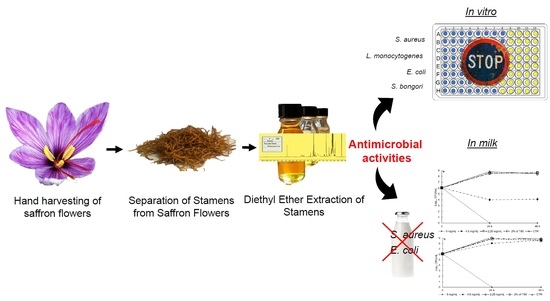
Antimicrobial Activity and Chemical Characterization of a Non-Polar Extract of Saffron Stamens in Food Matrix
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Extraction Method
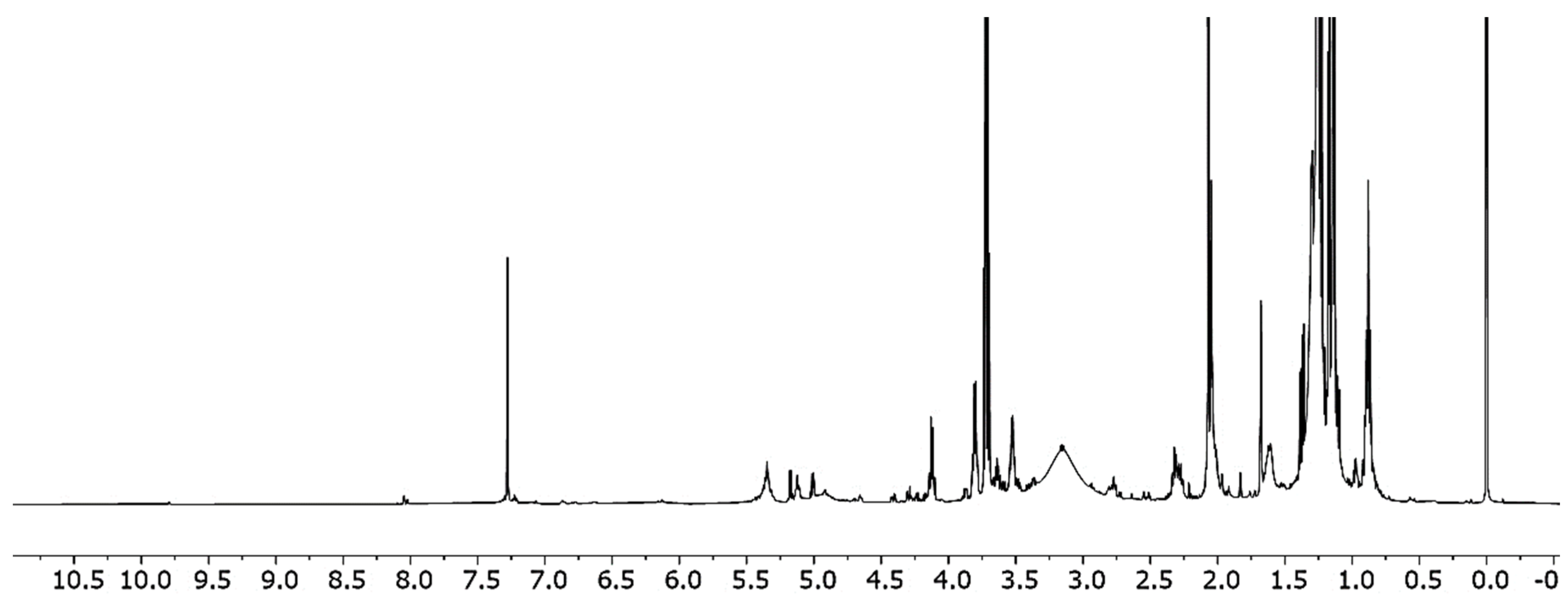
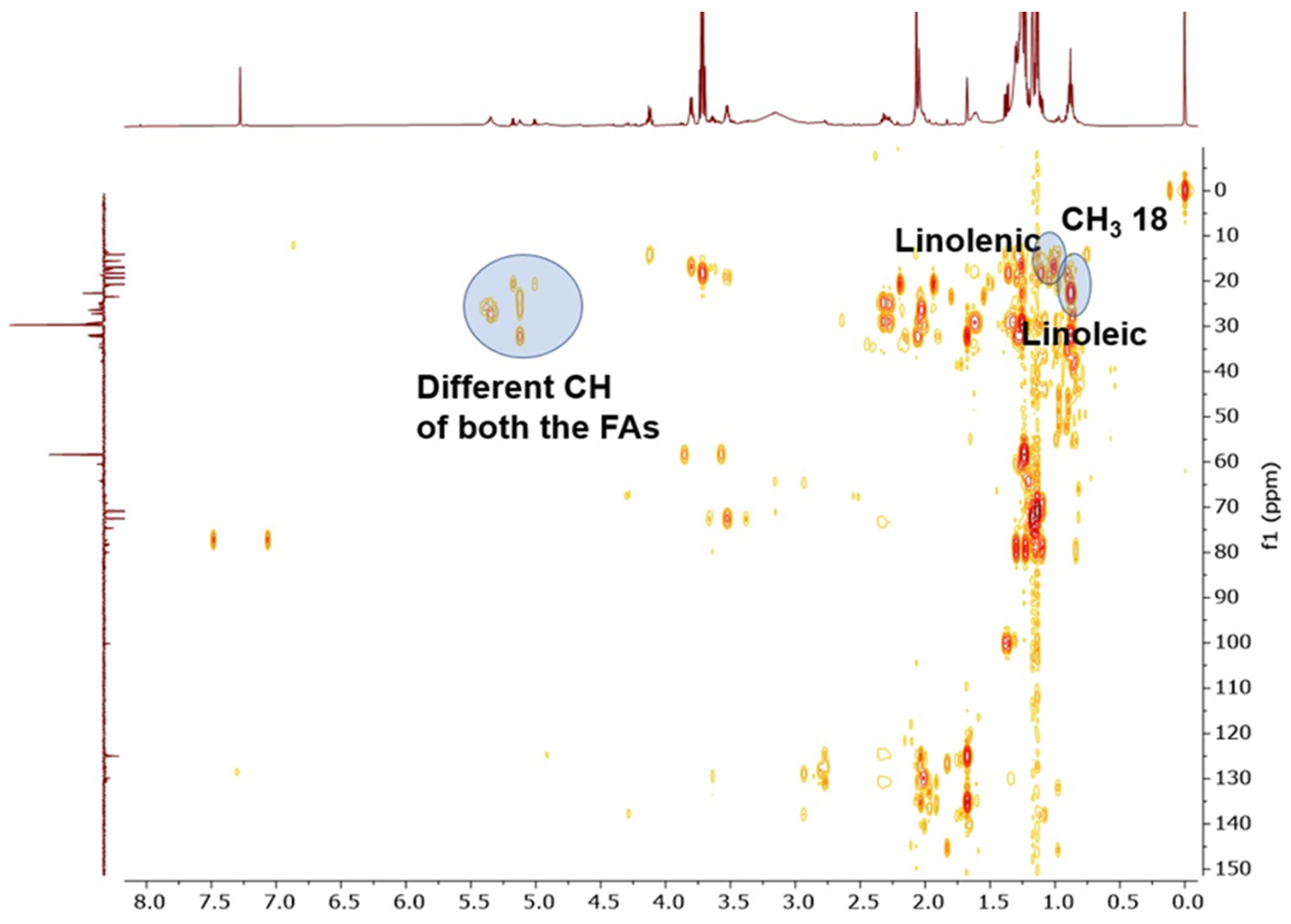
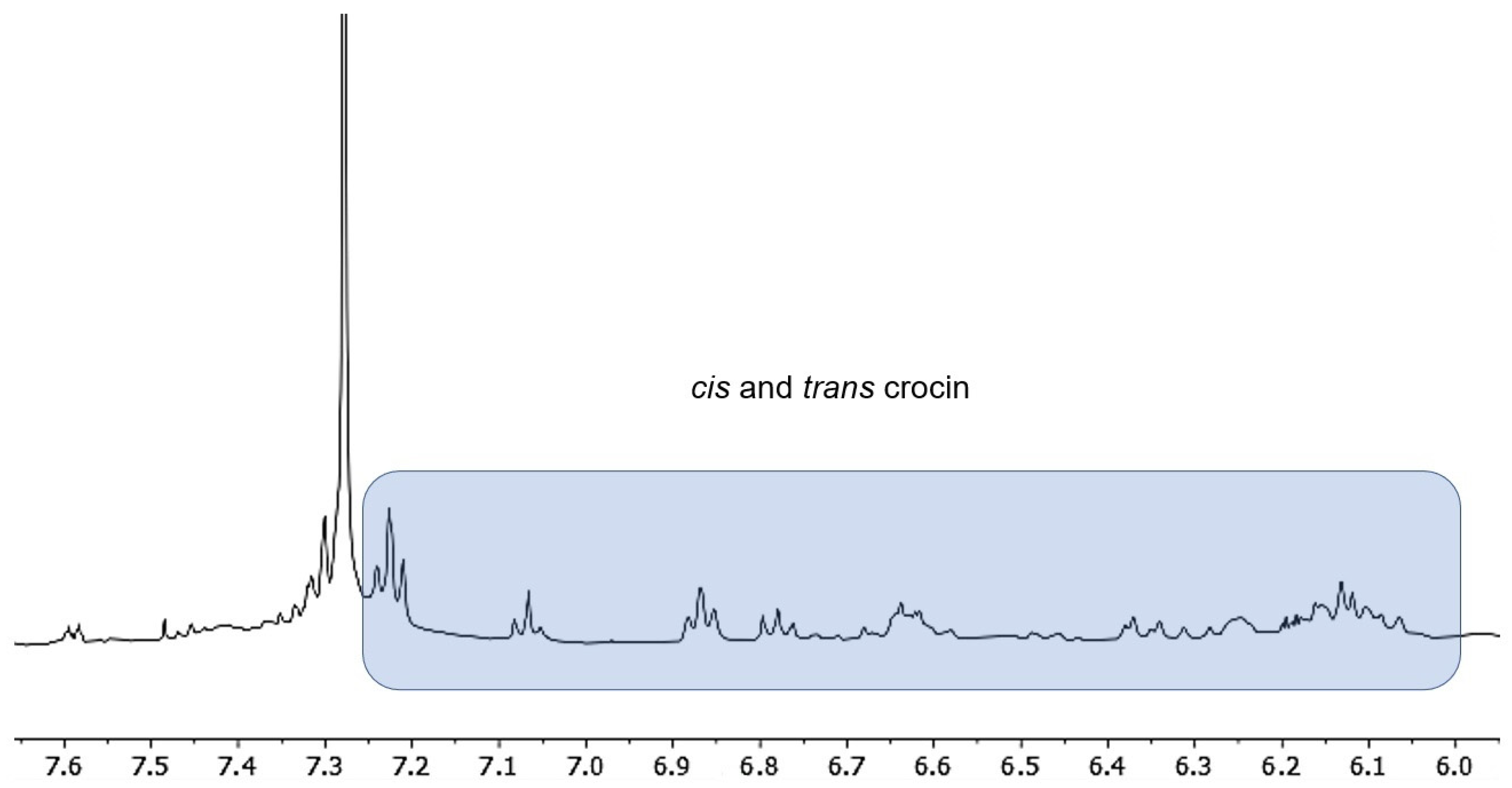
2.2. NMR Analysis
2.3. GC–MS Fatty Acid Analysis
2.4. Antimicrobial Activity
2.5. Evaluation of Antimicrobial Activity in a Food Matrix
2.5.1. Preparation of milk spiked with DES Extract
2.5.2. Preparation of Bacteria Inoculum
2.5.3. Inoculation and Growth Condition
2.6. Statistical Analysis
3. Results and Discussion
3.1. NMR Spectroscopy and GC Methyl Fatty Acids Esters Analysis
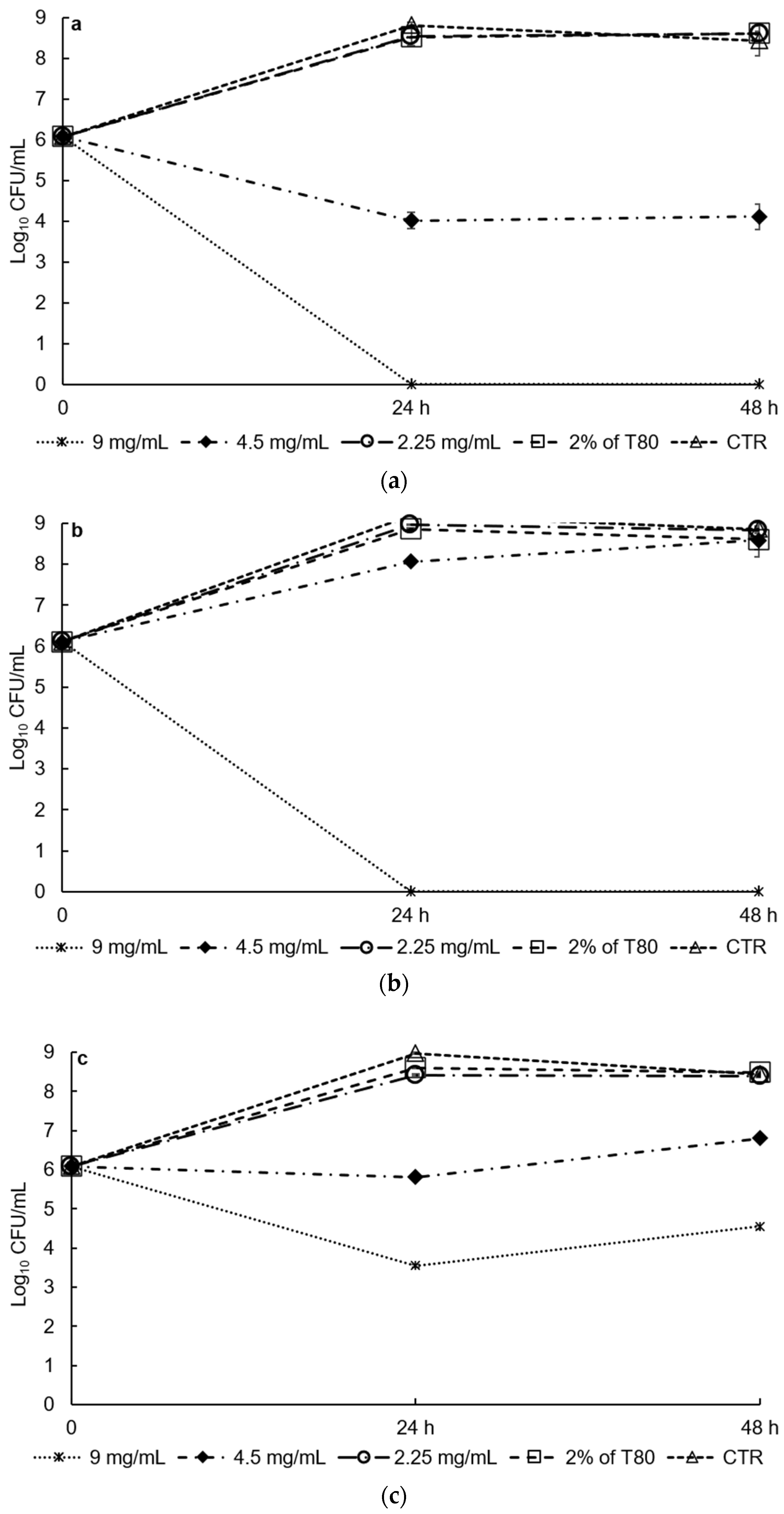
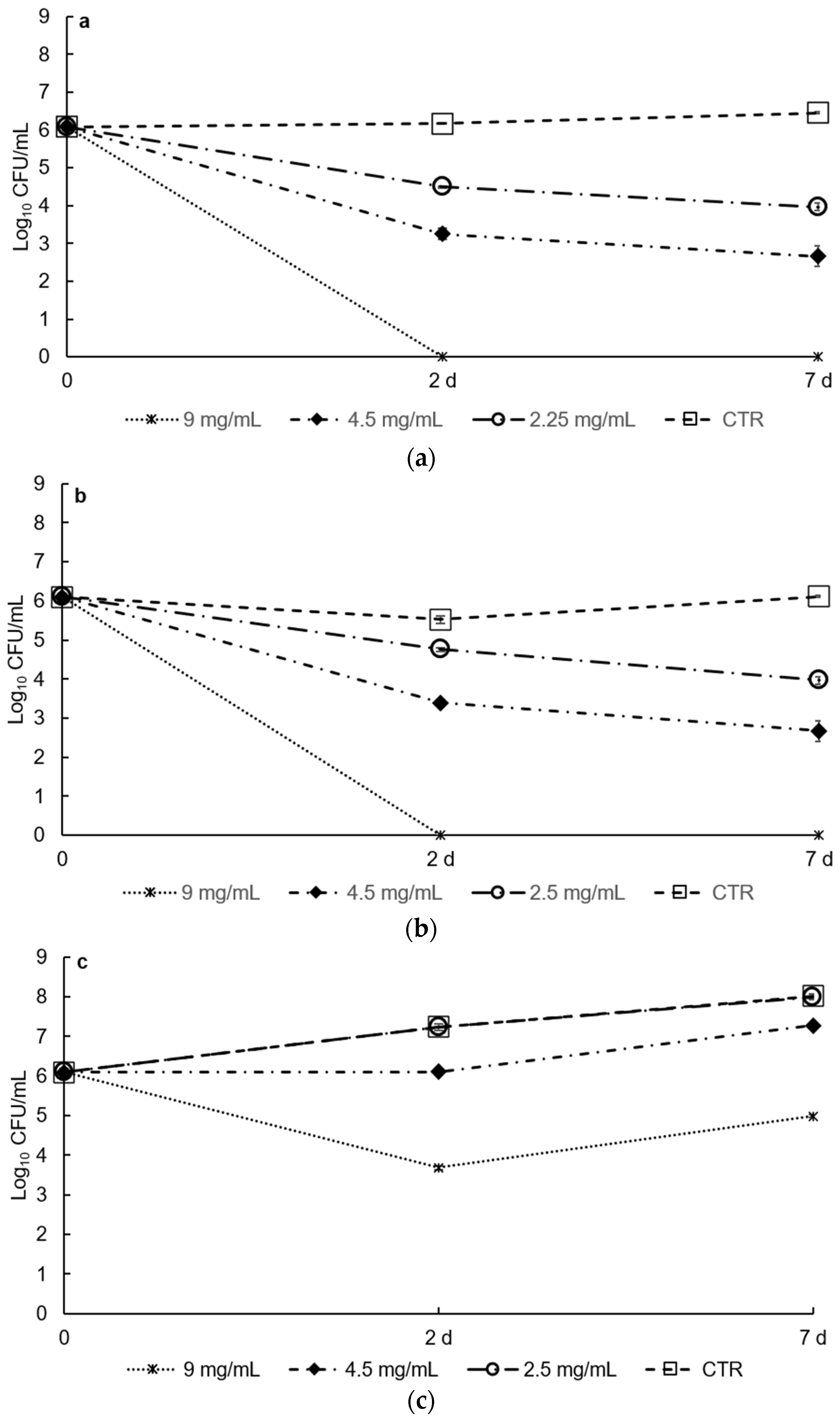
3.2. Antimicrobial Activities In Vitro and in Food Matrix
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cardone, L.; Castronuovo, D.; Perniola, M.; Cicco, N.; Candido, V. Saffron (Crocus sativus L.), the king of spices: An overview. Sci. Hortic. 2020, 272, 109560. [Google Scholar] [CrossRef]
- Moratalla-López, N.; Bagur, M.J.; Lorenzo, C.; Martínez-Navarro, M.E.; Salinas, M.R.; Alonso, G.L. Bioactivity and Bioavailability of the Major Metabolites of Crocus sativus L. Flower. Molecules 2019, 24, 2827. [Google Scholar] [CrossRef]
- Kyriakoudi, A.; Ocallaghan, Y.C.; Galvin, K.; Tsimidou, M.Z.; Obrien, N.M. Cellular Transport and Bioactivity of a Major Saffron Apocarotenoid, Picrocrocin (4-(β-D-Glucopyranosyloxy)-2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde). J. Agric. Food Chem. 2015, 63, 8662–8668. [Google Scholar] [CrossRef]
- Mir, A.M.; Rameashkannan, M.V.; Pala, R.A. A comparative study of phytochemical analysis and antimicrobial properties of stigmas and stamens of saffron (Crocus sativus L.) found in Kashmir. Adv. Biotechnol. 2011, 11, 35–38. [Google Scholar]
- Vahidi, H.; Kamalinejad, M.; Sedaghati, N. Antimicrobial properties of Crocus sativus L. Iran. J. Pharm. Res. 2002, 1, 33–35. [Google Scholar]
