A Multi-Elements Isotope Approach to Assess the Geographic Provenance of Manila Clams (Ruditapes philippinarum) via Recombining Appropriate Elements
Abstract
1. Introduction
2. Materials and Methods
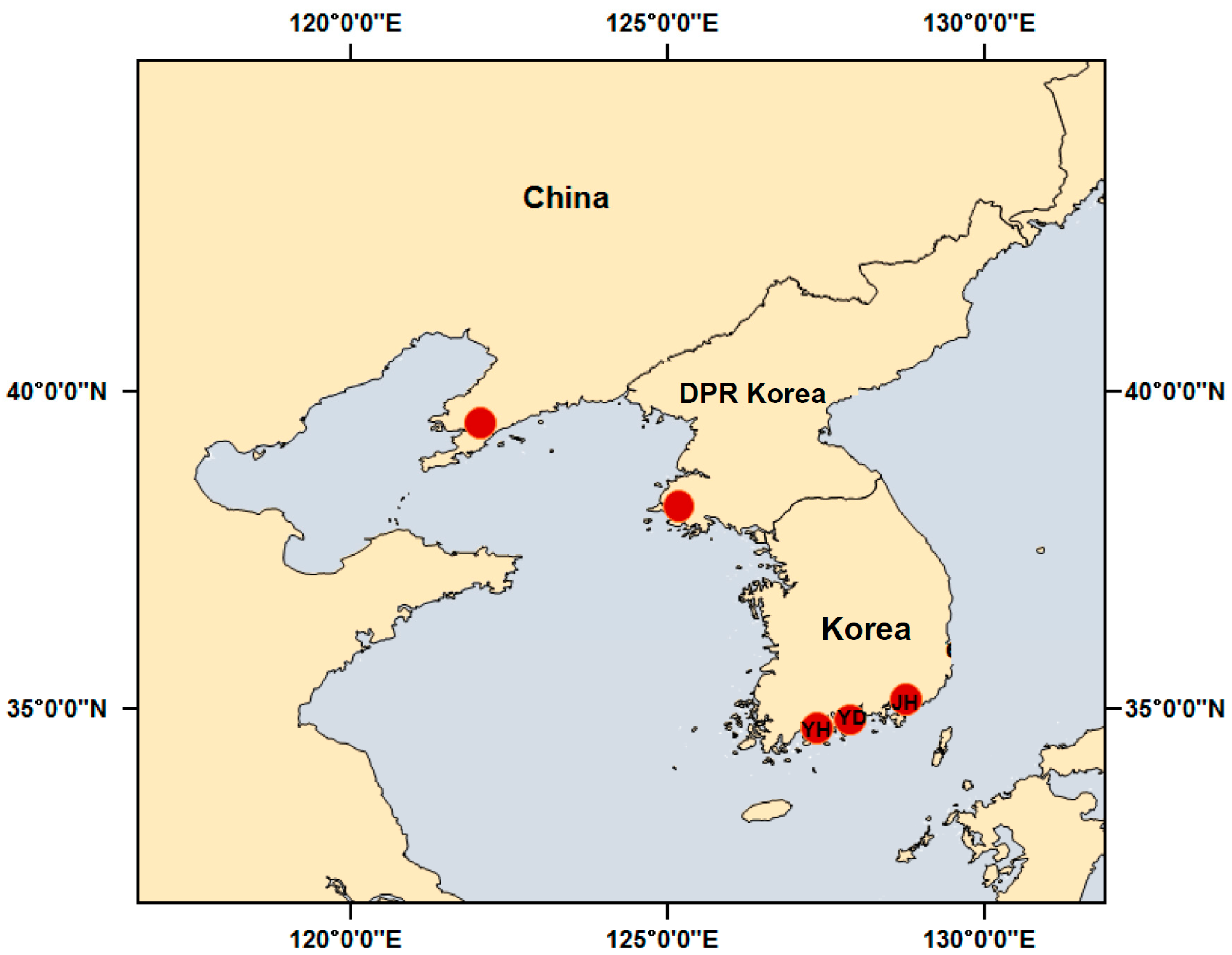
2.1. Sample Collection
2.2. Stable Isotope Analysis
δX = [(Rsample−Rstd)/Rstd] × 1000 (‰)
2.3. Radiogenic Sr and N Isotope Analysis
2.4. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kasai, A.; Horie, H.; Sakamoto, W. Selection of food sources by Ruditapes philippinarum and Mactra veneriformis (Bivalva: Mollusca) determined from stable isotope analysis. Fish. Sci. 2004, 70, 11–20. [Google Scholar] [CrossRef]
- Komorita, T.; Kajihara, R.; Tsutsumi, H.; Shibanuma, S.; Yamada, T.; Montani, S. Food sources for Ruditapes philippinarum in a coastal lagoon determined by mass balance and stable isotope approaches. PLoS ONE 2014, 9, e86732. [Google Scholar] [CrossRef]
- Zhao, L.; Yan, X.; Yang, F. Food sources of the Manila clam Ruditapes philippinarum in intertidal areas: Evidence from stable isotope analysis. Chin. J. Oceanol. Limnol. 2013, 31, 782–788. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO) of the United Nations. Fisheries and Aquaculture Department. Available online: http://www.fao.org/figis (accessed on 5 March 2021).
