Natural Drying of Astringent and Non-Astringent Persimmon “Rojo Brillante”. Drying Kinetics and Physico-Chemical Properties
Abstract
1. Introduction
2. Materials and Methods
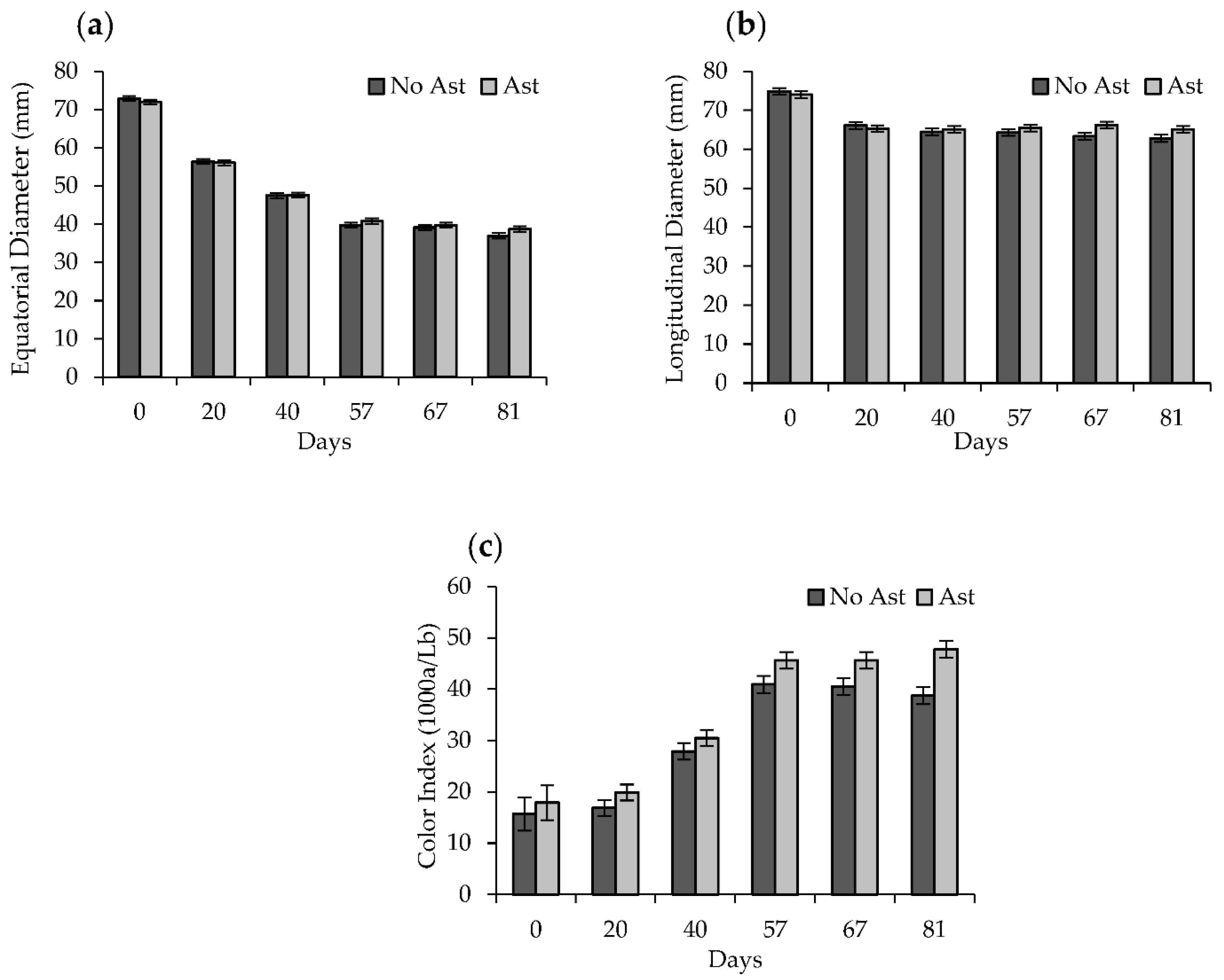
2.1. Weight Loss and Equatorial, Longitudinal Diameters
2.2. External Color
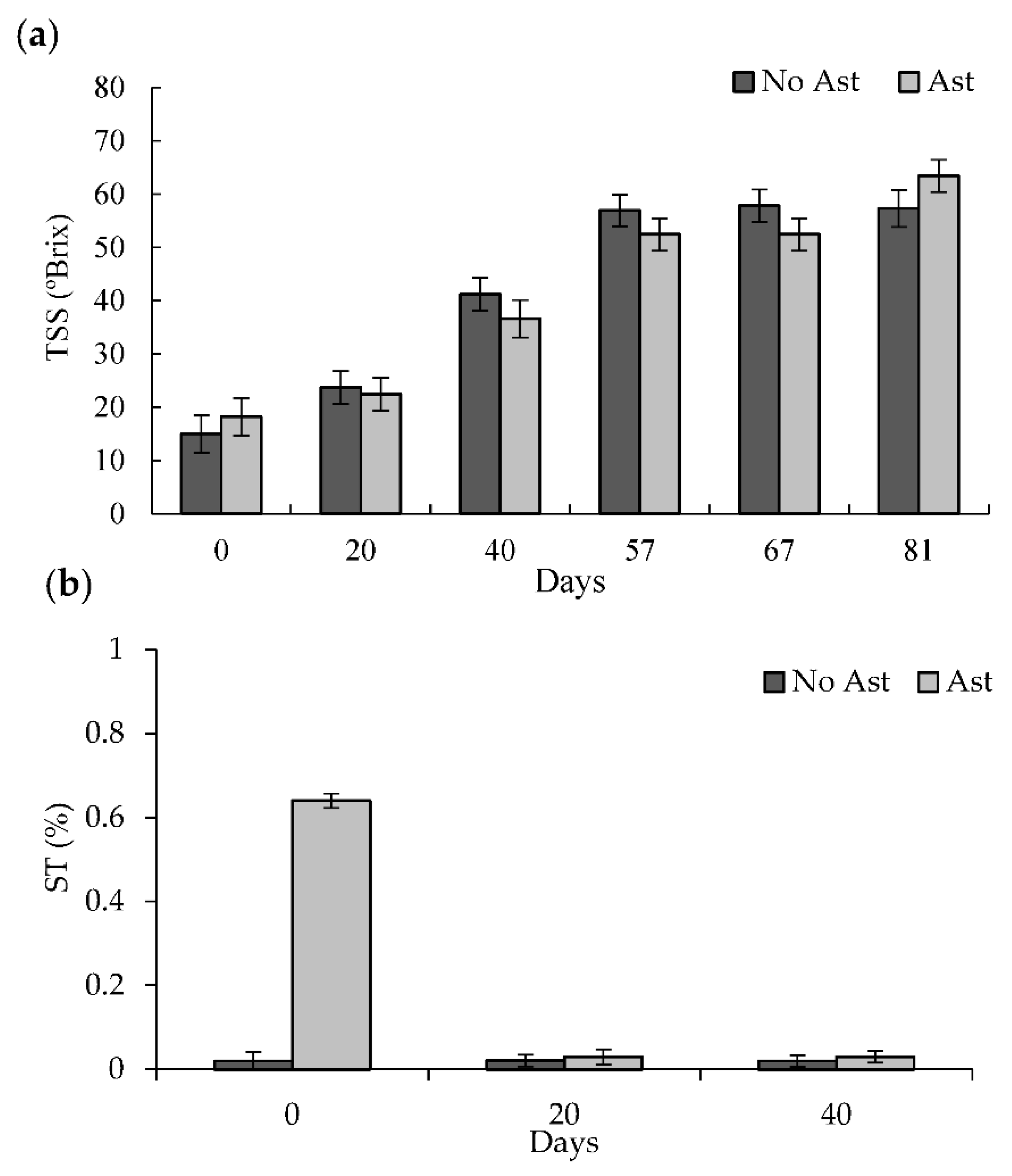
2.3. Total Soluble Solids (TSS) and Soluble Tannins (ST)
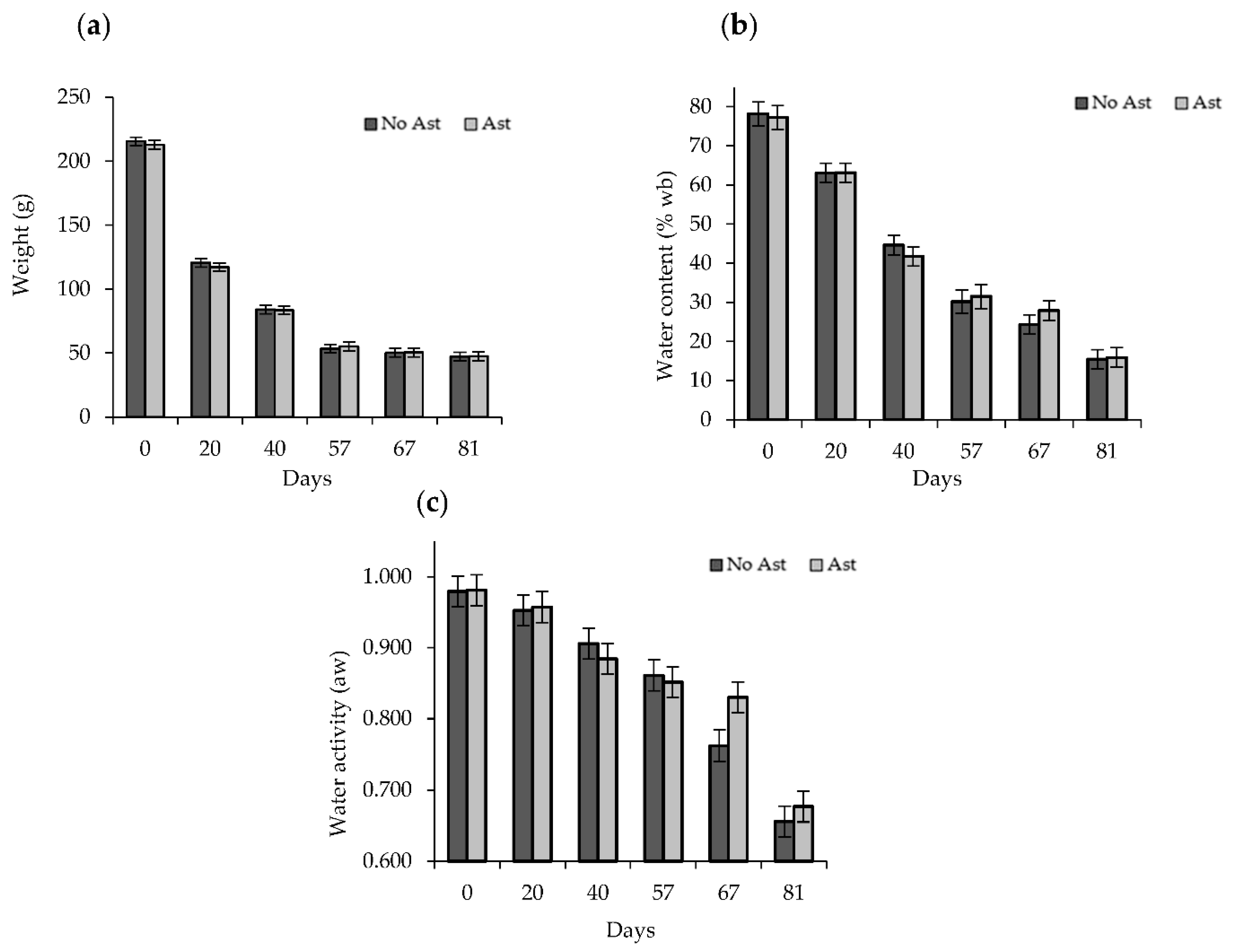
2.4. Water Content and Water Activity
