A Study of Overripe Seed Byproducts from Sun-Dried Grapes by Dispersive Raman Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Overripe Seeds from Sun-Dried Grape Byproducts
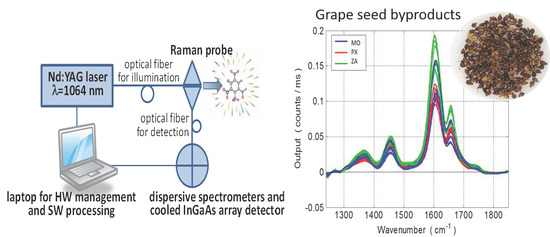
2.2. Instrumental Raman Analysis and Data Collection
2.3. Phenolic Extraction and Determination by RRLC Analysis
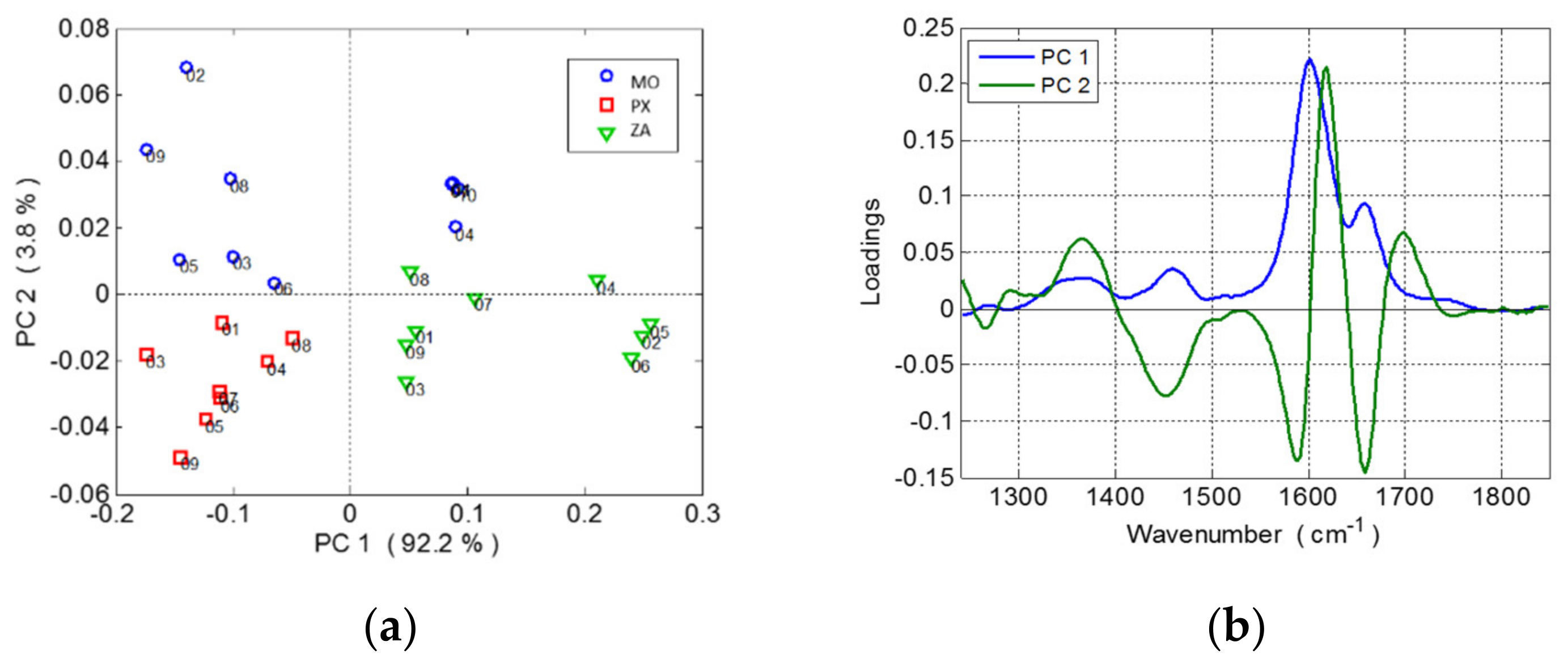
2.4. Chemometric Analysis
3. Results and Discussion
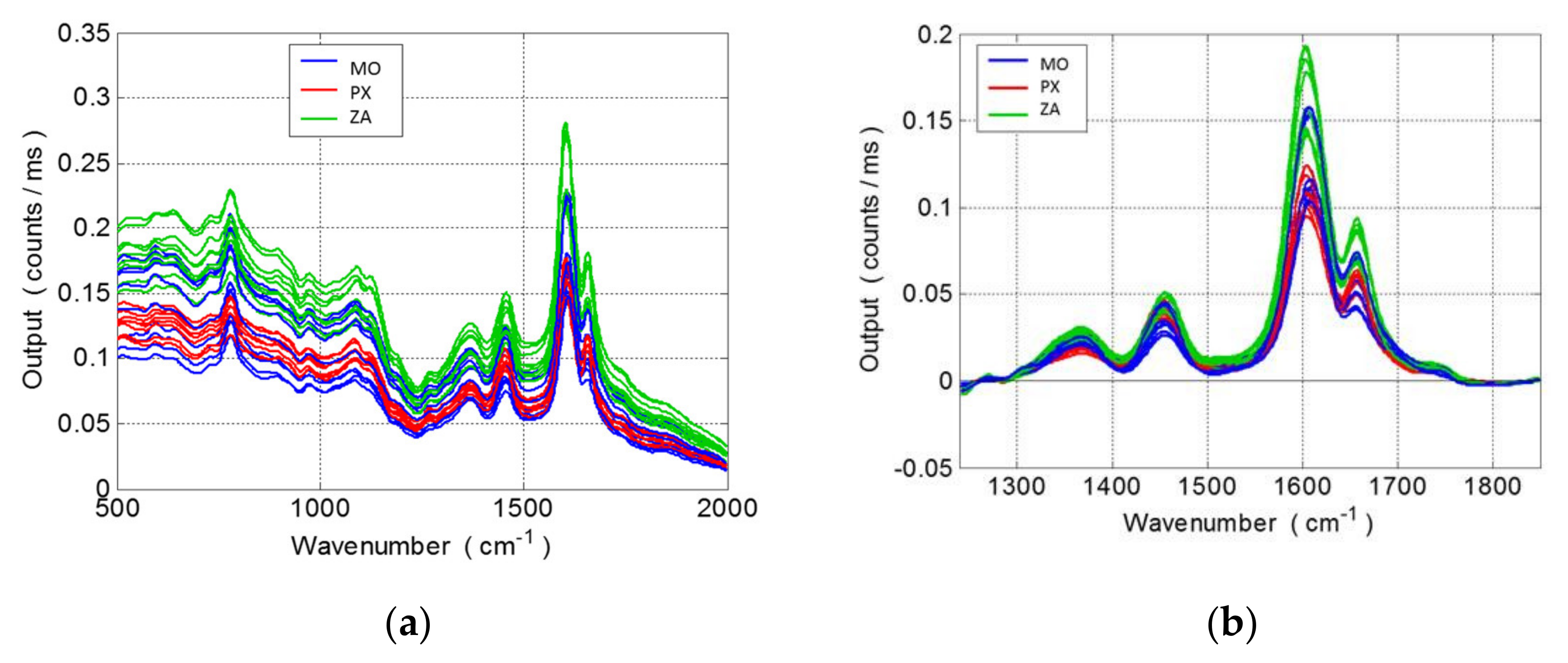
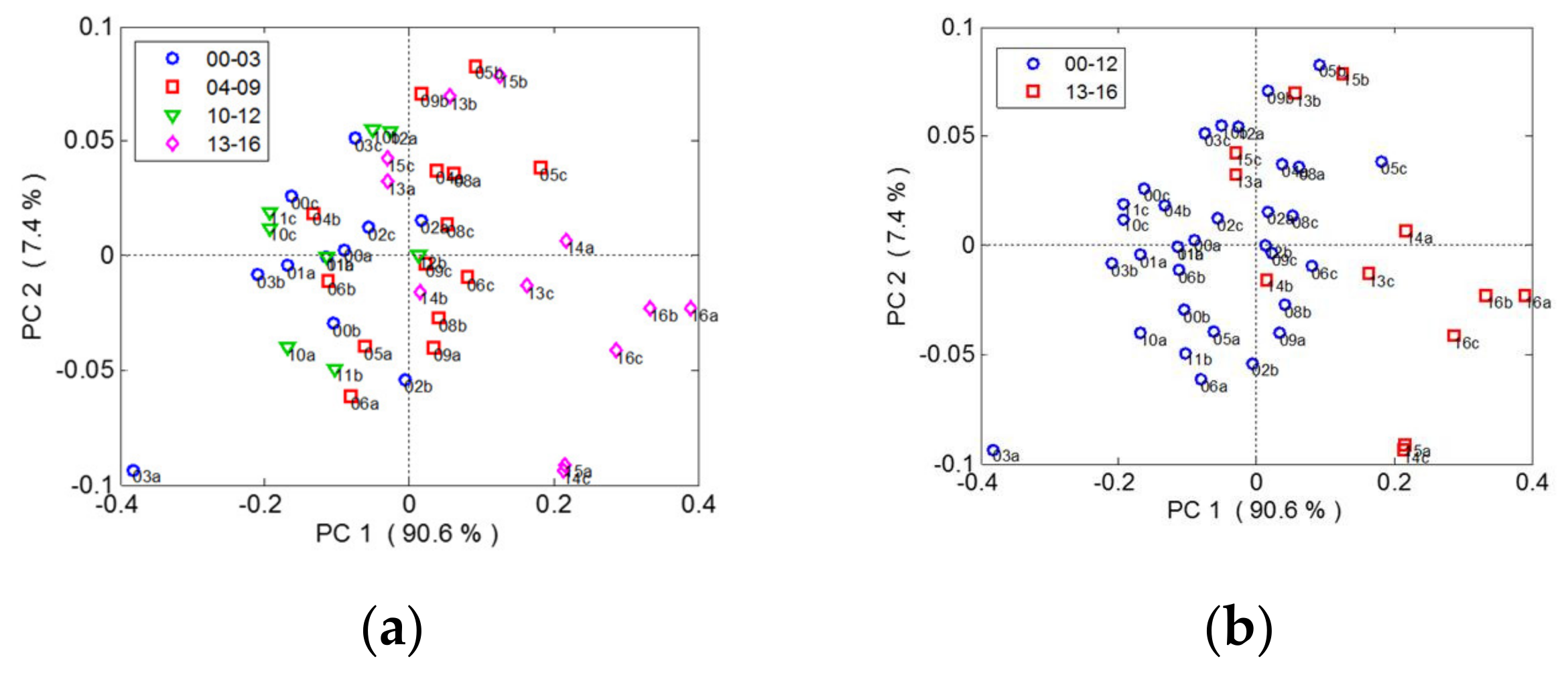
3.1. Raman Spectra and Data Processing of Overripe Seeds Varieties
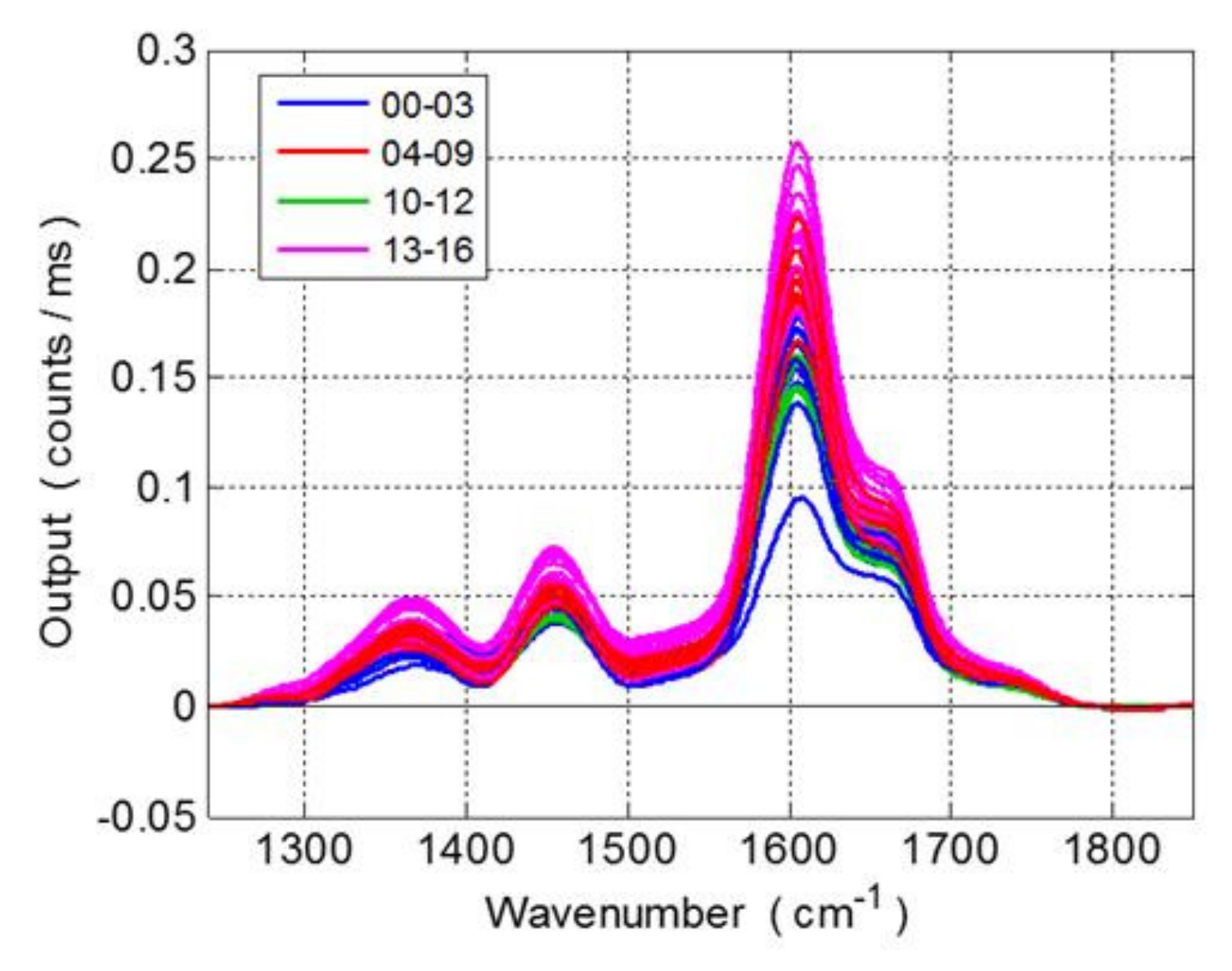
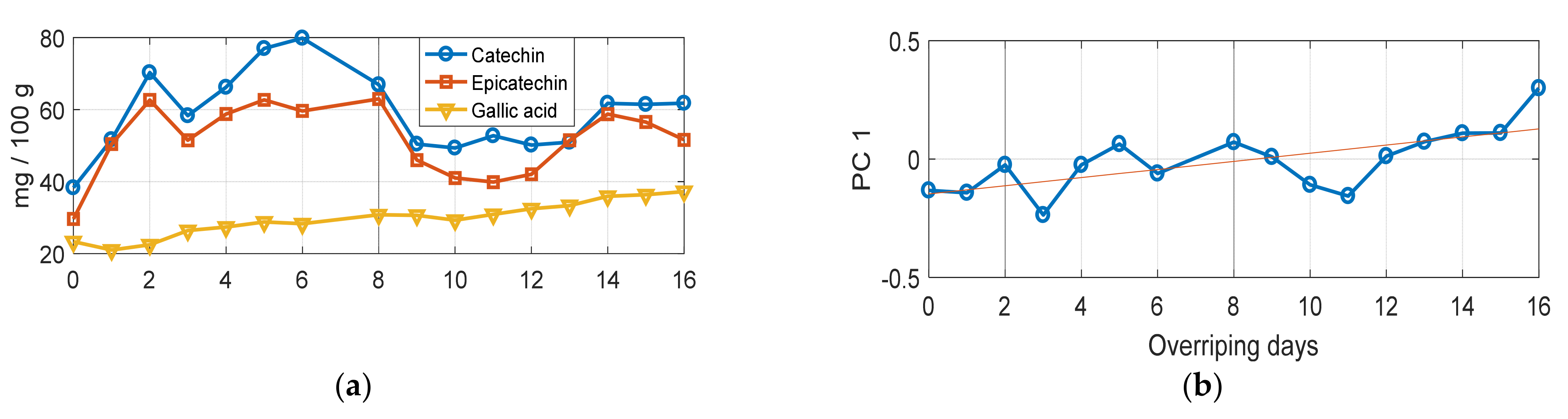
3.2. Monitoring the Grape Seed Overripening Process by Raman Spectroscopy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anastasiadi, M.; Pratsinis, H.; Kletsas, D.; Skaltsounis, A.L.S.; Haroutounian, A. Bioactive non-coloured polyphenols content of grapes, wines and vinification by-products: Evaluation of the antioxidant activities of their extracts. Food Res. Int. 2010, 43, 805–813. [Google Scholar] [CrossRef]
- Delgado-Vargas, D.; Jiménez, A.R.; Paredes-López, O.; Francis, F.J. Natural pigments: Carotenoids, anthocyanins, and betalains-characteristics, biosynthesis, processing, and stability. Crit. Rev. Food. Sci. Nutr. 2000, 40, 173–289. