Functional and Quality Characteristics of Ginger, Pineapple, and Turmeric Juice Mix as Influenced by Blend Variations
Abstract
1. Introduction
2. Materials and Methods
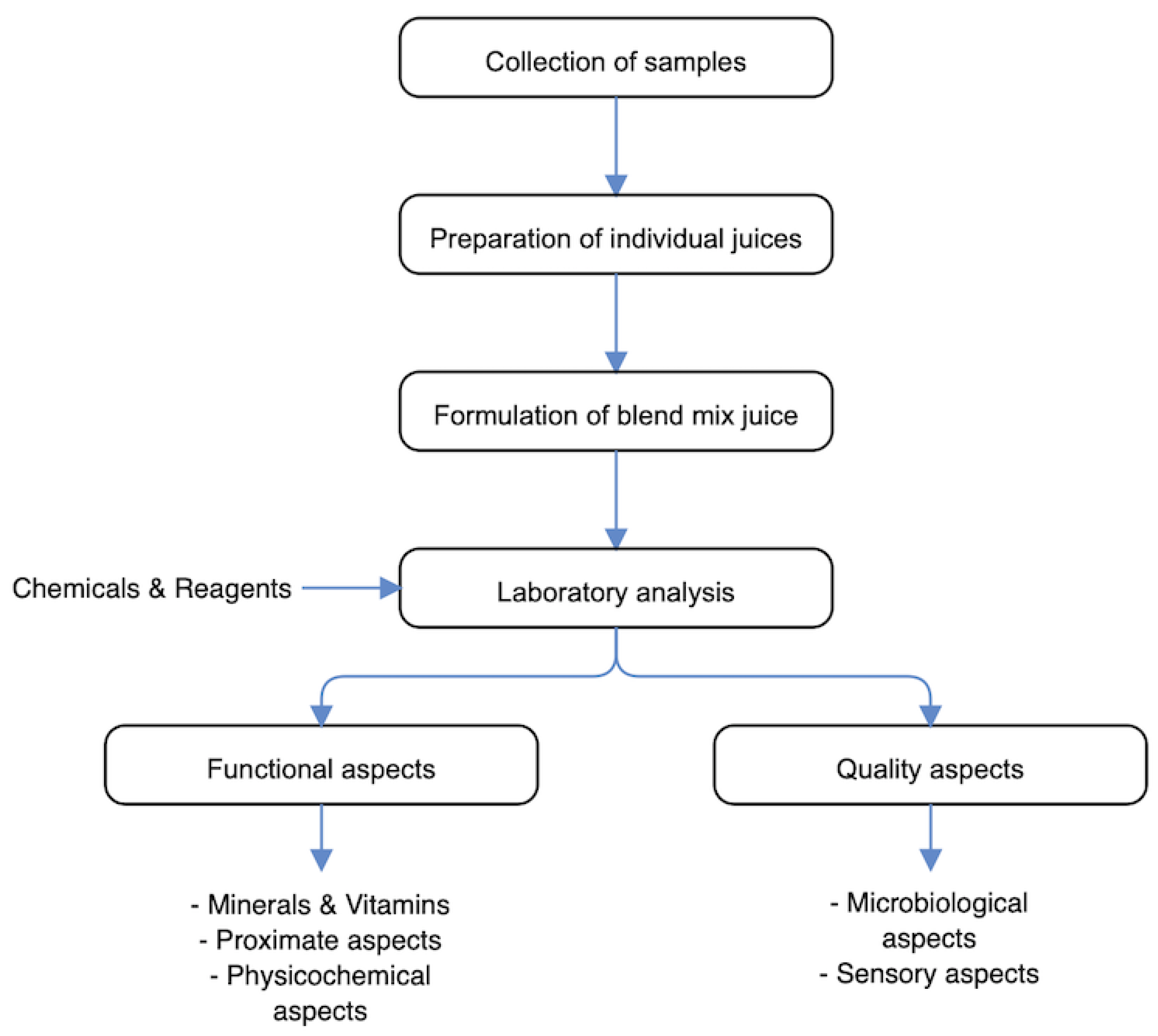
2.1. Overview of Experimental Program
2.2. Collection of Samples
2.3. Chemicals and Reagents
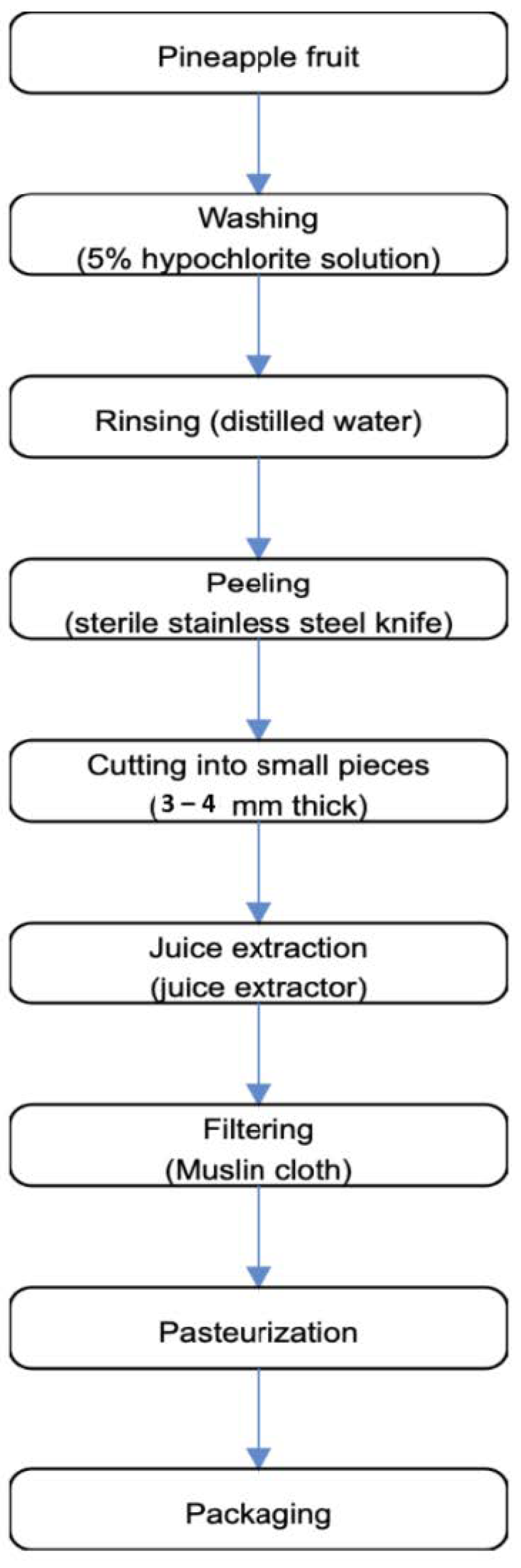
2.4. Preparation of Pineapple Fruit Juice
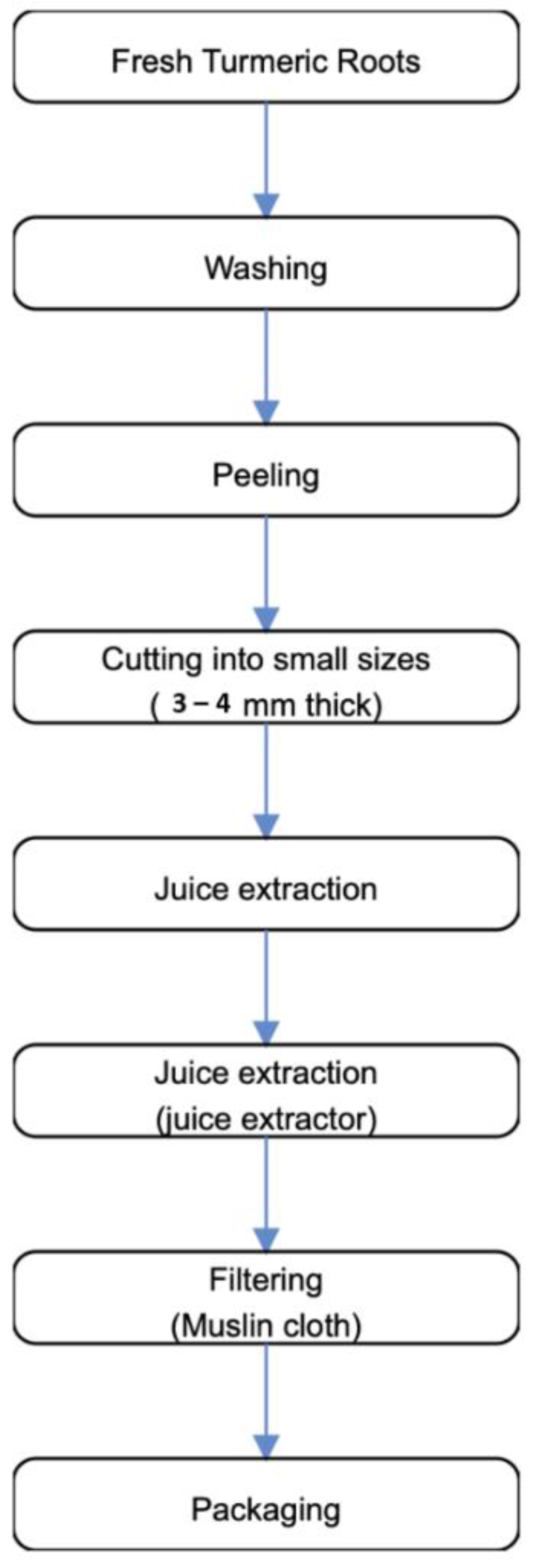
2.5. Preparation of Turmeric and Ginger Juice
2.6. Formulation of Pineapple, Turmeric and Ginger Blend Juice Mix
2.7. Functional Analysis.
2.7.1. Minerals and Vitamins Measurements
Determination of β-Carotene
Determination of Calcium and Magnesium
Determination of Vitamin C
2.7.2. Proximate Measurements
Determination of Moisture
Determination of Crude Protein
Determination of Crude Fat
Determination of Ash
2.7.3. Physicochemical Measurements
Determination of pH
Determination of Soluble Solids
Determination of Titratable Acid (TA)
Determination of Viscosity
2.8. Quality Analysis
2.8.1. Microbiological Evaluation
2.8.2. Sensory Evaluation
2.9. Statistical Analysis
3. Results and Discussion
3.1. Functional Aspects Minerals and Vitamins Variations
Proximate and Physicochemical Variations
3.2. Quality Aspects Microbiological Variations
Sensory Variations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, B. Fruit and Juice Processing. In Chemistry and Technology of Soft Drinks and Fruit Juices, 2nd ed.; Ashurst, R.P., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2005; pp. 35–67. [Google Scholar] [CrossRef]
- Mauseth, J.D. Botany, an Introduction to Plant Biology; Jomes and Barlet Publishers: Boston, MA, USA, 2003; p. 285. [Google Scholar]
- Agada, R.E. Quality Evaluation of Juice from Blends of Cucumber and Pineapple Fruits; Department of Food Science and Technology, Federal University of Agriculture: Makurdi, Benue State, Nigeria, 2018. [Google Scholar]
- Akusu, O.M.; Kiin-Kabari, D.B.; Ebere, C.O. Quality Characteristics of Orange/Pineapple Fruit Juice Blends. Am. J. Food. Sci. Technol. 2016, 4, 43–47. [Google Scholar] [CrossRef]