2.5. Mathematical Modeling of Drying Curves
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physico-Chemical Determinations
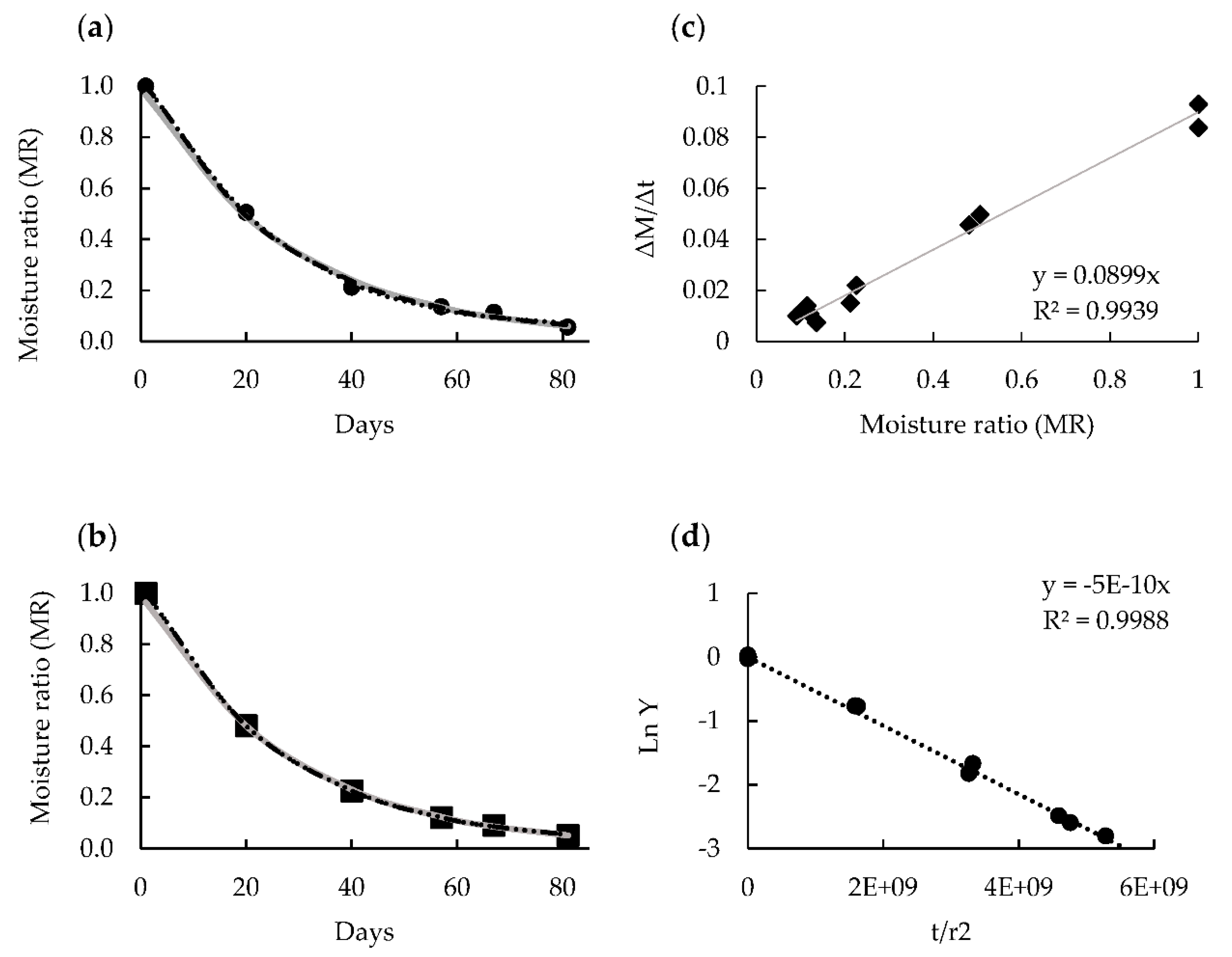
3.2. Fitting of Drying Curves and Drying Rate Determinations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perucho, R. Evolution of production of the ‘Rojo Brillante’ cultivar in Spain and its impact on markets. Acta Hortic. 2018, 1195, 1–8. [Google Scholar] [CrossRef]
- Tessmer, M.A.; Besada, C.; Hernando, I.; Appezzato-da-Glória, B.; Quiles, A.; Salvador, A. Microstructural changes while persimmon fruits mature and ripen. Comparison between astringent and non-astringent cultivars. Postharvest Biol. Technol. 2016, 120, 52–60. [Google Scholar] [CrossRef]
- Salvador, A.; Arnal, L.; Besada, C.; Larrea, V.; Quiles, A.; Pérez-Munuera, I. Physiological and structural changes during ripening and deastringency treatment of persimmon fruit cv. “Rojo Brillante”. Postharvest Biol. Technol. 2007, 46, 181–188. [Google Scholar] [CrossRef]
- Novillo, P.; Salvador, A.; Magalhaes, T.; Besada, C. Deastringency treatment with CO2 induces oxidative stress in persimmon fruit. Postharvest Biol. Technol. 2014, 92, 16–22. [Google Scholar] [CrossRef]
- UNECE Standard FFV-63 Concerning the Marketing and Commercial Quality Control of Persimmons. Available online: https://www.unece.org/fileadmin/DAM/trade/agr/standard/standard/fresh/FFV-Std/English/50Apples_2014.pdf (accessed on 10 March 2021).