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Gordillo, B.; Cejudo-Bastante, M.J.; Rodríguez-Pulido, F.J.; Jara-Palacios, M.J.; Ramírez-Pérez, P.; González-Miret, M.L.; Heredia, F.J. Impact of adding white pomace to red grapes on the phenolic composition and color stability of Syrah wines from a warm climate. J. Agric. Food Chem. 2014, 62, 2663–2671. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Bejarano, M.J.; Castro-Mejías, R.; Rodríguez-Dodero, M.C.; García-Barroso, C. Volatile composition of Pedro Ximénez and Muscat sweet Sherry wines from sun and chamber dried grapes: A feasible alternative to the traditional sun-drying. J. Food Sci. Technol. 2016, 53, 2519–2531. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu, D.; Peinado, R.A.; Peinado, J.; de Lerma, N.L. Grape pomace extract improves the in vitro and in vivo antioxidant properties of wines from sun light dried Pedro Ximénez grapes. J. Funct. Foods. 2015, 17, 380–387. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Hernanz, D.; Escudero-Gilete, M.L.; Heredia, F.J. The use of grape seed byproducts rich in flavonoids to improve the antioxidant potential of red wines. Molecules 2016, 21, 1526. [Google Scholar] [CrossRef] [PubMed]
- Rivero, F.J.; Jara-Palacios, M.J.; Gordillo, B.; Heredia, F.J.; González-Miret, M.L. Impact of a post-fermentative maceration with overripe seeds on the color stability of red wines. Food Chem. 2019, 272, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Brandon, M.; Lores, M.; Insam, H.; Domínguez, J. Strategies for recycling and valorization of grape marc. Crit Rev. Biotechnol. 