification of S and L extracts’ main phenolic compounds were performed by HPLC-DAD (Dionex Ultimate 3000 RSLC, Waltham, MA, USA), as previously described [20]. The phenolic determination was conducted using the Dionex Acclaim 120 C18 analytical column (3 µm, 150 × 3 mm) (Thermo Scientific, Waltham, MA, USA) set at 35 °C, a flow rate of 1 mL min−1, and an injection volume of 5 µL. Water/acetic acid (98:2, v/v) (A) and acetonitrile (B) were used as mobile phases, and the applied gradient was the following: 95% A and 5% B (5 min), 80% A and 20% B (10 min), 75% A and 25% B (15 min), 65% A and 35% B (20 min), 0% A and 100% B (25 min), and 95% A and 5% B (35 min). Quantification was performed by pure standard (Sigma-Aldrich Co. LLC, St. Louis, MO, USA) and data were expressed as mg g−1 of phenolic extract.
2.5. Cell Culture
Human umbilical vein endothelial cells (HUVECs) were acquired from Cell Application (San Diego, CA, USA) and grown as previously described [21]. Briefly, cells were grown in endothelial cell basal medium supplemented with cell growth supplement (EGM-V2 # 213K-500) as per company instructions. When confluent, cells were sub-culture at a split ratio of 1:2 and used within three passages. Unless specified in the text, cells were plated in 96-well plates (Corning, Lowell, MA, USA) at a concentration of 105 cells/mL and processed for experiments in a complete medium containing the different concentrations of the extracts.
Human pulmonary artery smooth muscle cells (HPASMCs) were acquired from Cell Application (San Diego, CA, USA) and grown as previously described [22]. Briefly, cells were grown in smooth muscle cells basal medium supplemented with cell growth supplement (HSMCs-kit #311K-500) as per company instructions. When confluent, HSMCs were sub-culture at a split ratio of 1:2 and used within three passages. Unless specified in the text, cells were plated in 96-well plates (Corning, Lowell, MA, USA) at a concentration of 105 cells/mL and processed for experiments in a complete medium containing the different concentrations of the extracts.
According to data obtained in our previous studies [23,24,25,26], we decided to test the extracts at the doses of 10, 25, 50, and 100 µg/mL.
2.6. Cell Metabolic Assay
Cell metabolic activity was evaluated using the oxidizable and reducible colorimetric probes 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) [23]. Cells were treated as indicated in figure legends and then processed for the MTT assay. After treatments, cells were added with 20 µL MTT solution (5 mg/mL) in medium M199 and placed at 37 °C in a cell incubator for 4 h. After that, the medium was discarded, and the converted dye was solubilized with acidic isopropanol (0.04 N HCl in absolute isopropanol), and the multi wells were read at 570 nm using a GENios plus microplate reader (Tecan) with background subtraction at 650 nm. Results were expressed as a percent of untreated control cells. Cell viability was calculated by the following equation: Cell viability (%) = (Abs of sample/Abs of control) × 100, where Abs of sample is the absorbance of the cells incubated with the different concentrations of the two extracts, and Abs of control is the absorbance of the cells incubated with the culture medium only (positive control).
2.7. Measurement of Intracellular ROS
Intracellular ROS levels were determined by using the ROS molecular probe 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA) (Molecular Probe, Eugene, OR, USA) as previously described with minor modification [24,25]. In this assay, ROS oxidize H2DCF, producing the fluorescent compound DCF, the fluorescence levels of which are proportional to the amount of intracellular ROS. Cells were treated as indicated in figure legends and then processed for the intracellular ROS assessment. For the ROS assay, cells were incubated for 30 min with Hank’s Balanced Salt Solution (HBSS) containing 5 µM H2DCF-DA, then washed twice with HBSS, and then fluorescence was measured by using a GENios plus microplate reader (Tecan, Mannedorf, Switzerland. Excitation and emission wavelengths used for fluorescence quantification were 485 and 535 nm, respectively. All fluorescence measurements were corrected for background fluorescence and protein concentration. Using untreated cells as a reference, the antioxidant and prooxidant outcomes were evaluated by comparing five measurements and expressed as a percentage of untreated control cells.
2.8. Cell Viability Assay
Cell viability was assessed as previously described [26] by using the CytoTox-ONE™ (Promega, Madison, WI, USA) kit. The CytoTox-ONE™ homogeneous membrane integrity assay is a fluorometric method for estimating the number of nonviable cells present in multi-well plates. The assay measures the release of lactate dehydrogenase (LDH) from cells with a damaged membrane. Then the LDH released into the culture medium is measured with a 10-min coupled enzymatic assay that results in the conversion of resazurin into a fluorescent resorufin product. The amount of fluorescence produced is proportional to the number of dead cells with the lysed membrane.