- Mat Desa, W.N.; Mohammad, M.; Fudholi, A. Review of drying technology of Figure. Trends. Food Sci. Technol. 2019, 88, 93–103. [Google Scholar] [CrossRef]
- Sijtsema, S.J.; Jesionkowska, K.; Symoneaux, R.; Konopacka, D.; Snoek, H. Perceptions of the health and convenience characteristics of fresh and dried fruits. LWT Food Sci. Technol. 2012, 49, 275–281. [Google Scholar] [CrossRef]
- Yaqub, S.; Farooq, U.; Shafi, A.; Akram, K.; Murtaza, M.A.; Kausar, T.; Siddique, F. Chemistry and functionality of bioactive compounds present in persimmon. J. Chem. 2016, 16, 1–13. [Google Scholar] [CrossRef]
- Matsumoto, T.; Matsuzaki, H.; Takata, K.; Tsurunaga, Y.; Takahashi, H.; Kurahashi, T.; Maki, S.; Fujiwara, K. Inhibition of astringency removal in semidried japanese persimmon fruit by 1-methylcyclopropene treatment. HortScience 2007, 42, 1493–1495. [Google Scholar] [CrossRef]
- Sun-wun, L.; Hye-Kyung, M.; Won-Young, L.; Jong-Kuk, K. Physicochemical characteristics of cold-air dried persimmons and traditional dried persimmons. Korean Soc. Food Preserv. 2011, 18, 481–487. [Google Scholar]
- Oh, H.J.; Park, M.S.; Cho, K.J.; Choi, S.I.; Kang, H.M.; Kim, H. Geographical indication and development plans in South Korea: A study on dried persimmons. Forest Sci. Technol. 2018, 14, 41–46. [Google Scholar] [CrossRef]
- Hur, S.; Kang, B.; Lee, D.; Lee, S.; Lee, J. Quality characteristics of domestic dried persimmon and imported dried persimmon. Korean Soc. Food Preserv. 2014, 21, 140–145. [Google Scholar] [CrossRef]
- Akpinar, E.K. Determination of suitable thin layer drying curve model for some vegetables and fruits. J. Food Eng. 2006, 73, 75–84. [Google Scholar] [CrossRef]
- Roberts, J.S.; Kidd, D.R.; Padilla-Zakour, O. Drying kinetics of grape seeds. J. Food Eng. 2008, 89, 460–465. [Google Scholar] [CrossRef]
- Sampaio, R.M.; Neto, J.P.M.; Perez, V.H.; Marcos, S.K.; Boizan, M.A.; Da Silva, L.R. Mathematical modeling of drying kinetics of persimmon fruits (Diospyros kaki cv. Fuyu). J. Food Process. Preserv. 2017, 41, 1–7. [Google Scholar] [CrossRef]
- García-Pérez, J.V.; Cárcel, J.A.; Benedito, J.; Mulet, A. Power ultrasound mass transfer enhancement on food drying. Food Bioprod. Process. 2007, 85, 247–254. [Google Scholar] [CrossRef]
- Bozkir, H.; Rayman Ergün, A.; Serdar, E.; Metin, G.; Baysal, T. Influence of ultrasound and osmotic dehydration pretreatments on drying and quality properties of persimmon fruit. Ultrason. Sonochem. 2019, 54, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Çelen, S. Effect of microwave drying on the drying characteristics, color, microstructure, and thermal properties of Trabzon persimmon. Foods 2019, 8, 84. [Google Scholar] [CrossRef]
- Doymaz, I. Evaluation of some thin-layer drying models of persimmon slices (Diospyros kaki L.). Energy Convers. Manag. 2012, 56, 199–205. [Google Scholar] [CrossRef]
- Demiray, E.; Tulek, Y. The effect of pretreatments on Air drying characteristics of persimmons. Heat Mass Transf. 2017, 53, 99–106. [Google Scholar] [CrossRef]
- Arnal, L.; Del Río, M.A. Effect of cold storage and removal astringency on quality of persimmon fruit (Diospyros kaki, L.) cv. Rojo brillante. Food Sci. Technol. Int. 2004, 10, 179–185. [Google Scholar] [CrossRef]
- Liu, Q.; Bakker-Arkema, F.W. Stochastic modelling of grain drying: Part 2. Model development. J. Agric. Eng. Res. 1997, 66, 275–280. [Google Scholar] [CrossRef]