2019, 39, 437–450. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Rodríguez-Pulido, F.J.; Hernanz, D.; Escudero-Gilete, M.L.; Heredia, F.J. Determination of phenolic substances of seeds, skins and stems from white grape marc by near-infrared hyperspectral imaging. Aust. J. Grape Wine Res. 2016, 22, 11–15. [Google Scholar] [CrossRef]
- Baca-Bocanegra, B.; Hernández-Hierro, J.M.; Nogales-Bueno, J.; Heredia, F.J. Feasibility study on the use of a portable micro near infrared spectroscopy device for the “in vineyard” screening of extractable polyphenols in red grape skins. Talanta 2019, 192, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Larkin, P. Infrared and Raman Spectroscopy, Principles and Spectral Interpretation; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Mignani, A.G.; Ciaccheri, L.; Mencaglia, A.A.; Di Sanzo, R.; Carabetta, S.; Russo, M. Dispersive Raman Spectroscopy for the Nondestructive and Rapid Assessment of the Quality of Southern Italian Honey Types. J. Light. Technol. 2016, 34, 4479. [Google Scholar] [CrossRef]
- Mandrile, L.; Zeppa, G.; Giovannozzi, A.M.; Rossi, A.M. Controlling protected designation of origin of wine by Raman spectroscopy. Food Chem. 2016, 211, 260–267. [Google Scholar] [CrossRef]
