Industrial Fruits By-Products and Their Antioxidant Profile: Can They Be Exploited for Industrial Food Applications?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruits By-Product Extraction
2.2. Antioxidant Activity
2.2.1. Free Radical DPPH Inhibition Assay
2.2.2. β-Carotene Bleaching Assay
2.3. Total Phenolic Compounds Content (TPC)
2.4. Total Flavonoid Compounds (TFC)
2.5. Identification of the Polyphenolic Compounds by UHPLC-ESI-MS/MS
2.6. Quantification of the Polyphenolic Compounds by HPLC-DAD/UV
2.7. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Capacity
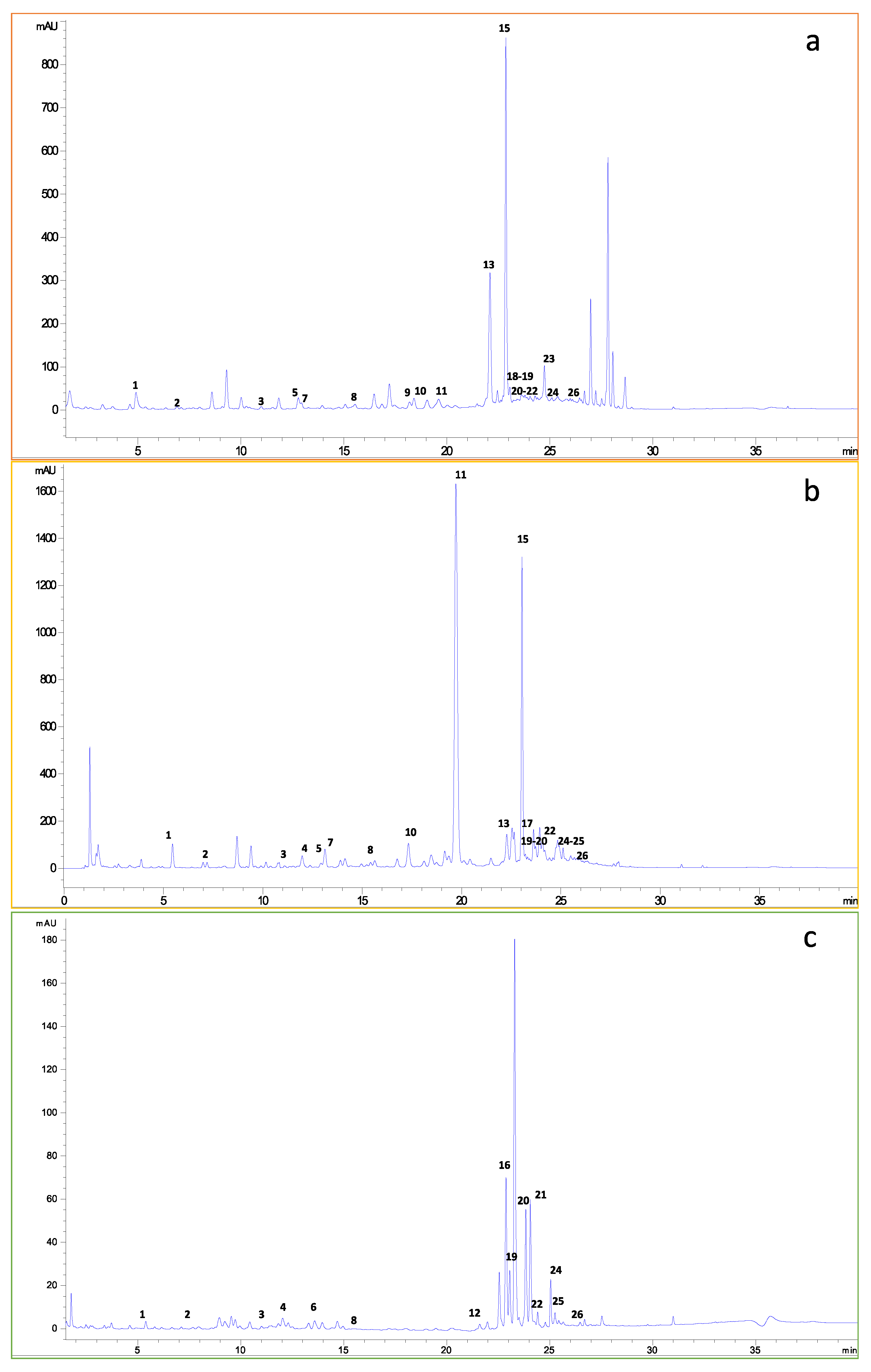
3.2. Chromatographic Polyphenolic Profile of the Fruit By-Products
3.2.1. Benzoic Acid Derivates
3.2.2. Hydroxycinnamic Acids and Their Glycosides
3.2.3. Flavanones Glycosides
3.2.4. Flavonols and Flavonol Glycosides
3.2.5. Others (Flavones and Glycosides, Dihydrochalcone Glycosides and Flavan-3-ols)
3.3. Quantitative Distribution of Polyphenolic Compounds in Fruit By-Products
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ayala-Zavala, J.F.; Vega-Vega, V.; Rosas-Domínguez, C.; Palafox-Carlos, H.; Villa-Rodriguez, J.A.; Siddiqui, M.W.; Dávila-Aviña, J.E.; González-Aguilar, G.A. Agro-industrial potential of exotic fruit byproducts as a source of food additives. Food Res. Int. 2011, 44, 1866–1874. [Google Scholar] [CrossRef]
- Andrade, M.A.; Lima, V.; Sanches Silva, A.; Vilarinho, F.; Castilho, M.C.; Khwaldia, K.; Ramos, F. Pomegranate and grape by-products and their active compounds: Are they a valuable source for food applications? Trends Food Sci. Technol. 2019, 86, 68–84. [Google Scholar] [CrossRef]
- de la Rosa, L.A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Phenolic Compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2019; pp. 253–271. [Google Scholar]
- Andrade, M.A.; Ribeiro-Santos, R.; Costa Bonito, M.C.; Saraiva, M.; Sanches-Silva, A. Characterization of rosemary and thyme extracts for incorporation into a whey protein based film. LWT Food Sci. Technol. 2018, 92, 497–508. [Google Scholar] [CrossRef]
- Ćetković, G.; Čanadanović-Brunet, J.; Djilas, S.; Savatović, S.; Mandić, A.; Tumbas, V. Assessment of polyphenolic content and in vitro antiradical characteristics of apple pomace. Food Chem. 2008, 109, 340–347. [Google Scholar] [CrossRef]
- Raudone, L.; Raudonis, R.; Liaudanskas, M.; Janulis, V.; Viskelis, P. Phenolic antioxidant profiles in the whole fruit, flesh and peel of apple cultivars grown in Lithuania. Sci. Hortic. 2017, 216, 186–192. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO) FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 30 December 2020).