- Page, G.E. Factors Influencing the Maximum rates of Air Drying Shelled Corn in Thin Layers. Master’s Thesis, Purdue University, West Lafayette, IN, USA, 1949; pp. 355–367. [Google Scholar]
- Midilli, A.; Kucuk, H.; Yapar, Z. A new model for single-layer drying. Dry. Technol. 2002, 20, 37–41. [Google Scholar] [CrossRef]
- Togrul, I.T.; Pehlivan, D. Mathematical modelling of solar drying of apricots in thin layers. J. Food Eng. 2002, 55, 209–216. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, J.; Liao, X.; Chen, F.; Zhao, G.; Wu, J.; Hu, X. Mathematical modeling on Hot Air drying of thin layer apple pomace. Food Res. Int. 2007, 40, 39–46. [Google Scholar] [CrossRef]
- Verma, L.R.; Bucklin, R.A.; Endan, J.B.; Wratten, F.T. Effects of drying air parameters on rice drying models. Trans. Am. Soc. Agric. Eng. 1985, 28, 296–301. [Google Scholar] [CrossRef]
- Fang, S.; Wang, Z.; Hu, X. Hot Air drying of whole fruit chinese jujube (Zizyphus jujuba Miller): Thin-layer mathematical modelling. Int. J. Food Sci. Technol. 2009, 44, 1818–1824. [Google Scholar] [CrossRef]
- Contreras, C.; Martín-Esparza, M.E.; Chiralt, A.; Martínez-Navarrete, N. Influence of microwave application on convective drying: Effects on drying kinetics, and optical and mechanical properties of apple and strawberry. J. Food Eng. 2008, 88, 55–64. [Google Scholar] [CrossRef]
- Martinez-Navarrete, N.; Chiralt, A. Water diffusivity and mechanical changes during hazelnut hydration. Food Res. Int. 1999, 32, 447–452. [Google Scholar] [CrossRef]
- Choi, J.Y.; Lee, H.J.; Cho, J.S.; Lee, Y.M.; Woo, J.H.; Moon, K.D. Prediction of shelf-life and changes in the quality characteristics of semidried persimmons stored at different temperatures. Food Sci. Biotechnol. 2017, 26, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Katsuno, N.; Nishizu, T. Changes in the physico-chemical properties of persimmon (Diospyros kaki thunb.) during drying and quality deterioration during storage. Rev. Agric. Sci. 2019, 8, 1–14. [Google Scholar] [CrossRef]
- Yamada, H.; Ando, T.; Tsutani, K.; Amano, S.; Yamamoto, Y. Mechanism of browning occurring during the processing of semi-dried persimmons. J. Jpn. Soc. Hortic. Sci. 2009, 78, 124–130. [Google Scholar] [CrossRef]
- Mamet, T.; Yao, F.; Li, K.; Li, C. mei Persimmon tannins enhance the gel properties of high and low methoxyl pectin. LWT Food Sci. Technol. 2017, 86, 594–602. [Google Scholar] [CrossRef]
- Im, J.; Lee, M. Physicochemical Compositions of Raw and Dried Wolha Persimmons. Korean Soc. Food Preserv. 2007, 14, 611–616. [Google Scholar]
- Arnal, L.; Del Río, M.A. Removing astringency by carbon dioxide and nitrogen-enriched atmospheres in persimmon fruit cv. “Rojo brillante”. J. Food Sci. 2003, 68, 1516–1518. [Google Scholar] [CrossRef]
- Taira, S.; Ono, M.; Matsumoto, N. Reduction of persimmon astringency by complex formation between pectin and tannins. Postharvest Biol. Technol. 1997, 12, 265–271. [Google Scholar] [CrossRef]
- Asgar, M.A.; Yamauchi, R.; Kato, K. Structural features of pectins from fresh and sun-dried Japanese persimmon fruit. Food Chem. 2004, 87, 247–251. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Puente-Díaz, L.; Lemus-Mondaca, R.; Miranda, M.; Torres, M.J. Mathematical Modeling of Thin-Layer Drying Kinetics of Cape Gooseberry (Physalis peruviana L.). J. Food Process. Preserv. 2014, 38, 728–736. [Google Scholar] [CrossRef]