Cells were treated as indicated in figure legends and then processed as per company instructions at each experimental point’s end. Fluorescence was measured by using a GENios plus microplate reader (Tecan, Mannedorf, Switzerland). Excitation and emission wavelengths used for fluorescence quantification were 535 and 620 nm, respectively. All fluorescence measurements were corrected for background fluorescence and protein concentration, and results were expressed as a percentage of untreated control cells.
2.9. Statistical Analysis
Data were expressed as means ± S.D. of the indicated number of experiments. One-way analysis of variance (ANOVA) followed by a post-hoc Newman–Keuls multiple comparison test were used to detect differences of means among treatments with significance defined as p < 0.05. Statistical analysis was performed using GraphPad Prism version 8.00 for Windows, GraphPad Software, San Diego, CA, USA.
3. Results
In this work, two extracts derived from OMW were assessed for their phenol contents and their potential biological activity on two human vascular cells. The two extracts were obtained employing a desolvented ethyl acetate extraction followed by water recovery.
Hydroxytyrosol and tyrosol were the principal quantified compounds in both OMW extracts, with significantly greater abundance in the S extract, mainly related to the first phenolic alcohol (4.55 mg g−1). The tyrosol concentration varied between 0.54 mg g−1 in L extract and 0.58 mg g−1 in L extract. The S extract also showed higher amounts of caffeic acid and oleuropein, respectively, of 0.36 and 0.15 mg g−1. Finally, among the identified flavonoids, apigenin-7-O-glucoside was more abundant in L extract with a concentration of 0.15 mg g−1 (Figure 1).

Figure 1.
Chromatogram of phenolic compounds in S olive mill wastewater (OMW) extract. (1) Hydroxytyrosol; (2) tyrosol; (3) vanillic acid; (4) caffeic acid; (5) ferulic acid; (7) apigenin-7-O-glucoside; (8) oleuropein; (9) luteolin-7-O-glucoside.
Next, we proceeded with the extracts study by assessing their potential biological activities on two human vascular cells, HUVECs and HSMC, respectively, endothelial and vascular smooth muscle cells. Based on data from our previous studies [27,28,29,30], cell treatments were performed using the following extracts’ concentrations: 10, 25, 50, and 100 µg/mL.
Although widely accepted as a provider of health benefits, natural antioxidants, including phenolic compounds, based on both dosages and redox environment status, have been reported to act as prooxidants, thus increasing intracellular ROS levels and inducing cell death [23,25,26,31,32,33,34]. Moreover, extracts deriving from food processing waste have been reported to be unsafe for the environment [7]. For this reason, we first investigated the potential harmful effects of OMW extracts by assessing their ability to affect cell viability, metabolic activity, and intracellular ROS production, three aspects tightly interconnected in maintaining the physiological functions of vascular cells [35,36]. As reported in Figure 2, 24-h exposition of HUVECs (Figure 2A) and HSMCs (Figure 2B) to the indicated concentrations of OMW failed to induce cell death in either cellular model. To further investigate potential harmful effects and corroborate the absence of cell toxicity, we assessed the extract’s effect on cellular metabolic activity. Figure 2C,D indicate that only the highest dose tested (100 µg/mL) was able to significantly affect the cell metabolic activity, while no effects were observed at the other tested concentrations.
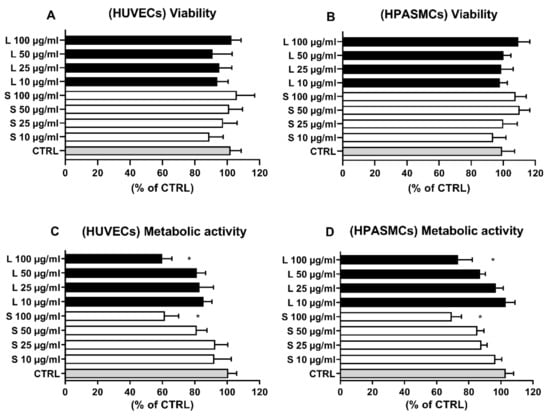
Figure 2.
Effect of OMW on HUVECs and HPASMCs viability and metabolic activity. Cells were exposed for 24 h in the absence (CTRL) or presence to the indicated concentrations of OMW extracts. Cell viability (A,B) and metabolic activity (C,D) were assessed as reported in the materials and methods. HUVECs, human umbilical vein endothelial cell; HPASMCs, human pulmonary artery smooth muscle cells; L, liquid extract; S, solid extract; CTRL, untreated cells; * significantly different from CTRL; Values are shown as mean ± SD and expressed as a percentage of the CTRL. (n = 4).