- Serrano-Díaz, J.; Sánchez, A.M.; Maggi, L.; Martínez-Tomé, M.; García-Diz, L.; Murcia, M.A.; Alonso, G.L. Increasing the Applications of Crocus sativus Flowers as Natural Antioxidants. J. Food Sci. 2012, 77, C1162–C1168. [Google Scholar] [CrossRef] [PubMed]
- Shadmehri Ahmadi, A.; Miri, H.; Namvar, F.; Nakhaei Moghaddam, M.; Yaghmaei, P. Cytotoxicity, antioxidant and antibacterial activities of Crocus sativus petal extract. Int. J. Res. Appl. Basic Med. Sci. 2019, 5, 69–76. [Google Scholar]
- Jadouali, S.M.; Atifi, H.; Bouzoubaa, Z.; Majourhat, K.; Gharby, S.; Achemchem, F.; Elmoslih, A.; Laknifli, A.; Mamouni, R. Chemical characterization, antioxidant and antibacterial activity of Moroccan Crocus sativus L. petals and leaves. J. Mater. Environ. Sci. 2018, 9, 113–118. [Google Scholar] [CrossRef]
- Lahmass, I.; Lamkami, T.; Delporte, C.; Sikdar, S.; van Antwerpen, P.; Saalaoui, E.; Megalizzi, V. The waste of saffron crop, a cheap source of bioactive compounds. J. Funct. Foods 2017, 35, 341–351. [Google Scholar] [CrossRef]
- Montoro, P.; Maldini, M.; Luciani, L.; Tuberoso, C.I.G.; Congiu, F.; Pizza, C. Radical scavenging activity and LC-MS metabolic profiling of petals, stamens, and flowers of Crocus sativus L. J. Food Sci. 2012, 77, C893–C900. [Google Scholar] [CrossRef]
- Sánchez-Vioque, R.; Rodríguez-Conde, M.F.; Reina-Ureña, J.V.; Escolano-Tercero, M.A.; Herraiz-Peñalver, D.; Santana-Méridas, O. In vitro antioxidant and metal chelating properties of corm, tepal and leaf from saffron (Crocus sativus L.). Ind. Crop. Prod. 2012, 39, 149–153. [Google Scholar] [CrossRef]
- Zheng, C.J.; Li, L.; Ma, W.H.; Han, T.; Qin, L.P. Chemical constituents and bioactivities of the liposoluble fraction from different medicinal parts of Crocus sativus. Pharm. Biol. 2011, 49, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Moshiri, E.; Basti, A.A.; Noorbala, A.A.; Jamshidi, A.H.; Hesameddin Abbasi, S.; Akhondzadeh, S. Crocus sativus L. (petal) in the treatment of mild-to-moderate depression: A double-blind, randomized and placebo-controlled trial. Phytomedicine 2006, 13, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Lee, E.J.; Wu, T.S. Antityrosinase Principles and Constituents of the Petals of Crocus sativus. J. Nat. Prod. 2004, 67, 437–440. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Younesi, H.M. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002, 2, 7. [Google Scholar] [CrossRef]