- Mundada, M.; Hathan, B.S.; Maske, S. Convective dehydration kinetics of osmotically pretreated pomegranate arils. Biosyst. Eng. 2010, 107, 307–316. [Google Scholar] [CrossRef]
- Mulet, A. Drying modelling and water diffusivity in carrots and potatoes. J. Food Eng. 1994, 22, 329–348. [Google Scholar] [CrossRef]
- Doymaz, I. Pretreatment effect on sun drying of mulberry fruits (Morus alba L.). J. Food Eng. 2004, 65, 205–209. [Google Scholar] [CrossRef]
- Doymaz, I. Sun drying of figs: An experimental study. J. Food Eng. 2005, 71, 403–407. [Google Scholar] [CrossRef]
- Doymaz, I. Convective drying kinetics of strawberry. Chem. Eng. Process. Process Intensif. 2008, 47, 914–919. [Google Scholar] [CrossRef]
- Zogzas, N.; Maroulis, Z.B. Moisture diffusivity data compilation in foodstuff. Dry. Technol. 1996, 14, 2225–2253. [Google Scholar] [CrossRef]

| Model | Mathematical Equation | References |
|---|---|---|
| Newton | [22] | |
| Page | [23] | |
| Midilli et al. | [24] | |
| Logarithmic | [25] | |
| Henderson and Pabis | [26] | |
| Verma model | [27] |
| Models Parameters | Statistical Parameters | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Models | k | n | a | g | c | b | R2 | X2 | MBE | RMSE | PE% |
| Newton | 0.035 | - | - | - | - | - | 0.997 | 0.001 | 0.017 | 0.000 | 7.071 |
| Page | 0.030 | 1.043 | - | - | - | - | 0.997 | 0.001 | 0.017 | 0.000 | 9.021 |
| Midilli et al. | 0.027 | 1.101 | 1.028 | - | - | 0.000 | 0.998 | 0.000 | 0.011 | 0.000 | 10.243 |
| Logarithmic | 0.039 | - | 1.022 | - | 0.019 | - | 0.998 | 0.000 | 0.012 | 0.000 | 7.965 |
| Henderson and Pabis | 0.037 | - | 1.037 | - | - | - | 0.998 | 0.000 | 0.012 | 0.000 | 7.586 |
| Verma model | 0.036 | - | 0.007 | 0.036 | - | - | 0.998 | 0.001 | 0.018 | 0.000 | 7.663 |
| Models Parameters | Statistical Parameters | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Models | k | n | a | g | c | b | R2 | X2 | MBE | RMSE | PE% |
| Newton | 0.037 | - | - | - | - | - | 0.999 | 0.000 | 0.008 | 0.000 | 2.305 |
| Page | 0.032 | 1.040 | - | - | - | - | 0.999 | 0.000 | 0.010 | 0.000 | 4.632 |
| Midilli et al. | 0.041 | 0.983 | 1.042 | - | - | 0.000 | 0.999 | 0.000 | 0.002 | 0.000 | 1.943 |
| Logarithmic | 0.039 | - | 1.028 | - | 0.011 | - | 0.999 | 0.000 | 0.002 | 0.000 | 2.032 |
| Henderson & Pabis | 0.038 | - | 1.037 | - | - | - | 0.999 | 0.000 | 0.004 | 0.000 | 3.095 |
| Verma model | 0.037 | - | 0.017 | 0.037 | - | - | 0.999 | 0.000 | 0.008 | 0.000 | 2.305 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, C.M.; Gil, R.; Moraga, G.; Salvador, A. Natural Drying of Astringent and Non-Astringent Persimmon “Rojo Brillante”. Drying Kinetics and Physico-Chemical Properties. Foods 2021, 10, 647. https://doi.org/10.3390/foods10030647
González CM, Gil R, Moraga G, Salvador A. Natural Drying of Astringent and Non-Astringent Persimmon “Rojo Brillante”. Drying Kinetics and Physico-Chemical Properties. Foods. 2021; 10(3):647. https://doi.org/10.3390/foods10030647
Chicago/Turabian StyleGonzález, Cristina M., Rebeca Gil, Gemma Moraga, and Alejandra Salvador. 2021. "Natural Drying of Astringent and Non-Astringent Persimmon “Rojo Brillante”. Drying Kinetics and Physico-Chemical Properties" Foods 10, no. 3: 647. https://doi.org/10.3390/foods10030647
APA StyleGonzález, C. M., Gil, R., Moraga, G., & Salvador, A. (2021). Natural Drying of Astringent and Non-Astringent Persimmon “Rojo Brillante”. Drying Kinetics and Physico-Chemical Properties. Foods, 10(3), 647. https://doi.org/10.3390/foods10030647