- Korea Institute of Marine Science and Technology Promotion (KIMST). Development of Practical Technique to Establish Fisheries Forensic Center; KIMST: Seoul, Korea, 2017. [Google Scholar]
- Ministry of Oceans and Fisheries. Ministry of Oceans and Fisheries, Portal of Fisheries Information. Statistics on Marine Products Import and Export. 2020. Available online: http://www.fips.go.kr/p/Main/ (accessed on 3 February 2021).
- Nagalakshmi, K.; Annam, P.-K.; Venkateshwarlu, G.; Pathakota, G.-B.; Lakra, W.S. Mislabeling in Indian seafood: An inves-tigation using DNA barcoding. Food Control 2016, 59, 196–200. [Google Scholar] [CrossRef]
- Hofherr, J.; Martinsohn, J.; Cawthorn, D.; Rasco, B.; Naaum, A.M. Regulatory Frameworks for Seafood Authenticity and Traceability; Elsevier BV: Amsterdam, The Netherlands, 2016; pp. 47–82. [Google Scholar]
- Camin, F.; Bontempo, L.; Perini, M.; Piasentier, E. Stable isotope ratio analysis for assessing the authenticity of food of animal origin. Compr. Rev. Food Sci. Food Saf. 2016, 15, 868–877. [Google Scholar] [CrossRef]
- Department of Primary Industries and Regions. Government of South Australia, Authenticity for the Australian Seafood Sector: A Review of Available Tools to Identify Substitution and Mislabelling Information. 2018. Available online: https://www.safefish.com.au/reports/technical-reports/seafood-authenticity (accessed on 10 March 2021).