- Mottaghipisheh, J.; Sourestani, M.M.; Kiss, T.; Horváth, A.; Tóth, B.; Ayanmanesh, M.; Khamushi, A.; Csupor, D. Comprehensive chemotaxonomic analysis of saffron crocus tepal and stamen samples, as raw materials with potential antidepressant activity. J. Pharm. Biomed. Anal. 2020, 184, 113183. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Nile, S.H.; Zhang, Y.; Qin, L.; El-Seedi, H.R.; Daglia, M.; Kai, G. Novel Insight into Utilization of Flavonoid Glycosides and Biological Properties of Saffron (Crocus sativus L.) Flower Byproducts. J. Agric. Food Chem. 2020, 68, 10685–10696. [Google Scholar] [CrossRef] [PubMed]
- Tirillini, B.; Pagiotti, R.; Menghini, L.; Miniati, E. The Volatile Organic Compounds from Tepals and Anthers of Saffron Flowers (Crocus sativus L.). J. Essent. Oil Res. 2006, 18, 298–300. [Google Scholar] [CrossRef]
- Zeka, K.; Ruparelia, K.C.; Continenza, M.A.; Stagos, D.; Vegliò, F.; Arroo, R.R. Petals of Crocus sativus L. as a potential source of the antioxidants crocin and kaempferol. Fitoterapia 2015, 107, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Ahrazem, O.; Argandoña, J.; Fiore, A.; Aguado, C.; Luján, R.; Rubio-Moraga, Á.; Marro, M.; Araujo-Andrade, C.; Loza-Alvarez, P.; Diretto, G.; et al. Transcriptome analysis in tissue sectors with contrasting crocins accumulation provides novel insights into apocarotenoid biosynthesis and regulation during chromoplast biogenesis. Sci. Rep. 2018, 8, 1–17. [Google Scholar] [CrossRef]
- Abbasvali, M.; Ranaei, A.; Shekarforoush, S.S.; Moshtaghi, H. The Effects of Aqueous and Alcoholic Saffron (Crocus sativus) Tepal Extracts on Quality and Shelf-Life of Pacific White Shrimp (Litopeneous vannamei) During Iced Storage. J. Food Qual. 2016, 39, 732–742. [Google Scholar] [CrossRef]