- Offia-Olua, B.I.; Ekwunife, O. Production and evaluation of the physico-chemical and sensory qualities of mixed fruit leather and cakes produced from apple (Musa Pumila), banana (Musa Sapientum), pineapple (Ananas Comosus). Niger. Food J. 2015, 33, 22–28. [Google Scholar] [CrossRef][Green Version]
- Bhaskar, J.J.; Chilkunda, N.D.; Salimath, P.V. Banana (Musa sp. var. elakki bale) Flower and Pseudostem: Dietary Fiber and Associated Antioxidant Capacity. J. Agric. Food Chem. 2012, 60, 427–432. [Google Scholar] [CrossRef]
- Ogawa, E.M.; Costa, H.B.; Ventura, J.A.; Caetano, L.C.; Pinto, F.E.; Oliveira, B.G.; Barroso, M.E.S.; Scherer, R.; Endringer, D.C.; Romão, W. Chemical profile of pineapple cv. Vitória in different maturation stages using electrospray ionization mass spectrometry. J. Sci. Food Agric. 2018, 98, 1105–1116. [Google Scholar] [CrossRef]
- Ajav, E.; Kolapo, A.; Ogunlade, C.A. Physical Properties of Ginger (Zingiber officinale). Glob. J. Sci. Front. Res. 2007, 14, 2249–4626. [Google Scholar]
- Bailey-Shaw, Y.A.; Williams, L.A.D.; Junor, G.-A.O.; Green, C.E.; Hibbert, S.L.; Salmon, C.N.A.; Smith, A.M. Changes in the Contents of Oleoresin and Pungent Bioactive Principles of Jamaican Ginger (Zingiber officinale Roscoe.) during Maturation. J. Agric. Food Chem. 2008, 56, 5564–5571. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Bioactives in spices, and spice oleoresins: Phytochemicals and their beneficial effects in food preservation and health promotion. J. Food Bioact. 2018, 3, 8–75. [Google Scholar] [CrossRef]
- Varakumar, S.; Umesh, K.V.; Singhal, R.S. Enhanced extraction of oleoresin from ginger (Zingiber officinale) rhizome powder using enzyme-assisted three phase partitioning. Food Chem. 2017, 216, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Samaniego-Sánchez, C.; Inurreta-Salinas, Y.; Quesada-Granados, J.; Blanca-Herrera, R.; Villalón-Mir, M.; De La Serrana, H.L.-G.; Martínez, M.L. The influence of domestic culinary processes on the Trolox Equivalent Antioxidant Capacity of green tea infusions. J. Food Compos. Anal. 2011, 24, 79–86. [Google Scholar] [CrossRef]