- Gopi, K.; Mazumder, D.; Sammut, J.; Saintilan, N.; Crawford, J.; Gadd, P. Combined use of stable isotope analysis and elemental profiling to determine provenance of black tiger prawns (Penaeus monodon). Food Control. 2019, 95, 242–248. [Google Scholar] [CrossRef]
- Kang, X.; Zhao, Y.; Shang, D.; Zhai, Y.; Ning, J.; Ding, H.; Sheng, X. Identification of the geographical origins of sea cucumbers in China: The application of stable isotope ratios and compositions of C, N, O and H. Food Control. 2020, 111, 107036. [Google Scholar] [CrossRef]
- Sacco, D.; Brescia, M.A.; Buccolieri, A.; Jambrenghi, A.C. Geographical origin and breed discrimination of Apilian lamb meat samples by means of analytical and spectroscopic determinations. Meat Sci. 2015, 71, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Zanden, H.B.V.; Soto, D.X.; Bowen, G.J.; Hobson, K.A. Expanding the isotopic toolbox: Applications of hydrogen and oxygen stable isotope ratios to food web studies. Front. Ecol. Evol. 2016, 4, 1–19. [Google Scholar] [CrossRef]
- Kim, H.; Kumar, K.S.; Shin, K.-H. Applicability of stable C and N isotope analysis in inferring the geographical origin and authentication of commercial fish (Mackerel, Yellow Croaker and Pollock). Food Chem. 2015, 172, 523–527. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Valenzuela, L.O.; Chesson, L.A.; Tipple, B.J.; Martinelli, L.A. Stable isotopes trace the truth: From adulterated foods to crime scenes. Elements 2015, 11, 259–264. [Google Scholar] [CrossRef]
- Li, L.; Boyd, C.E.; Sun, Z. Authentication of fishery and aquaculture products by multi-element and stable isotope analysis. Food Chem. 2016, 194, 1238–1244. [Google Scholar] [CrossRef]
- Mai, Z.; Lai, B.; Sun, M.; Shao, J.; Guo, L. Food adulteration and traceability tests using stable carbon isotope techniques. Trop. J. Pharm. Res. 2019, 18, 1771–1784. [Google Scholar]
- Tanz, N.; Schmidt, H.-L. δ34S-Value measurements in food origin assignments and sulfur isotope fractionations in plants and animals. J. Agric. Food Chem. 2010, 58, 3139–3146. [Google Scholar] [CrossRef] [PubMed]
- Bartelink, E.J.; Chesson, L.A. Recent applications of isotope analysis to forensic anthropology. Forensic Sci. Res. 2018, 4, 29–44. [Google Scholar] [CrossRef]
- Voerkelius, S.; Lorenz, G.D.; Rummel, S.; Quétel, C.R.; Heiss, G.; Baxter, M.; Brach-Papa, C.; Deters-Itzelsberger, P.; Hoelzl, S.; Hoogewerff, J.; et al. Strontium isotopic signatures of natural mineral waters, the reference to a simple geological map and its potential for authentication of food. Food Chem. 2010, 118, 933–940. [Google Scholar] [CrossRef]
- Palmer, M.; Edmond, J. The strontium isotope budget of the modern ocean. Earth Planet. Sci. Lett. 1989, 92, 11–26. [Google Scholar] [CrossRef]
- Goldstein, S.; Hemming, S. Long-lived isotopic tracers in oceanography, paleoceanography, and ice-sheet dynamics. Treatise Geochem. 2003, 6, 453–489. [Google Scholar] [CrossRef]
- Frank, M. Radiogenic isotopes: Tracers of past ocean circulation and erosional input. Rev. Geophys. 2002, 40, 1–38. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, J.; Han, D.; Zhao, X.; Chen, X.; Liu, Y. Geographical origin traceability and species identification of three scallops (Patinopecten yessoensis, Chlamys farreri, and Argopecten irradians) using stable isotope analysis. Food Chem. 2019, 299, 125107. [Google Scholar] [CrossRef]
- Carter, J.F.; Chesson, L.A. Food Forensics: Stable Isotope as a Guide to Authenticity and Origin Florida; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Bouvier, A.; Vervoort, J.D.; Patchett, P.J. The Lu–Hf and Sm–Nd isotopic composition of CHUR: Constraints from unequilibrated chondrites and implications for the bulk composition of terrestrial planets. Earth Planet. Sci. Lett. 2008, 273, 48–57. [Google Scholar] [CrossRef]
- Esteki, M.; Shahsavari, Z.; Simal-Gandara, J. Review Use of spectroscopic methods in combination with linear discriminant analysis for authentication of food products. Food Control 2018, 91, 100–112. [Google Scholar] [CrossRef]
- Jiang, D.; Du, L.; Guo, Y.; Ma, J.; Li, X.; Han, L.; Xu, Y.; Qian, Y. Potential use of stable isotope and multi-element analyses for regional geographical traceability of bone raw materials for gelatin production. Food Anal. Methods 2020, 13, 762–769. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, B.; Chen, G.; Chen, A.; Yang, S.; Ye, Z. Tracing the geographic origin of beef in China on the basis of the combination of stable isotopes and multielement analysis. J. Agric. Food Chem. 2013, 61, 7055–7060. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. 2019, Vegan: Community Ecology Package. R Package Version 2.5-6. Available online: http://CRAN.Rproject.org/package=vegan (accessed on 10 February 2021).