- Kondo, T.; Hashimoto, R.; Ohrui, Y.; Ryoji Sekioka, R.; Nogami, T.; Fumihito Muta, F.; Seto, Y. Analysis of chemical warfare agents by portable Raman spectrometer with both 785 nm and 1064 nm excitation. Forensic Sci. Int. 2018, 291, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.S. Raman Spectroscopy for Soft Matter Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Rygula, A.; Majzner, K.; Marzec, K.M.; Kaczor, A.; Pilarczyk, M.; Baranska, M. Raman spectroscopy of proteins: A review. J. Raman Spect. 2013, 44, 1061–1076. [Google Scholar] [CrossRef]
- Lee, H.; Cho, B.K.; Kim, M.S.; Lee, W.H.; Tewari, J.; Bae, H.; Sohn, S.I.; Chi, H.Y. Prediction of crude protein and oil content of soybeans using Raman spectroscopy. Sens Actuators B Chem. 2013, 185, 694–700. [Google Scholar] [CrossRef]
- Gallego, A.L.; Guesalaga, A.R.; Bordeu, E.; González, A.S. Rapid measurement of phenolics compounds in red wine using Raman spectroscopy. IEEE T Instrum Meas. 2011, 60, 507–512. [Google Scholar] [CrossRef]
- Ciaccheri, L.; Yuan, T.; Zhang, S.; Mencaglia, A.A.; Trono, A.C.; Yuan, L.; Mignani, A.G. Blueberry juices: A rapid multi-analysis of quality indicators by means of dispersive Raman spectroscopy excited at 1064 nm. SPIE—The International Society for Optical Engineering. In Proceedings of the 25th International Conference on Optical Fiber Sensors, Jeju, Korea, 24–28 April 2017; Volume 10323, p. 32. [Google Scholar]