- Charles, D.J. Antioxidant Properties of Spices, Herbs and Other Sources; Springer Science & Business Media: New York, NY, USA, 2013; ISBN 978-1-4614-4310-0. [Google Scholar]
- Parthasarathy, V.A.; Chempakam, B.; Zachariah, T.J. Chemistry of Spices; CABI: Oxfordshire, UK, 2008; p. 464. ISBN 978-1-84593-405-7. [Google Scholar]
- Peter, K.V. (Ed.) Handbook of Herbs and Spices, 2nd ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2016; Volume 2, p. 624. ISBN 9780081016176. [Google Scholar]
- Cheng, X.-L.; Liu, Q.; Peng, Y.-B.; Qi, L.-W.; Li, P. Steamed ginger (Zingiber officinale): Changed chemical profile and increased anticancer potential. Food Chem. 2011, 129, 1785–1792. [Google Scholar] [CrossRef]
- Embuscado, M.E. Spices and herbs: Natural sources of antioxidants—A mini review. J. Funct. Foods 2015, 18, 811–819. [Google Scholar] [CrossRef]
- Leja, K.B.; Czaczyk, K. The industrial potential of herbs and spices—A mini review. Acta Sci. Pol. Technol. Aliment. 2015, 15, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Yashin, A.; Yashin, Y.; Xia, X.; Nemzer, B. Antioxidant Activity of Spices and Their Impact on Human Health: A Review. Antioxidants 2017, 6, 70. [Google Scholar] [CrossRef]
- Nisar, T.; Iqbal, M.; Raza, A. Turmeric: A promising spice for phytochemical and antimicrobial activities. Am. Eurasian J. Agric. Environ. Sci. 2015, 15, 1278–1288. [Google Scholar] [CrossRef]
- Amalraj, A.; Pius, A.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; El Rayess, Y.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef]
- Idowu-Adebayo, F.; Toohey, M.J.; Fogliano, V.; Linnemann, A.R. Enriching street-vended zobo (Hibiscus sabdariffa) drink with turmeric (Curcuma longa) to increase its health-supporting properties. Food Funct. 2021, 12, 761–770. [Google Scholar] [CrossRef]
- Muogbo, P.C.; Gbabo, A.; Nwakuba, N.R.; Onyekwere, I.; Obasa, P.A. Livelihood enhancement through mechanization of turmeric production in Nigeria. In Proceedings of the 3rd NIAE-SE Regional Conference, University of Nigeria Nsukka, Enugu State, Nigeria, 27–30 August 2018. [Google Scholar]