- Kakouri, E.; Daferera, D.; Paramithiotis, S.; Astraka, K.; Drosinos, E.H.; Polissiou, M.G. Crocus sativus L. tepals: The natural source of antioxidant and antimicrobial factors. J. Appl. Res. Med. Aromat. Plants 2017, 4, 66–74. [Google Scholar] [CrossRef]
- Tuberoso, C.I.; Rosa, A.; Montoro, P.; Fenu, M.A.; Pizza, C. Antioxidant activity, cytotoxic activity and metabolic profiling of juices obtained from saffron (Crocus sativus L.) floral by-products. Food Chem. 2016, 199, 18–27. [Google Scholar] [CrossRef]
- Chichiriccò, G.; Ferrante, C.; Menghini, L.; Recinella, L.; Leone, S.; Chiavaroli, A.; Brunetti, L.; Di Simone, S.; Ronci, M.; Piccone, P.; et al. Crocus sativus by-products as sources of bioactive extracts: Pharmacological and toxicological focus on anthers. Food Chem. Toxicol. 2019, 124, 7–14. [Google Scholar] [CrossRef]
- Straubinger, M.; Bau, B.; Eckstein, S.; Fink, M.; Winterhalter, P. Identification of novel glycosidic aroma precursors in saffron (Crocus sativus L.). J. Agric. Food Chem. 1998, 46, 3238–3243. [Google Scholar] [CrossRef]
- Assimiadis, M.K.; Tarantilis, P.A.; Polissiou, M.G. UV-Vis, FT-Raman, and 1H NMR spectroscopies of cis-trans carotenoids from saffron (Crocus sativus L.). Appl. Spectrosc. 1998, 52, 519–522. [Google Scholar] [CrossRef]
- Sobolev, A.P.; Carradori, S.; Capitani, D.; Vista, S.; Trella, A.; Marini, F.; Mannina, L. Saffron samples of different origin: An NMR study of microwave-assisted extracts. Foods 2014, 3, 403–419. [Google Scholar] [CrossRef]
- Dowlatabadi, R.; Farshidfar, F.; Zare, Z.; Pirali, M.; Rabiei, M.; Khoshayand, R.M.; Vogel, H.J. Detection of adulteration in Iranian saffron samples by 1H NMR spectroscopy and multivariate data analysis techniques. Metabolomics 2017, 13, 19. [Google Scholar] [CrossRef]
- Menghini, L.; Leporini, L.; Vecchiotti, G.; Locatelli, M.; Carradori, S.; Ferrante, C.; Zengin, G.; Recinella, L.; Chiavaroli, A.; Leone, S.; et al. Crocus sativus L. stigmas and byproducts: Qualitative fingerprint, antioxidant potentials and enzyme inhibitory activities. Food Res. Int. 2018, 109, 91–98. [Google Scholar] [CrossRef]