- Carrera, M.; Gallardo, J.M. Determination of the geographical origin of all commercial hake species by stable isotope ratio (SIR) analysis. J. Agric. Food Chem. 2017, 65, 1070–1077. [Google Scholar] [CrossRef]
- Yi, W.; Jiang, H.; Lihui, A.; Wei, A.; Min, Y.; Mitsuaki, I.; Tatsuya, H.; Shu, T. Determination of trophic relationships within a Bohai bay food web using stable δ15N and δ13C analysis. Chin. Sci. Bull. 2015, 50, 1021–1025. [Google Scholar]
- Dang, C.; De Montaudouin, X.; Savoye, N.; Caill-Milly, N.; Martinez, P.; Sauriau, P.G. Stable isotopes changes in the adductor muscle of diseased bivalve Ruditapes philippinarum. Mar. Biol. 2009, 156, 611–618. [Google Scholar] [CrossRef]
- Park, H.J.; Choy, E.J.; Lee, K.-S.; Kang, C.-K. Trophic transfer between coastal habitats in a seagrass-dominated macrotidal embayment system as determined by stable isotope and fatty acid signatures. Mar. Freshw. Res. 2013, 64, 1169. [Google Scholar] [CrossRef]
- Choi, B.; Lee, C.; Takizawa, Y.; Chikaraishi, Y.; Oh, H.; Chang, K.; Jang, M.; Kim, H.; Lee, K.; Shin, K. Trophic response to ecological conditions of habitats: Evidence from trophic variability of freshwater fish. Ecol. Evol. 2020, 10, 7250–7260. [Google Scholar] [CrossRef]
- Watanabe, S.; Katayama, S.; Kodama, M.; Cho, N.; Nakata, K.; Fukuda, M. Small-scale variation in feeding environments for the Manila clam Ruditapes philippinarum in a tidal flat in Tokyo Bay. Fish. Sci. 2009, 75, 937–945. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.; Li, D.J.; Wei, H.; Lu, R.X. Isotope variability of particulate organic matter at the PN section in the East China Sea. Biogeochemistry 2003, 65, 31–49. [Google Scholar] [CrossRef]
- Luo, D.; Dong, H.; Luo, H.; Xian, Y.; Wan, J.; Guo, X.; Wu, Y. The application of stable isotope ratio analysis to determine the geographical origin of wheat. Food Chem. 2015, 174, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Bowen, G.J.; Ehleringer, J.R.; Chesson, L.A.; Stange, E.; Cerling, T.E. Stable isotope ratios of tap water in the contiguous United States. Water Resour. Res. 2007, 43, 03419. [Google Scholar] [CrossRef]
- Hondula, K.L.; Pace, M.L.; Cole, J.J.; Batt, R.D. Hydrogen isotope discrimination in aquatic primary producers: Implications for aquatic food web studies. Aquat. Sci. 2013, 76, 217–229. [Google Scholar] [CrossRef]