- Kalaycıoğlu, Z.; Torlak, E.; Akın-Evingür, G.; Özen, I.; Erim, F.B. Antimicrobial and physical properties of chitosan films incorporated with turmeric extract. Int. J. Biol. Macromol. 2017, 101, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Sakhya, K.S. Ethnopharmacological properties of Curcuma longa: A review. Int. J. Pharm. Sci. Res. 2013, 4, 103–112. [Google Scholar]
- Sabale, P.; Modi, A.; Sabale, V. Curcuma longa Linn. A phytochemical and phytopharmacological review. Res. J. Pharmacogn. Phytochem. 2013, 5, 59–68. [Google Scholar]
- Okwori, E.; Onu, R.; Adamu, M.; Chindo, H.; Dikko, H.; Odunze, I.; Baidu, A.; Natala, C.; Eze, P. Production and shelf life determination of fruit/vegetable juices using watermelon, cucumber, pineapple and carrot. Afr. J. Food Sci. Technol. 2017, 8, 34–39. [Google Scholar] [CrossRef]
- Top 10 Home Remedies Team. Available online: https://www.top10homeremedies.com/author/ap (accessed on 21 December 2020).
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- AOAC. Official Methods of Analysis of Association of Official Analytical Chemistry International, 5th ed.; AOAC International: Washington, DC, USA, 1990. [Google Scholar]
- Ikewuchi, C.J.; Ikewuchi, C.C. Iodometric determination of the ascorbic acid (vitamin C) content of some fruits con-sumed in a university community in Nigeria. Global J. Pure Appl. Sci. 2011, 17, 47–49. [Google Scholar]
- Iwe, M.O. Handbook of Sensory Methods and Analysis; Rejoint Communication Services Ltd.: Enugu, Nigeria, 2002. [Google Scholar]
- Adegoke, G.O. Understanding Food Microbiology, 2nd ed.; Alleluian Ventures: Ibadan, Nigeria, 2004; p. 216. [Google Scholar]
- Abubakar, S.; Akanbi, B.O.; Nasir-Naeem, K.O.; Abdulsalam, Z.N. Phytochemical and In-vitro Antibacterial Activity of the Leaves and Stem Extracts of Sesbania grandiflora (L.) against Some Clinical Isolates. Br. Microbiol. Res. J. 2015, 8, 424–433. [Google Scholar] [CrossRef]