- Angioni, A.; Addis, P. Characterization of the lipid fraction of wild sea urchin from the Sardinian Sea (Western Mediterranean). J. Food Sci. 2014, 79, C155–C162. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard: M07-A9, 9th ed.; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2012. [Google Scholar]
- Chichiriccò, F.; Lanza, B.; Piccone, P.; Poma, A. Nutrients and heavy metals in flowers and corms of the Saffron Crocus (Crocus sativus L.). Med. Arom. Plants 2016, 5, 4. [Google Scholar] [CrossRef]
- Chen, K.; Wang, X.M.; Chen, F.; Bai, J. In vitro antimicrobial and free radical scavenging activities of the total flavonoid in petal and stamen of Crocus sativus. Indian J. Pharm. Sci. 2017, 79, 482–487. [Google Scholar] [CrossRef]
- Kakouri, E.; Hatziagapiou, K.; Bethanis, K.; Nikola, O.A.; Lambrou, G.I.; Tarantilis, P.A. Tumor-suppressing properties of Crocus sativus L.: Nature as an anti-cancer agent. Crit. Rev. Oncog. 2017, 22, 263–273. [Google Scholar] [CrossRef]
- Didaras, N.A.; Karatasou, K.; Dimitriou, T.G.; Amoutzias, G.D.; Mossialos, D. Antimicrobial Activity of Bee-Collected Pollen and Beebread: State of the Art and Future Perspectives. Antibiotics 2020, 9, 811. [Google Scholar] [CrossRef]
- Mir, M.A.; Rameashkannan, M.V.; Raj, J.A.; Malik, A.H.; Rajesh, T.S. Phytochemical and pharmacological profile of Crocus sativus L. by-products found in Kashmir. Acta Hortic. 2018, 1200, 213–225. [Google Scholar] [CrossRef]
- Dilika, F.; Bremner, P.D.; Meyer, J.J. Antibacterial activity of linoleic and oleic acids isolated from Helichrysum pedunculatum: A plant used during circumcision rites. Fitoterapia 2000, 71, 450–452. [Google Scholar] [CrossRef]
- Chanda, W.; Joseph, T.P.; Guo, X.F.; Wang, W.D.; Liu, M.; Vuai, M.S.; Padhiar, A.A.; Zhong, M.T. Effectiveness of omega-3 polyunsaturated fatty acids against microbial pathogens. J. Zhejiang Univ. Sci. B 2018, 19, 253–262. [Google Scholar] [CrossRef]