Since the two OMW extracts showed a remarkable quantity of antioxidant phenolic compounds, we sought to investigate their potential antioxidant activity by assessing the ability to counteract H2O2-elicited oxidative changes. First, we evaluated the ability of our ROS assay to reveal intracellular ROS level variations within a range of selected H2O2 concentrations. As reported in Figure 3A,B, the probe displayed both a significant dynamic range and linear response when challenged with increasing concentrations of H2O2, a well-known cellular prooxidant in these vascular cells. [37,38]. Therefore, based on these results, 75 µM was the dosage of H2O2 employed for the experiments concerning potential extract antioxidant activity. In this experiment, cells were treated for 4 h with the four extract concentrations followed by a 2 h-treatment with 75 μM H2O2. Then, the levels of intracellular ROS were measured as indicated in the Section 2.
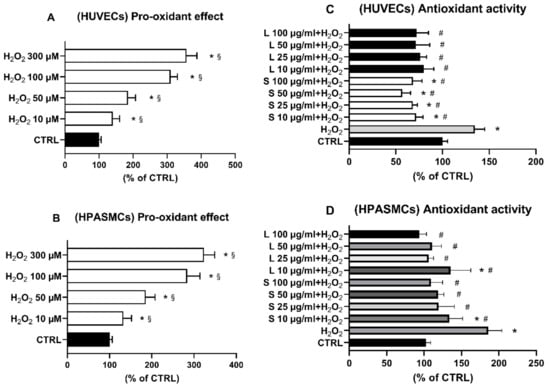
Figure 3.
(A,B) Dose–response effect of H2O2 on intracellular ROS levels of HUVECs (A) and HPASMCs (B). Cells were exposed for 6 h to the indicated concentrations of H2O2. Intracellular ROS levels were assessed as reported in Materials and Methods. (C,D) OMW extracts counteract H2O2-induced ROS increase in both HUVECs (C) and HPASMCs (D). Cells were exposed for 3 h to the indicated concentrations of OMW extracts and then incubated for 6 h in the presence of 75 µM H2O2. Intracellular ROS levels were assessed, as reported in Materials and Methods. HUVECs, human umbilical vein endothelial cell; HPASMCs, human pulmonary artery smooth muscle cells; L, liquid extract; S, solid extract; CTRL, untreated cells; H2O2, hydrogen peroxide; * significantly different from CTRL; § significantly different from each other; # significantly different from H2O2. Values are shown as mean ± SD and expressed as a percentage of the CTRL. (n = 5).
Exposure of the HUVECs to increasing concentrations of the two extracts significantly counteracted the H2O2-elicited increase of ROS in all the tested concentrations (Figure 3C). Similarly, compared to H2O2-treated cells, exposure of HSMCs to the two extracts induced a significant antioxidant effect in the whole concentration range (Figure 3D). Since oxidative stress is recognized to cause cell injury and even death, we next hypothesized that the observed extracts’ antioxidant effects could provide cellular protection against cells damaged elicited by H2O. To this end, cells were pre-treated for 3 h with the indicated extract concentrations, and then 75 µM H2O2 was added for 24 h before cell viability determination. In agreement with the displayed antioxidant effect on H2O2-increased ROS production (Figure 3C,D), cell pre-treatments with increasing concentrations of the two OMW extracts significantly protected both cell lines from H2O2-elicited oxidative cell death.
4. Discussion
World globalization requires sustainable growth. In this context, the processing of different types of waste, derived from food treatment or processing, to produce value-added products is becoming a challenging reality that we must pursue. Waste processing or transformation may involve using different technologies, and the obtained products may or may not be safe for the environment and human health. However, since transformation may give rise to new, unknown, or unwanted compounds, it is useful to assess their safety, especially in terms of their impact on human health.