- Rahman, M.M.; Sheikh, M.M.I.; Sharmin, S.A.; Islam, S.; Rahman, M.A.; Rahman, M.M.; Alam, M.F. Antibacterial activity of leaf juice and extracts of Moringa oleifera Lam. against some human pathogenic bacteria. CMU J. Nat. Sci. 2009, 8, 219–227. [Google Scholar]
- Balasubramanian, K. Molecular Orbital Basis for Yellow Curry Spice Curcumin’s Prevention of Alzheimer’s Disease. J. Agric. Food Chem. 2006, 54, 3512–3520. [Google Scholar] [CrossRef]
- Vyas, K. The Cure is in the Roots: Turmeric. J. Nutr. Disord. Ther. 2015, 5, 3. [Google Scholar] [CrossRef]
- Oboh, G.; Akinyemi, A.J.; Ademiluyi, A.O. Antioxidant and inhibitory effect of red ginger (Zingiber officinale var. Rubra) and white ginger (Zingiber officinale Roscoe) on Fe2+ induced lipid peroxidation in rat brain in vitro. Exp. Toxicol. Pathol. 2012, 64, 31–36. [Google Scholar] [CrossRef]
- Achinewhu, S.C.; Hart, A.D. Effect of processing and storage on the ascorbic acid (vitamin C) content of some pineapple varieties grown in the Rivers State of Nigeria. Plant Foods Hum. Nutr. 1994, 46, 335–337. [Google Scholar] [CrossRef]
- Rodríguez, Ó.; Gomes, W.; Rodrigues, S.; Fernandes, F.A. Effect of acoustically assisted treatments on vitamins, antioxidant activity, organic acids and drying kinetics of pineapple. Ultrason. Sonochem. 2017, 35, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Rao, P.S.; Mishra, H.N. Effect of combined high pressure–temperature treatments on color and nutritional quality attributes of pineapple (Ananas comosus L.) puree. Innov. Food Sci. Emerg. Technol. 2015, 28, 10–21. [Google Scholar] [CrossRef]
- Uckiah, A.; Goburdhun, D.; Ruggoo, A. Vitamin C content during processing and storage of pineapple. Nutr. Food Sci. 2006, 39, 398–412. [Google Scholar] [CrossRef]
- Nwaekpe, J.O.; Anyaegbunam, H.N.; Okoye, B.C.; Asumugha, G.N. Promotion of Turmeric for the Food/Pharmaceutical Industry in Nigeria. Am. J. Exp. Agric. 2015, 8, 335–341. [Google Scholar] [CrossRef]
- Ekeledo, E.N.; Omodamiro, R.M.; Oti, E. Development and evaluation of turmeric: Ginger based pineapple drinks and food flavourings. Asian J. Plant Sci. Res. 2013, 3, 139–141. Available online: www.pelagiaresearchlibrary.com (accessed on 2 December 2020).