- Gandomi Nasrabadi, H.; Azami Sarokelaei, L.; Misaghi, A.; Abbaszadeh, S.; Shariatifar, N.; Tayyar Hashtjin, N. Antibacterial effect of aqueous and alcoholic extracts from petal of saffron (Crocus sativus L.) on some foodborne bacterial pathogens. J. Med. Plants 2012, 2, 189–196. [Google Scholar]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems—A review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid. Based Complement. Altern. Med. 2016, 3012462, 1–21. [Google Scholar] [CrossRef]
- Bezerra Dos Santos, A.T.; Araújo, T.F.D.S.; Nascimento da Silva, L.C.; Silva, C.B.D.; Oliveira, A.F.M.D.; Araújo, J.M.; Correia, M.T.D.S.; Lima, V.L.D.M. Organic extracts from Indigofera suffruticosa leaves have antimicrobial and synergic actions with erythromycin against Staphylococcus aureus. Front. Microbiol. 2015, 6, 1–7. [Google Scholar] [CrossRef]
- Fancello, F.; Petretto, G.L.; Zara, S.; Sanna, M.L.; Addis, R.; Maldini, M.; Foddai, M.; Rourke, J.P.; Chessa, M.; Pintore, G. Chemical characterization, antioxidant capacity and antimicrobial activity against food related microorganisms of Citrus limon var. pompia leaf essential oil. LWT Food Sci. Technol. 2016, 69, 579–585. [Google Scholar] [CrossRef]
- Petretto, G.L.; Fancello, F.; Bakhy, K.; Faiz, C.A.; Sibawayh, Z.; Chessa, M.; Zara, S.; Sanna, M.L.; Maldini, M.; Rourke, J.P.; et al. Chemical composition and antimicrobial activity of essential oils from Cuminum cyminum L. collected in different areas of Morocco. Food Biosci. 2018, 22, 50–58. [Google Scholar] [CrossRef]
- Fancello, F.; Petretto, G.L.; Marceddu, S.; Venditti, T.; Pintore, G.; Zara, G.; Mannazzu, I.; Budroni, M.; Zara, S. Antimicrobial activity of gaseous Citrus limon var pompia leaf essential oil against Listeria monocytogenes on ricotta salata cheese. Food Microbiol. 2020, 87, 103386. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Yessoufou, K.; Mahmoud, E.A.; Skalicka-Woźniak, K. In vitro antioxidant and antimicrobial effects of Ceratostigma plumbaginoides. Nat. Prod. Commun. 2016, 11, 1455–1458. [Google Scholar] [CrossRef] [PubMed]
- Jastaniah, S.D. The antimicrobial activity of some plant extracts, commonly used by Saudi people, against multidrug resistant bacteria. Life Sci. J. 2014, 11, 78–84. [Google Scholar]