The olive (Olea europaea L.) is native to the Mediterranean area where it can be found in the wild form in the Middle East. Olea europaea L. is widely spread throughout the world, especially in the Mediterranean regions, where it accounts for nearly 96 percent of global olive production (FAOSTAT Food and Agriculture Data [20]). The production of olive oil forms a significant quantity of residue, such as aqueous waste called olive mill wastewater (OMW), and olive leaves. Other wastes are also produced during the filtration processes and during the storage time when the solid components migrate to the tank bottoms, generating sediments. These waste products are rich in bioactive compounds and can be therefore considered valuable byproducts to be exploited for further uses [39,40]. Although OMWs are effluents capable of degrading soil and water quality, with serious negative effects on the environment [7], studies have revealed that they contain high concentrations of antioxidants, such as phenolic molecules [41]. However, since the quality of OMW compounds differs according to the olive variety, the technological process of olive oil production, storage time, and climatic conditions [40], it is imperative to evaluate the technological processes employed in OMW processing in order to obtain valuable bioactive compounds. In this regard, phenolic compounds derived from OMW have been reported to show interesting biological properties [10,39,40]. Nonetheless, yet a large amount of crusher residues remain without actual application, since only small quantities are used as natural fertilizers, biomass fuel, additives in animal feed, and activated carbon. However, these residues are a precious starting material to produce extracts of phenolic molecules that could be used in various industrial fields [7]. Different technologies are proposed rather than classical solid–liquid extraction to enhance phenolic compounds’ extraction from different olive oil byproducts.
We used a liquid–liquid extraction procedure followed by acidic hydrolysis [18], which provided two extracts, L and S, harboring a remarkable amount of total phenol content. The quantitative analysis indicated the S extract had a higher phenol content compared to the L extract. Moreover, hydroxytyrosol and tyrosol had the primary phenols in both OMW extracts, with hydroxytyrosol significantly more abundant in the S extract than the L extract (Table 1).

Table 1.
Main phenolic compounds in liquid (L) and freeze dried (S) OMW extracts (mg g−1).
In the cellular experiments, we first evaluated potential extracts’ harmful effects by treating endothelial and smooth muscle cells with different OMW dosages for 24 h. As reported in Figure 2A,B, the extracts were unable to induce cell death at all the concentrations tested. However, sometimes compound effects may affect cell metabolism without resulting in cell death. For this reason, we also assessed the extract effect on the two cells’ metabolic activities. In this analysis, the extract showed no effect, except for the highest concentration, 100 µg/mL, where we found a slight but significant decrease in cell metabolic activity, an aspect that requires further investigations as it has been reported for other phenol compounds [23,25,26,31,32,33,34]. However, the finding that the concentration of 100 µg/mL affects only the cell metabolism without inducing cell death indicated that the extracts, even at high concentrations, appeared not to be toxic, being unable to induce cell death. Considering hydroxytyroso as the main compound present in the OMW, our findings align with In Vivo experiments reporting no toxic effects in rodents fed with HT concentrations as high as 2 g/kg b.wt. [42,43]. Similarly, no adverse clinical, biochemical, or gross necropsy effects were also reported in rats orally administered with HIDROXTM, a hydrolyzed aqueous olive pulp extract with a phenol composition closer to our OMW [44]. Moreover, In Vitro experiments, performed with different cell lines, also confirmed our findings reporting lack of cytotoxicity with HT concentrations as high as 500 µM [45,46,47].
Although chemical tests such as TBARS, ABTS, and DPPH are often used to determine compounds’ antioxidant properties, a likely better method to investigate the antioxidant activity/effect of a molecule or extract would be In Vivo or in a cellular model. Indeed, a cellular model would provide the proper environment for studying potential interactions (e.g., compound(s)–cellular signal transduction pathway(s), compound(s)–cellular receptor(s)) that would be otherwise missed in a solely chemical setting. In this regard, the intracellular oxidation of H2DCF-DA is mainly caused by H2O2, an oxidizing molecule physiologically present in the cell and therefore functional to induce oxidative insults and study their effects on cell functions [48,49]. Thus we created H2O2-induced oxidative stress and investigated whether the extracts could counteract it by following the variation of intracellular levels of DFC, the oxidized form of H2DCF-DA, and assessing cell viability (Figure 3 and Figure 4). In our experimental conditions, the different extracts’ concentrations significantly inhibited the H2O2-induced increase of ROS in both HUVECs (Figure 3C) and in HAPVSCs (Figure 3D), bringing the oxidative stress values back to those of the controls. Consonant with observed extracts’ antioxidant activity are the findings reported in Figure 4. The extracts were indeed capable of dose-dependently counteracting H2O2-induced cell death and restoring cell viability completely at the dosages of 50 and 100 µg/mL. Our current findings support and confirm previously performed experiments; indeed, similarly to EVOO-contained phenolic compounds [9,10,45,46,50], olive oil processing byproducts such as OMW are a source of valuable compounds harboring health benefit properties.
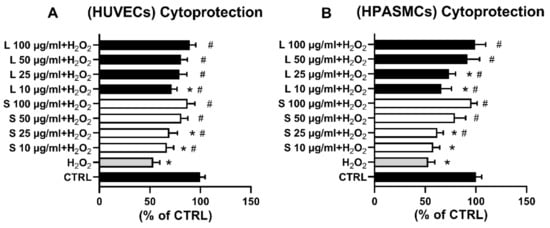
Figure 4.