- Biology—LibreTexts. Module 8.3. The Effects of pH and Temperature on Microbial Growth. Available online: https://bio.libretexts.org/Courses/Manchester_Community_College_(MCC)/Remix_of_Openstax%3AMicrobiology_by_Parker_Schneegurt_et_al/08%3A_Microbial_Growth/8.03%3A_The_Effects_of_pH_on_Microbial_Growth (accessed on 15 February 2021).
- Ehirim, F.N.; Mokorie, S.U.; Ebiringa, D.C. Effect of pineapple juice dilution of varying levels of yoghurt quality. In Proceedings of the NIFST Annual Conference, Lagos, Nigeria, 23–27 October 2006; pp. 35–36. [Google Scholar]
- Kuttigounder, D.; Lingamallu, J.R.; Bhattacharya, S. Turmeric Powder and Starch: Selected Physical, Physicochemical, and Microstructural Properties. J. Food Sci. 2011, 76, C1284–C1291. [Google Scholar] [CrossRef]
- Andrews, W. Manuals of Food Quality Control. 4. Microbiological Analysis; FAO Food & Nutrition Paper 1414, Rev. 1; Food and Agriculture Organization of the United Nations: Rome, Italy, 1992; p. 344. ISBN 92-5-103189-4. [Google Scholar]
- Gupta, A.; Mahajan, S.; Sharma, R. Evaluation of antimicrobial activity of Curcuma longa rhizome extract against Staphylococcus aureus. Biotechnol. Rep. 2015, 6, 51–55. [Google Scholar] [CrossRef]
- Lourenço, T.; Mendonça, E.; Nalevaiko, P.; Melo, R.; Silva, P.; Rossi, D. Antimicrobial effect of turmeric (Curcuma longa) on chicken breast meat contamination. Braz. J. Poult. Sci. 2013, 15, 79–82. [Google Scholar] [CrossRef][Green Version]
- Pankaj, S.; Balqees, A.T.; Najat, A.N.; Rahma, A.M.; Sah, P.; Al-Tamimi, B.; Al-Nassri, N.; Al-Mamari, R. Effect of temperature on antibiotic properties of garlic (Allium sativum L.) and ginger (Zingiber officinale Rosc.). Afr. J. Biotechnol. 2012, 11, 16192–16195. [Google Scholar] [CrossRef]
- Yassen, D.; Ibrahim, E.A. Antibacterial activity of crude extracts of ginger (Zingiber officinale Roscoe) on Escherichia coli and Staphylococcus aureus: A study in vitro. Indo Am. J. Pharm. Res. 2016, 6, 5830–5835. [Google Scholar]
| Samples (PJ:TJ:GJ) | Pineapple (mL) | Turmeric (mL) | Ginger (mL) | Total (mL) |