- Jaffrés, J.B.; Shields, G.A.; Wallmann, K. The oxygen isotope evolution of seawater: A critical review of a long-standing controversy and an improved geological water cycle model for the past 3.4 billion years. Earth Sci. Rev. 2007, 83, 83–122. [Google Scholar] [CrossRef]
- Portarena, S.; Gavrichkova, O.; Lauteri, M.; Brugnoli, E. Authentication and traceability of Italian extra-virgin olive oils by means of stable isotopes techniques. Food Chem. 2014, 164, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Page, H.M.; Cooper, S.D.; Wiseman, S.W.; Bennett, D.; Klose, K.; Sadro, S.; Nelson, C.; Even, T. Comparisons of stable isotope (C, H, N) signatures for revealing organic matter sources and trophic relationships in headwater streams. Can. J. Fish. Aquat. Sci. 2017, 74, 2110–2121. [Google Scholar] [CrossRef]
- Connolly, R.M.; Guest, M.A.; Melville, A.J.; Oakes, J.M. Sulfur stable isotopes separate producers in marine food-web analysis. Oecologia 2004, 138, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Buchardt, B.; Bunch, V.; Helin, P. Fingernails and diet: Stable isotope signatures of a marine hunting community from modern Uummannaq, North Greenland. Chem. Geol. 2007, 244, 316–329. [Google Scholar] [CrossRef]
- Weber, P.K.; Hutcheon, I.D.; McKeegan, K.D.; Ingram, B.L. Otolith sulfur isotope method to reconstruct salmon (Oncorhynchus tshawytscha) life history. Can. J. Fish. Aquat. Sci. 2002, 59, 587–591. [Google Scholar] [CrossRef][Green Version]
- Ariyama, K.; Shinozaki, M.; Kawasaki, A. Determination of the geographic origin of rice by chemometrics with strontium and lead isotope ratios and multielement concentrations. J. Agric. Food Chem. 2012, 60, 1628–1634. [Google Scholar] [CrossRef] [PubMed]
- Faure, G.; Mensing, T.M. Isotopes: Principles and Applications, 3rd ed.; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Kennedy, B.P.; Klaue, A.; Blum, J.D.; Folt, C.L.; Nislow, K.H. Reconstructing the lives of fish using Sr isotopes in otoliths. Can. J. Fish. Aquat. Sci. 2002, 59, 925–929. [Google Scholar] [CrossRef]

| Country | δ13C | δ15N | δ18O | δD | δ34S | 143Nd/144Nd | 87Sr/86Sr |
|---|---|---|---|---|---|---|---|
| China, Dalian (n = 10) | −17.7 (0.3) B,C | 8.8 (0.20) B,C | 22.0 (0.3) B,C | −86.8 (4.9) B,C | 21.1 (0.5) A,C | 0.5119 (0.00006) A,B,C | 0.7094 (0.00011) |
| DPR Korea (n = 10) | −17.7 (0.4) B,C | 9.0 (0.2) A,B,C | 23.0 (1.29) A,B,C | −81.3 (6.2) B,C | 21.9 (0.9) B | 0.5118 (0.0002) B,C | 0.7093 (0.000056) |
| Korea (n = 10) | −16.8 (0.4) A | 9.4 (0.6) A,B | 23.9 (2.2) A,B | −97.1 (10.6) A | 20.6 (0.2) A,C | 0.5120 (0.0001) A,C | 0.7093 (0.000025) |