OMW extracts protect HUVECs (A) and HPASMCs (B) from H2O2-induced cell death. Cells were exposed for 3 h to the indicated concentrations of OMW extracts and then incubated in the absence (CTRL) or presence of 75 µM H2O2. Cell viability was assessed as reported in Materials and Methods. HUVECs, human umbilical vein endothelial cell; HPASMCs, human pulmonary artery smooth muscle cells; L, liquid extract; S, solid extract; CTRL, untreated cells; H2O2, hydrogen peroxide; * significantly different from CTRL; # significantly different from H2O2. Values are shown as mean ± SD and expressed as a percentage of the CTRL. (n = 4).
5. Conclusions
The OMW extracts obtained by the production of olive oil showed interesting antioxidant activity on both the employed cell models. The tested extracts were capable of effectively protecting cells from oxidative stress-indued cell death, failing indeed to interfere with cell viability and even with the metabolism, except for the highest tested concentration. Our results indicates a different point of view concerning the food processing residues, which should not be considered waste but precious material containing molecules capable of modulating essential functions of cellular biological models, such as the vascular model. Further studies are required to establish these substances’ fates in the organism and to understand their biological functions and fine molecular mechanisms.
Author Contributions
Conceptualization, A.M.P., A.P. (Antonio Piga) and G.P.; Formal analysis, A.P. (Amalia Piscopo) and G.P.; Funding acquisition, A.P. (Antonio Piga) and G.P.; Investigation, A.M.P., A.C. and R.G.; Methodology, A.M.P., A.C., R.G. and A.P. (Amalia Piscopo); Resources, A.P. (Antonio Piga) and G.P.; Validation, G.P.; Writing—original draft, A.M.P. and A.P. (Amalia Piscopo); Writing—review & editing, A.M.P., A.P. (Amalia Piscopo), W.M.A.-R., A.P. (Antonio Piga) and G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the AGER 2 Project, grant no. 2016-0105, and by the Fondo UNISS di Ateneo per la Ricerca 2020 to GP and AP.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data are reported in the article.
Acknowledgments
The authors thank the firm Olearia San Giorgio for supplying OMW.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stillitano, T.; Falcone, G.; De Luca, A.I.; Piga, A.; Conte, P.; Strano, A.; Gulisano, G. A life cycle perspective to assess the environmental and economic impacts of innovative technologies in extra virgin olive oil extraction. Foods 2019, 8, 209. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.A.; Bita, E.-C.; Banilas, G.; Hajjar, S.E.; Sellianakis, V.; Aksoy, U.; Hepaksoy, S.; Chamoun, R.; Talhook, S.N.; Metzidakis, I. AFLP reveals structural details of genetic diversity within cultivated olive germplasm from the Eastern Mediterranean. Theor. Appl. Genet. 2005, 110, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Hatzopoulos, P.; Banilas, G.; Giannoulia, K.; Gazis, F.; Nikoloudakis, N.; Milioni, D.; Haralampidis, K. Breeding, molecular markers and molecular biology of the olive tree. Eur. J. Lipid Sci. Technol. 2002, 104, 574–586. [Google Scholar] [CrossRef]
- Sivakumar, G.; Uccella, N.A.; Gentile, L. Probing downstream olive biophenol secoiridoids. Int. J. Mol. Sci. 2018, 19, 2892. [Google Scholar] [CrossRef]
- Rodis, P.S.; Karathanos, V.T.; Mantzavinou, A. Partitioning of olive oil antioxidants between oil and water phases. J. Agric. Food Chem. 2002, 50, 596–601. [Google Scholar] [CrossRef]
- Obied, H.K.; Allen, M.S.; Bedgood, D.R.; Prenzler, P.D.; Robards, K.; Stockmann, R. Bioactivity and analysis of biophenols recovered from olive mill waste. J. Agric. Food Chem. 2005, 53, 823–837. [Google Scholar] [CrossRef]