|---|---|---|---|---|
| 100:0:0 | 100 | 0 | 0 | 100 |
| 80:10:10 | 80 | 10 | 10 | 100 |
| 70:20:10 | 70 | 20 | 10 | 100 |
| 60:30:10 | 60 | 30 | 10 | 100 |
| 50:40:10 | 50 | 40 | 10 | 100 |
| Samples | Minerals and Vitamin Composition | |||
|---|---|---|---|---|
| PJ:TJ:GJ | Ca (mg/100 mL) | Mg (mg/100 mL) | Vitamin C (mg/100 mL) | β-Carotene (mg/100 mL) |
| 100:0:0 | 7.37 e ± 0.09 | 5.37 e ± 0.07 | 73.60 d ± 0.71 | 67.92 e ± 0.76 |
| 80:10:10 | 8.78 d ± 0.04 | 6.59 d ± 0.01 | 86.74 c ± 0.23 | 83.19 d ± 3.30 |
| 70:20:10 | 12.73 c ± 0.04 | 7.67 c ± 0.02 | 101.81 b ± 0.27 | 199.14 c ± 1.15 |
| 60:30:10 | 15.63 b ± 0.19 | 7.89 b ± 0.01 | 103.22 b ± 0.82 | 1318.10 b ± 1.97 |
| 50:40:10 | 18.09 a ± 0.01 | 8.54 a ± 0.01 | 122.98 a ± 1.45 | 1454.10 a ± 1.69 |
| LSD | 0.257 | 0.081 | 2.65 | 5.089 |
| Samples | Tested Parameters (%) | |||
|---|---|---|---|---|
| P:T:G | Moisture | Protein | Fat | Ash |
| PTG100:0:0 | 95.89 b ± 0.00 | 0.008 e ± 0.001 | 0.051 e ± 0.001 | 0.125 d ± 0.004 |
| PTG80:10:10 | 96.86 ab ± 1.37 | 0.013 d ± 0.001 | 0.061 d ± 0.001 | 0.287 c ± 0.006 |
| PTG70:20:10 | 97.91 a ± 0.01 | 0.018 c ± 0.001 | 0.072 c ± 0.001 | 0.322 c ± 0.001 |
| PTG60:30:10 | 98.12 a ± 0.21 | 0.087 b ± 0.001 | 0.101 b ± 0.001 | 0.456 b ± 0.063 |
| PTG50:40:10 | 98.18 a ± 0.07 | 0.261 a ± 0.001 | 0.168 a ± 0.002 | 0.585 a ± 0.049 |
| LSD | 1.597 | 0.003 | 0.007 | 0.081 |
| Samples | Tested Parameters | |||
|---|---|---|---|---|
| PJ:TJ:GJ | pH | SS (°Brix) | TA (%) | Viscosity (mPa.s) |
| 100:0:0 | 3.81 d ± 0.007 | 11.95 a ± 0.07 | 0.9005 a ± 0.07 | 300.11 d ± 0.12 |
| 80:10:10 | 3.83 c ± 0014 | 9.32 b ± 0.028 | 0.8425 b ± 0.35 | 301.68 d ± 0.78 |
| 70:20:10 | 3.85 c ± 0.001 | 8.10 c ± 0.001 | 0.6727 c ± 0.09 | 351.06 c ± 1.35 |
| 60:30:10 | 3.89 b ± 0.014 | 7.42 d ± 0.016 | 0.5775 d ± 0.21 | 501.61 b ± 2.27 |
| 50:40:10 | 4.01 a ± 0.076 | 4.90 e ± 0.004 | 0.5425 e ± 0.35 | 850.06 a ± 0.06 |
| LSD | 0.024 | 0.081 | 0.0640 | 3.178 |
| Samples (PJ:TJ:GJ) | Bacterial Count (log cfu/mL) | Fungi Count (log cfu/mL) |
|---|---|---|