| -Yeosu Hwayang (YH) (n = 3) | −17.1 (0.02) a,b | 9.4 (0.05) a | 24.9 (0.7) | −107.1 (7.9) a,b | 20.7 (0.1) a,b,c | 0.5119 (0.00003) a | 0.7093 (0.000026) |
| - Yeosu Dolsan (YD) (n = 4) | −17.1 (0.2) a,b | 8.7 (0.3) b | 22.3 (2.8) | −96.8 (9.3) a,b,c | 20.7 (0.2) a,b | 0.5120 (0.00003) b | 0.7093 (0.000015) |
| - Jinhae (JH) (n = 3) | −16.3 (0.05) c | 10.1 (0.2) c | 25.2 (0.6) | −87.5 (4.8) b,c | 20.4 (0.1) a,c | 0.5121 (0.00003) c | 0.7093 (0.000023) |
| Sample | ID | δ13C | δ15N | δ34S | δ18O | δD | εNd | 87Sr/86Sr |
|---|---|---|---|---|---|---|---|---|
| Korea | KR1 | 0.6539 | 0.8719 | −0.6792 | 1.417 | −2.805 | −0.4344 | −0.4868 |
| KR2 | 0.5998 | 0.7397 | −0.5018 | 0.6678 | −1.395 | −0.09674 | −0.6146 | |
| KR3 | 0.6539 | 0.9381 | −0.7385 | 1.411 | −1.458 | −0.4884 | 0.02455 | |
| KR4 | 2.078 | 2.657 | −1.009 | 1.137 | −0.4215 | 1.227 | −0.6146 | |
| KR5 | 2.150 | 2.239 | −0.9072 | 1.082 | 0.5346 | 1.598 | −1.126 | |
| KR6 | 1.988 | 1.908 | −1.237 | 1.758 | 0.1487 | 1.261 | −0.6146 | |
| KR7 | 0.8883 | −1.156 | −0.3896 | −1.652 | 0.5005 | 0.5738 | −0.1307 | |
| KR8 | 0.1310 | −0.5168 | −0.7679 | −0.9099 | −1.191 | 0.7136 | −0.3590 | |
| KR9 | 0.7440 | 0.1665 | −0.7679 | 2.112 | −1.024 | 1.018 | −0.3590 | |
| KR10 | 0.4736 | −1.399 | −0.3878 | −1.184 | −1.657 | 0.9838 | −0.6146 | |
| China | CHN1 | −0.1394 | −1.156 | −0.1628 | −0.8429 | 1.121 | −0.02921 | −0.3590 |
| CHN2 | −0.1665 | −1.141 | 0.6598 | −0.3590 | 0.3592 | 0.04729 | −0.4625 | |
| CHN3 | 0.6222 | 0.02967 | 0.5214 | −0.7548 | 0.2213 | −0.2804 | −0.6719 | |
| CHN4 | −0.1711 | −0.9010 | 0.4765 | −0.5418 | 0.3978 | 0.004361 | −0.1179 | |
| CHN5 | −1.213 | −0.9114 | −0.5979 | −0.7958 | −0.1410 | −0.2603 | 4.073 | |
| CHN6 | −0.08372 | −0.5090 | −0.7320 | −0.3374 | −0.3770 | −0.1837 | 1.471 | |
| CHN7 | −0.9140 | −0.6960 | −0.1665 | −0.6114 | −0.3036 | −1.018 | 1.140 | |
| CHN8 | −0.4917 | −0.2347 | −0.5720 | −0.8027 | −0.5137 | 0.3004 | 0.09759 | |
| CHN9 | −1.456 | −0.2082 | −0.09640 | −0.3861 | 0.2868 | 0.2342 | −0.3590 | |
| CHN10 | −1.257 | −0.09797 | −0.9579 | −0.6175 | 0.5296 | 0.2477 | −0.1033 | |
| DPR Korea | NK1 | 0.6028 | 0.04868 | 1.962 | −0.1968 | 1.310 | −0.6541 | 0.2359 |
| NK2 | 0.1674 | −0.8804 | 1.876 | −0.3604 | 1.208 | −0.5670 | 0.01865 | |
| NK3 | −0.5489 | −0.3236 | 2.033 | −0.1838 | 0.9059 | −3.999 | 0.06630 | |
| NK4 | 0.06214 | 0.6149 | 1.647 | −0.3802 | 1.146 | 0.3288 | −0.6583 | |
| NK5 | −1.430 | −0.08148 | 1.194 | −0.5140 | 0.9097 | −0.3504 | −0.8709 | |