- Muruzović, M.Ž.; Mladenović, K.G.; Stefanović, O.D.; Vasić, S.M.; Čomić, L.R. Extracts of Agrimonia eupatoria L. as sources of biologically active compounds and evaluation of their antioxidant, antimicrobial, and antibiofilm activities. J. Food Drug Anal. 2016, 24, 539–547. [Google Scholar] [CrossRef]
- Al-Hadid, K.J. Quantitative analysis of antimicrobial activity of Foeniculum vulgare: A review. Plant Omics 2017, 10, 28–36. [Google Scholar] [CrossRef]
- Dekić, M.S.; Radulović, N.S.; Ranđelović, V.N.; Stojanović-Radić, Z.Z.; Veljković, B.P. Essential oils and diethyl ether extracts of serbian Xeranthemum cylindraceum and X. annum: Chemical composition, antimicrobial activity, and chemotaxonomic implications. Chem. Biodivers. 2015, 12, 1378–1397. [Google Scholar] [CrossRef]
- Asgarpanah, J.; Darabi-Mahboub, E.; Mahboubi, A.; Mehrab, R.; Hakemivala, M. In-Vitro evaluation of Crocus sativus L. petals and stamens as natural antibacterial agents against food-borne bacterial strains. Iran. J. Pharm. Sci. 2013, 9, 69–82. [Google Scholar]
- Sarkar, P.; Bhunia, A.K.; Yao, Y. Impact of starch-based emulsions on the antibacterial efficacies of nisin and thymol in cantaloupe juice. Food Chem. 2017, 217, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Syed, I.; Sarkar, P. Ultrasonication-assisted formation and characterization of geraniol and carvacrol-loaded emulsions for enhanced antimicrobial activity against food-borne pathogens. Chem. Pap. 2018, 72, 2659–2672. [Google Scholar] [CrossRef]
- Shah, B.; Davidson, P.M.; Zhong, Q. Nanocapsular dispersion of thymol for enhanced dispersibility and increased antimicrobial effectiveness against Escherichia coli O157:H7 and Listeria monocytogenes in model food systems. Appl. Environ. Microbiol. 2012, 78, 8448–8453. [Google Scholar] [CrossRef]
- Shah, B.; Davidson, P.M.; Zhong, Q. Nanodispersed eugenol has improved antimicrobial activity against Escherichia coli O157:H7 and Listeria monocytogenes in bovine milk. Int. J. Food Microbiol. 2013, 161, 53–59. [Google Scholar] [CrossRef]
| Bacteria | Source | Medium | Temperature and Time of Incubation |