- Nogales-Bueno, J.; Baca-Bocanegra, B.; Rooney, A.; Hernández-Hierro, J.M.; Heredia, F.J.; Byrne, H.J. Linking ATR-FTIR and Raman features to phenolic extractability and other attributes in grape skin. Talanta 2017, 167, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Nogales-Bueno, J.; Baca-Bocanegra, B.; Rooney, A.; Hernández-Hierro, J.M.; Byrne, H.J.; Heredia, F.J. Study of phenolic extractability in grape seeds by means of ATR-FTIR and Raman spectroscopy. Food Chem. 2017, 232, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Gebrekidan, M.T.; Knipfer, C.; Stelzle, F.; Poop, J.; Will, S.; Braeuer, A. A shifted-excitation Raman difference spectroscopy (SERDS) evaluation strategy for the efficient isolation of Raman spectra from extreme fluorescence interference. J. Raman Spectrosc. 2016, 47, 198–209. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Hernanz, D.; Gonzalez-Manzano, S.; Santos-Buelga, C.; Escudero-Gilete, M.L.; Heredia, F.J. Detailed phenolic composition of white grape by-products by RRLC/MS and measurement of the antioxidant activity. Talanta 2014, 125, 51–57. [Google Scholar] [CrossRef]
- Rivero, F.J.; González-Miret, M.L.; Jara-Palacios, M.J.; García-Estévez, I.; Escribano-Bailón, M.T.; Heredia, F.J.; Gordillo, B. Copigmentation potential of overripe seeds from sun-dried white grapes on anthocyanins colour and stability by differential colorimetry. Int. J. Food Sci. Technol. 2020, 55, 389–396. [Google Scholar] [CrossRef]