- Araújo, M.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P. Phenolic compounds from olive mill wastes: Health effects, analytical approach and application as food antioxidants. Trends Food Sci. Technol. 2015, 45, 200–211. [Google Scholar] [CrossRef]
- Zbakh, H.; El Abbassi, A. Potential use of olive mill wastewater in the preparation of functional beverages: A review. J. Funct. Foods 2012, 4, 53–65. [Google Scholar] [CrossRef]
- Tripoli, E.; Giammanco, M.; Tabacchi, G.; Di Majo, D.; Giammanco, S.; La Guardia, M. The phenolic compounds of olive oil: Structure, biological activity and beneficial effects on human health. Nutr. Res. Rev. 2005, 18, 98–112. [Google Scholar] [CrossRef]
- Giuffrè, A.; Sicari, V.; Piscopo, A.; Louadj, L. Antioxidant activity of olive oil mill wastewater obtained from different thermal treatments. Grasas y Aceites 2012, 63, 209–213. [Google Scholar] [CrossRef]
- Zarkovic, N. Roles and Functions of ROS and RNS in Cellular Physiology and Pathology. Cells 2020, 9, 767. [Google Scholar] [CrossRef]
- Costa, T.J.; Barros, P.R.; Arce, C.; Santos, J.D.; da Silva-Neto, J.; Egea, G.; Dantas, A.P.; Tostes, R.C.; Jimenez-Altayó, F. The homeostatic role of hydrogen peroxide, superoxide anion and nitric oxide in the vasculature. Free Radic. Biol. Med. 2021, 162, 615–635. [Google Scholar] [CrossRef]
- Sabbatino, F.; Conti, V.; Liguori, L.; Polcaro, G.; Corbi, G.; Manzo, V.; Tortora, V.; Carlomagno, C.; Vecchione, C.; Filippelli, A. Molecules and Mechanisms to Overcome Oxidative Stress Inducing Cardiovascular Disease in Cancer Patients. Life 2021, 11, 105. [Google Scholar] [CrossRef]
- Sauer, H.; Wartenberg, M. Reactive oxygen species as signaling molecules in cardiovascular differentiation of embryonic stem cells and tumor-induced angiogenesis. Antioxid. Redox Signal. 2005, 7, 1423–1434. [Google Scholar] [CrossRef]
- Burtenshaw, D.; Hakimjavadi, R.; Redmond, E.M.; Cahill, P.A. Nox, reactive oxygen species and regulation of vascular cell fate. Antioxidants 2017, 6, 90. [Google Scholar] [CrossRef]
- Gentile, L.; Uccella, N.A.; Sivakumar, G. Oleuropein: Molecular dynamics and computation. Curr. Med. Chem. 2017, 24, 4315–4328. [Google Scholar] [CrossRef]
- Guzmán, E.; Baeten, V.; Pierna, J.A.F.; García-Mesa, J.A. Determination of the olive maturity index of intact fruits using image analysis. J. Food Sci. Technol. 2015, 52, 1462–1470. [Google Scholar] [CrossRef]
- De Marco, E.; Savarese, M.; Paduano, A.; Sacchi, R. Characterization and fractionation of phenolic compounds extracted from olive oil mill wastewaters. Food Chem. 2007, 104, 858–867. [Google Scholar] [CrossRef]
- De Bruno, A.; Romeo, R.; Fedele, F.L.; Sicari, A.; Piscopo, A.; Poiana, M. Antioxidant activity shown by olive pomace extracts. J. Environ. Sci. Health Part B 2018, 53, 526–533. [Google Scholar] [CrossRef]
- Centrone, M.; D’Agostino, M.; Difonzo, G.; De Bruno, A.; Di Mise, A.; Ranieri, M.; Montemurro, C.; Valenti, G.; Poiana, M.; Caponio, F. Antioxidant Efficacy of Olive By-Product Extracts in Human Colon HCT8 Cells. Foods 2021, 10, 11. [Google Scholar] [CrossRef]
- Zinellu, A.; Sotgia, S.; Scanu, B.; Pintus, G.; Posadino, A.M.; Cossu, A.; Deiana, L.; Sengupta, S.; Carru, C. S-homocysteinylated LDL apolipoprotein B adversely affects human endothelial cells In Vitro. Atherosclerosis 2009, 206, 40–46. [Google Scholar] [CrossRef]
- Boin, F.; Erre, G.L.; Posadino, A.M.; Cossu, A.; Giordo, R.; Spinetti, G.; Passiu, G.; Emanueli, C.; Pintus, G. Oxidative stress-dependent activation of collagen synthesis is induced in human pulmonary smooth muscle cells by sera from patients with scleroderma-associated pulmonary hypertension. Orphanet J. Rare Dis. 2014, 9, 1–5. [Google Scholar] [CrossRef]