|---|---|---|---|
| Staphylococcus aureus DSM 20231 | DSMZ | BHI | 37 °C × 24 h |
| Listeria monocytogenes B | UNISS | BHI | 37 °C × 24 h |
| Listeria monocytogenes E | UNISS | BHI | 37 °C × 24 h |
| Listeria monocytogenes C | UNISS | BHI | 37 °C × 24 h |
| Listeria monocytogenes DSM 20600 | DSMZ | BHI | 37 °C × 24 h |
| Salmonella enterica subsp. bongori DSM 13772 | DSMZ | BHI | 37 °C × 24 h |
| Escherichia coli DSM 30083 | DSMZ | BHI | 37 °C × 24 h |
| Bacteria | MIC * | MBC * |
|---|---|---|
| Staphylococcus aureus DSM 20231 | 4.5 | 9 |
| Listeria monocytogenes B | 9 | 9 |
| Listeria monocytogenes E | 9 | 9 |
| Listeria monocytogenes C | 9 | 9 |
| Listeria monocytogenes DSM 20600 | 9 | 9 |
| Salmonella enterica subsp. bongori DSM 13772 | 9 | 18 |
| Escherichia coli DSM 30083 | 9 | 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zara, S.; Petretto, G.L.; Mannu, A.; Zara, G.; Budroni, M.; Mannazzu, I.; Multineddu, C.; Pintore, G.; Fancello, F. Antimicrobial Activity and Chemical Characterization of a Non-Polar Extract of Saffron Stamens in Food Matrix. Foods 2021, 10, 703. https://doi.org/10.3390/foods10040703
Zara S, Petretto GL, Mannu A, Zara G, Budroni M, Mannazzu I, Multineddu C, Pintore G, Fancello F. Antimicrobial Activity and Chemical Characterization of a Non-Polar Extract of Saffron Stamens in Food Matrix. Foods. 2021; 10(4):703. https://doi.org/10.3390/foods10040703
Chicago/Turabian StyleZara, Severino, Giacomo L. Petretto, Alberto Mannu, Giacomo Zara, Marilena Budroni, Ilaria Mannazzu, Chiara Multineddu, Giorgio Pintore, and Francesco Fancello. 2021. "Antimicrobial Activity and Chemical Characterization of a Non-Polar Extract of Saffron Stamens in Food Matrix" Foods 10, no. 4: 703. https://doi.org/10.3390/foods10040703
APA StyleZara, S., Petretto, G. L., Mannu, A., Zara, G., Budroni, M., Mannazzu, I., Multineddu, C., Pintore, G., & Fancello, F. (2021). Antimicrobial Activity and Chemical Characterization of a Non-Polar Extract of Saffron Stamens in Food Matrix. Foods, 10(4), 703. https://doi.org/10.3390/foods10040703