- Quijada-Morín, N.; García-Estévez, I.; Nogales-Bueno, J.; Rodríguez-Pulido, F.J.; Heredia, F.J.; Escribano-Bailón, M.T.; Hernández-Hierro, J.M. Trying to set up the flavanolic phases during grape seed ripening: A Spectral and chemical approach. Talanta 2016, 160, 556–561. [Google Scholar] [CrossRef] [PubMed]
| Grape Varieties | |||
|---|---|---|---|
| Phenolic Compounds | MO | PX | ZA |
| Sum of benzoic acids | 80.78 ± 5.01 a | 55.20 ± 1.37 b | 62.21 ± 1.98 c |
| Sum of monomeric flavanols | 189.85 ± 17.16 a | 46.32 ± 6.14 b | 105.00 ± 8.67 c |
| Sum of procyanidins | 7.26 ± 2.39 a | 5.28 ± 0.73 b | 8.21 ± 0.88 a |
| Sum of oligomeric flavanols | 2.94 ± 1.17 a | 1.60 ± 0.13 b | 3.46 ± 0.36 a |
| Sum of total phenolics | 280.85 ± 20.43 a | 108.41 ± 7.16 b | 178.89 ± 11.44 c |
| Benzoic acids (280 nm) | |||
| Gallic acid | 47.21 ± 5.22 a | 22.13 ± 1.23 b | 30.27 ± 1.40 c |
| Protocatechuic acid | 16.13 ± 0.49 a | 15.22 ± 0.27 b | 15.17 ± 0.52 b |
| Protocatechuic acid derivative | 17.45 ± 1.79 a | 17.83 ± 0.46 a | 16.77 ± 0.97 a |
| Monomeric flavanols (280 nm) | |||
| (+)-Catechin | 105.18 ± 15.15 a | 24.75 ± 4.47 b | 57.48 ± 3.93 c |
| (-)-Epicatechin | 84.66 ± 10.53 a | 21.56 ± 2.31 b | 47.51 ± 6.14 c |
| Procyanidins (280 nm) | |||
| Procyanidin B1 | 2.23 ± 1.42 a | 1.86 ± 0.41 a | 3.71 ± 0.46 b |
| Procyanidin B2 | 1.45 ± 0.48 a | 0.99 ± 0.28 b | 1.83 ± 0.25 a |
| Procyanidin B2 3-O-gallate | 0.72 ± 0.11 a | 0.66 ± 0.04 a | 0.93 ± 0.10 b |
| Procyanidin B7 | 0.86 ± 0.22 a | 0.67 ± 0.05 b | 0.90 ± 0.09 a |
| Procyanidin EC gallate | 1.98 ± 0.99 a | 1.09 ± 0.17 b | 0.75 ± 0.05 b |