- Posadino, A.M.; Phu, H.T.; Cossu, A.; Giordo, R.; Fois, M.; Thuan, D.T.B.; Piga, A.; Sotgia, S.; Zinellu, A.; Carru, C. Oxidative stress-induced Akt downregulation mediates green tea toxicity towards prostate cancer cells. Toxicol. In Vitro 2017, 42, 255–262. [Google Scholar] [CrossRef]
- Fois, A.G.; Posadino, A.M.; Giordo, R.; Cossu, A.; Agouni, A.; Rizk, N.M.; Pirina, P.; Carru, C.; Zinellu, A.; Pintus, G. Antioxidant activity mediates pirfenidone antifibrotic effects in human pulmonary vascular smooth muscle cells exposed to sera of idiopathic pulmonary fibrosis patients. Oxidative Med. Cell. Longev. 2018, 2639081. [Google Scholar] [CrossRef]
- Posadino, A.M.; Giordo, R.; Cossu, A.; Nasrallah, G.K.; Shaito, A.; Abou-Saleh, H.; Eid, A.H.; Pintus, G. Flavin oxidase-induced ROS generation modulates PKC biphasic effect of resveratrol on endothelial cell survival. Biomolecules 2019, 9, 209. [Google Scholar] [CrossRef]
- Posadino, A.M.; Cossu, A.; Giordo, R.; Zinellu, A.; Sotgia, S.; Vardeu, A.; Hoa, P.T.; Deiana, L.; Carru, C.; Pintus, G. Coumaric acid induces mitochondrial damage and oxidative-mediated cell death of human endothelial cells. Cardiovasc. Toxicol. 2013, 13, 301–306. [Google Scholar] [CrossRef]
- Posadino, A.M.; Biosa, G.; Zayed, H.; Abou-Saleh, H.; Cossu, A.; Nasrallah, G.K.; Giordo, R.; Pagnozzi, D.; Porcu, M.C.; Pretti, L. Protective effect of cyclically pressurized solid–liquid extraction polyphenols from Cagnulari grape pomace on oxidative endothelial cell death. Molecules 2018, 23, 2105. [Google Scholar] [CrossRef]
- Posadino, A.M.; Porcu, M.C.; Marongiu, B.; Cossu, A.; Piras, A.; Porcedda, S.; Falconieri, D.; Cappuccinelli, R.; Biosa, G.; Pintus, G. Antioxidant activity of supercritical carbon dioxide extracts of Salvia desoleana on two human endothelial cell models. Food Res. Int. 2012, 46, 354–359. [Google Scholar] [CrossRef]
- Cossu, A.; Posadino, A.M.; Giordo, R.; Emanueli, C.; Sanguinetti, A.M.; Piscopo, A.; Poiana, M.; Capobianco, G.; Piga, A.; Pintus, G. Apricot melanoidins prevent oxidative endothelial cell death by counteracting mitochondrial oxidation and membrane depolarization. PLoS ONE 2012, 7, e48817. [Google Scholar] [CrossRef]
- Posadino, A.M.; Cossu, A.; Piga, A.; Madrau, M.A.; Del Caro, A.; Colombino, M.; Paglietti, B.; Rubino, S.; Iaccarino, C.; Crosio, C. Prune melanoidins protect against oxidative stress and endothelial cell death. Front. Biosci. 2011, 3, 1034–1041. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K. Potential adverse effects of resveratrol: A literature review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef] [PubMed]
- Posadino, A.M.; Cossu, A.; Giordo, R.; Zinellu, A.; Sotgia, S.; Vardeu, A.; Hoa, P.T.; Carru, C.; Pintus, G. Resveratrol alters human endothelial cells redox state and causes mitochondrial-dependent cell death. Food Chem. Toxicol. 2015, 78, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Giordo, R.; Cossu, A.; Pasciu, V.; Hoa, P.T.; Posadino, A.M.; Pintus, G. Different redox response elicited by naturally occurring antioxidants in human endothelial cells. Open Biochem. J. 2013, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Pasciu, V.; Posadino, A.M.; Cossu, A.; Sanna, B.; Tadolini, B.; Gaspa, L.; Marchisio, A.; Dessole, S.; Capobianco, G.; Pintus, G. Akt downregulation by flavin oxidase–induced ROS generation mediates dose-dependent endothelial cell damage elicited by natural antioxidants. Toxicol. Sci. 2010, 114, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Harrison, D.G. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gines, J.; Lopez-Ongil, S.; Gonzalez-Rubio, M.; Gonzalez-Santiago, L.; Rodriguez-Puyol, M.; Rodriguez-Puyol, D. Reactive oxygen species induce proliferation of bovine aortic endothelial cells. J. Cardiovasc. Pharmacol. 2000, 35, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Haendeler, J.; Popp, R.; Goy, C.; Tischler, V.; Zeiher, A.M.; Dimmeler, S. Cathepsin D and H2O2 stimulate degradation of thioredoxin-1: Implication for endothelial cell apoptosis. J. Biol. Chem. 2005, 280, 42945–42951. [Google Scholar] [CrossRef]