| NK6 | −0.7793 | −0.6909 | 1.336 | −0.3869 | 1.050 | −0.00767 | −0.6713 | |
| NK7 | −1.323 | 0.1682 | 0.9653 | −0.7450 | 0.08906 | 0.09464 | 1.353 | |
| NK8 | −0.9508 | 0.2988 | −0.4765 | 1.502 | 0.1739 | 0.5651 | −0.2311 | |
| NK9 | −0.7524 | 0.6074 | −0.6792 | 1.417 | 0.9981 | 0.2949 | −0.1033 | |
| NK10 | −0.1394 | −0.3845 | −0.8439 | 0.05863 | −0.6029 | −1.123 | 1.047 |
| Combination | China (n = 10) | DPR Korea (n = 10) | Korea (n = 10) |
|---|---|---|---|
| C-N | 6 (6) | 6 (5) | 8 (8) |
| C-N-O | 7 (7) | 7 (3) | 8 (7) |
| C-N-D | 8 (6) | 8 (7) | 9 (9) |
| C-N-S | 9 (8) | 7 (7) | 10 (8) |
| C-N-O-D | 8 (6) | 8 (6) | 9 (9) |
| C-N-D-S | 9 (9) | 7 (2) | 9 (9) |
| C-N-S-O | 10 (7) | 9 (8) | 9 (7) |
| C-N-S-Sr | 9 (7) | 7 (7) | 10 (10) |
| C-N-S-O-D | 9 (7) | 9 (9) | 10 (9) |
| C-N-S-O-Sr | 10 (6) | 9 (7) | 10 (9) |
| C-N-S-D-Sr | 9 (7) | 7 (7) | 9 (9) |
| C-N-O-D-Sr | 6 (5) | 8 (6) | 9 (9) |
| C-N-O-D-Nd | 8 (7) | 8 (7) | 9 (9) |
| C-N-S-O-D-Sr | 10 (7) | 9 (8) | 10 (9) |
| C-N-S-O-D-Nd | 9 (8) | 9 (9) | 9 (9) |
| C-N-S-O-D-Sr-Nd | 10 (6) | 9 (7) | 10 (9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Won, E.-J.; Kim, S.H.; Go, Y.-S.; Kumar, K.S.; Kim, M.-S.; Yoon, S.-H.; Bayon, G.; Kim, J.-H.; Shin, K.-H. A Multi-Elements Isotope Approach to Assess the Geographic Provenance of Manila Clams (Ruditapes philippinarum) via Recombining Appropriate Elements. Foods 2021, 10, 646. https://doi.org/10.3390/foods10030646
Won E-J, Kim SH, Go Y-S, Kumar KS, Kim M-S, Yoon S-H, Bayon G, Kim J-H, Shin K-H. A Multi-Elements Isotope Approach to Assess the Geographic Provenance of Manila Clams (Ruditapes philippinarum) via Recombining Appropriate Elements. Foods. 2021; 10(3):646. https://doi.org/10.3390/foods10030646
Chicago/Turabian StyleWon, Eun-Ji, Seung Hee Kim, Young-Shin Go, K. Suresh Kumar, Min-Seob Kim, Suk-Hee Yoon, Germain Bayon, Jung-Hyun Kim, and Kyung-Hoon Shin. 2021. "A Multi-Elements Isotope Approach to Assess the Geographic Provenance of Manila Clams (Ruditapes philippinarum) via Recombining Appropriate Elements" Foods 10, no. 3: 646. https://doi.org/10.3390/foods10030646
APA StyleWon, E.-J., Kim, S. H., Go, Y.-S., Kumar, K. S., Kim, M.-S., Yoon, S.-H., Bayon, G., Kim, J.-H., & Shin, K.-H. (2021). A Multi-Elements Isotope Approach to Assess the Geographic Provenance of Manila Clams (Ruditapes philippinarum) via Recombining Appropriate Elements. Foods, 10(3), 646. https://doi.org/10.3390/foods10030646