| Oligomeric flavanols (280 nm) | |||
| Trimer | 1.31 ± 0.40 a | 0.93 ± 0.12 b | 1.03 ± 0.08 ab |
| Tetramer | 1.64 ± 0.83 a | 0.68 ± 0.05 b | 2.43 ± 0.30 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivero, F.J.; Ciaccheri, L.; González-Miret, M.L.; Rodríguez-Pulido, F.J.; Mencaglia, A.A.; Heredia, F.J.; Mignani, A.G.; Gordillo, B. A Study of Overripe Seed Byproducts from Sun-Dried Grapes by Dispersive Raman Spectroscopy. Foods 2021, 10, 483. https://doi.org/10.3390/foods10030483
Rivero FJ, Ciaccheri L, González-Miret ML, Rodríguez-Pulido FJ, Mencaglia AA, Heredia FJ, Mignani AG, Gordillo B. A Study of Overripe Seed Byproducts from Sun-Dried Grapes by Dispersive Raman Spectroscopy. Foods. 2021; 10(3):483. https://doi.org/10.3390/foods10030483
Chicago/Turabian StyleRivero, Francisco J., Leonardo Ciaccheri, M. Lourdes González-Miret, Francisco J. Rodríguez-Pulido, Andrea A. Mencaglia, Francisco J. Heredia, Anna G. Mignani, and Belén Gordillo. 2021. "A Study of Overripe Seed Byproducts from Sun-Dried Grapes by Dispersive Raman Spectroscopy" Foods 10, no. 3: 483. https://doi.org/10.3390/foods10030483
APA StyleRivero, F. J., Ciaccheri, L., González-Miret, M. L., Rodríguez-Pulido, F. J., Mencaglia, A. A., Heredia, F. J., Mignani, A. G., & Gordillo, B. (2021). A Study of Overripe Seed Byproducts from Sun-Dried Grapes by Dispersive Raman Spectroscopy. Foods, 10(3), 483. https://doi.org/10.3390/foods10030483