- Park, W.H. Exogenous H2O2 induces growth inhibition and cell death of human pulmonary artery smooth muscle cells via glutathione depletion. Mol. Med. Rep. 2016, 14, 936–942. [Google Scholar] [CrossRef]
- Ricelli, A.; Gionfra, F.; Percario, Z.; De Angelis, M.; Primitivo, L.; Bonfantini, V.; Antonioletti, R.; Bullitta, S.M.; Saso, L.; Incerpi, S. Antioxidant and Biological Activities of Hydroxytyrosol and Homovanillic Alcohol Obtained from Olive Mill Wastewaters of Extra-Virgin Olive Oil Production. J. Agric. Food Chem. 2020, 68, 15428–15439. [Google Scholar] [CrossRef]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Alvarino, T.; Cortina, J.L.; Saurina, J.; Granados, M. Olive mill and winery wastes as viable sources of bioactive compounds: A study on polyphenols recovery. Antioxidants 2020, 9, 1074. [Google Scholar] [CrossRef]
- Obied, H.K.; Allen, M.S.; Bedgood, D.R.; Prenzler, P.D.; Robards, K. Investigation of Australian olive mill waste for recovery of biophenols. J. Agric. Food Chem. 2005, 53, 9911–9920. [Google Scholar] [CrossRef]
- D’Angelo, S.; Manna, C.; Migliardi, V.; Mazzoni, O.; Morrica, P.; Capasso, G.; Pontoni, G.; Galletti, P.; Zappia, V. Pharmacokinetics and metabolism of hydroxytyrosol, a natural antioxidant from olive oil. Drug Metab. Dispos. 2001, 29, 1492–1498. [Google Scholar]
- Auñon-Calles, D.; Canut, L.; Visioli, F. Toxicological evaluation of pure hydroxytyrosol. Food Chem. Toxicol. 2013, 55, 498–504. [Google Scholar] [CrossRef]
- Christian, M.S.; Sharper, V.A.; Hoberman, A.M.; Seng, J.E.; Fu, L.; Covell, D.; Diener, R.M.; Bitler, C.M.; Crea, R. The toxicity profile of hydrolyzed aqueous olive pulp extract. Drug Chem. Toxicol. 2004, 27, 309–330. [Google Scholar] [CrossRef]
- Crupi, R.; Palma, E.; Siracusa, R.; Fusco, R.; Gugliandolo, E.; Cordaro, M.; Impellizzeri, D.; De Caro, C.; Calzetta, L.; Cuzzocrea, S. Protective effect of Hydroxytyrosol against oxidative stress induced by the Ochratoxin in kidney cells: In Vitro and In Vivo study. Front. Vet. Sci. 2020, 7, 136. [Google Scholar] [CrossRef]
- Calahorra, J.; Martínez-Lara, E.; De Dios, C.; Siles, E. Hypoxia modulates the antioxidant effect of hydroxytyrosol in MCF-7 breast cancer cells. PLoS ONE 2018, 13, e0203892. [Google Scholar] [CrossRef]
- Gugliandolo, E.; Fusco, R.; Licata, P.; Peritore, A.F.; D’amico, R.; Cordaro, M.; Siracusa, R.; Cuzzocrea, S.; Crupi, R. Protective Effect of Hydroxytyrosol on LPS-Induced Inflammation and Oxidative Stress in Bovine Endometrial Epithelial Cell Line. Vet. Sci. 2020, 7, 161. [Google Scholar] [CrossRef]
- Schmidt, K.N.; Traenckner, E.B.-M.; Meier, B.; Baeuerle, P.A. Induction of oxidative stress by okadaic acid is required for activation of transcription factor NF-κB. J. Biol. Chem. 1995, 270, 27136–27142. [Google Scholar] [CrossRef]
- Zulueta, J.J.; Sawhney, R.; Yu, F.S.; Cote, C.C.; Hassoun, P.M. Intracellular generation of reactive oxygen species in endothelial cells exposed to anoxia-reoxygenation. Am. J. Physiol. Lung Cell. Mol. Physiol. 1997, 272, L897–L902. [Google Scholar] [CrossRef]
- Hormozi, M.; Marzijerani, A.S.; Baharvand, P. Effects of Hydroxytyrosol on Expression of Apoptotic Genes and Activity of Antioxidant Enzymes in LS180 Cells. Cancer Manag. Res. 2020, 12, 7913. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).