| 100:0:0 | 5.0 × 103 | 5.0 × 103 |
| 80:10:10 | 3.0 × 104 | 1.0 × 104 |
| 70:20:10 | 1.2 × 104 | 8.0 × 103 |
| 60:30:10 | 1.6 × 104 | 2.8 × 104 |
| 50:40:10 | 8.0 × 103 | 4.2 × 103 |
| Samples (PJ:TJ:GJ) | Inhibition Zones of Juice Samples (mm) | Control (mm) |
|---|---|---|
| 100:0:0 | 12.50 | 19.90 |
| 80:10:10 | 12.00 | 19.90 |
| 70:20:10 | 8.00 | 19.85 |
| 60:30:10 | NSI | 19.90 |
| 50:40:10 | NSI | 20.00 |
| Samples PJ:TJ:GJ | Appearance | Taste | Aroma | Mouth Feel | General Acceptability |
|---|---|---|---|---|---|
| 100:0:0 | 7.87 ab | 7.27 a | 7.33 a | 7.40 a | 7.60 a |
| 80:10:10 | 8.27 a | 5.87 b | 6.67 a | 6.20 b | 6.33 b |
| 70:20:10 | 7.73 ab | 4.80 bc | 6.47 a | 5.93 b | 5.87 bc |
| 60:30:10 | 7.93 ab | 4.47 c | 5.93 a | 5.73 b | 5.33 bc |
| 50:40:10 | 6.73 b | 3.80 c | 5.87 a | 4.20 c | 5.00 c |
| LSD | 1.27 | 1.18 | 1.32 | 1.15 | 1.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogori, A.F.; Amove, J.; Aduloju, P.; Sardo, G.; Okpala, C.O.R.; Bono, G.; Korzeniowska, M. Functional and Quality Characteristics of Ginger, Pineapple, and Turmeric Juice Mix as Influenced by Blend Variations. Foods 2021, 10, 525. https://doi.org/10.3390/foods10030525
Ogori AF, Amove J, Aduloju P, Sardo G, Okpala COR, Bono G, Korzeniowska M. Functional and Quality Characteristics of Ginger, Pineapple, and Turmeric Juice Mix as Influenced by Blend Variations. Foods. 2021; 10(3):525. https://doi.org/10.3390/foods10030525
Chicago/Turabian StyleOgori, Akama Friday, Julius Amove, Precious Aduloju, Giacomo Sardo, Charles Odilichukwu R. Okpala, Gioacchino Bono, and Małgorzata Korzeniowska. 2021. "Functional and Quality Characteristics of Ginger, Pineapple, and Turmeric Juice Mix as Influenced by Blend Variations" Foods 10, no. 3: 525. https://doi.org/10.3390/foods10030525
APA StyleOgori, A. F., Amove, J., Aduloju, P., Sardo, G., Okpala, C. O. R., Bono, G., & Korzeniowska, M. (2021). Functional and Quality Characteristics of Ginger, Pineapple, and Turmeric Juice Mix as Influenced by Blend Variations. Foods, 10(3), 525. https://doi.org/10.3390/foods10030525