- Cruz, M.G.; Bastos, R.; Pinto, M.; Ferreira, J.M.; Santos, J.F.; Wessel, D.F.; Coelho, E.; Coimbra, M.A. Waste mitigation: From an effluent of apple juice concentrate industry to a valuable ingredient for food and feed applications. J. Clean. Prod. 2018, 193, 652–660. [Google Scholar]
- Kalinowska, M.; Bielawska, A.; Lewandowska-Siwkiewicz, H.; Priebe, W.; Lewandowski, W. Apples: Content of phenolic compounds vs. variety, part of apple and cultivation model, extraction of phenolic compounds, biological properties. Plant Physiol. Biochem. 2014, 84, 169–188. [Google Scholar] [CrossRef]
- Grigoras, C.G.; Destandau, E.; Fougère, L.; Elfakir, C. Evaluation of apple pomace extracts as a source of bioactive compounds. Ind. Crops Prod. 2013, 49, 794–804. [Google Scholar] [CrossRef]
- Yates, M.; Gomez, M.R.; Martin-Luengo, M.A.; Ibañez, V.Z.; Martinez Serrano, A.M. Multivalorization of apple pomace towards materials and chemicals. Waste to wealth. J. Clean. Prod. 2017, 143, 847–853. [Google Scholar] [CrossRef] [Green Version]
- M’hiri, N.; Ghali, R.; Ben Nasr, I.; Boudhrioua, N. Effect of different drying processes on functional properties of industrial lemon byproduct. Process Saf. Environ. Prot. 2018, 116, 450–460. [Google Scholar] [CrossRef]
- Yazgan, H.; Ozogul, Y.; Kuley, E. Antimicrobial influence of nanoemulsified lemon essential oil and pure lemon essential oil on food-borne pathogens and fish spoilage bacteria. Int. J. Food Microbiol. 2019, 306, 108266. [Google Scholar] [CrossRef]
- AL-Jabri, N.N.; Hossain, M.A. Chemical composition and antimicrobial potency of locally grown lemon essential oil against selected bacterial strains. J. King Saud Univ. Sci. 2018, 30, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Larrauri, J.A.; Rupérez, P.; Bravo, L.; Saura-Calixto, F. High dietary fibre powders from orange and lime peels: Associated polyphenols and antioxidant capacity. Food Res. Int. 1996, 29, 757–762. [Google Scholar] [CrossRef]
- Chen, X.M.; Tait, A.R.; Kitts, D.D. Flavonoid composition of orange peel and its association with antioxidant and anti-inflammatory activities. Food Chem. 2017, 218, 15–21. [Google Scholar] [CrossRef]
- de Moraes Crizel, T.; Jablonski, A.; de Oliveira Rios, A.; Rech, R.; Flôres, S.H. Dietary fiber from orange byproducts as a potential fat replacer. LWT-Food Sci. Technol. 2013, 53, 9–14. [Google Scholar] [CrossRef]
- Pereira de Abreu, D.A.; Losada, P.P.; Maroto, J.; Cruz, J.M. Evaluation of the effectiveness of a new active packaging film containing natural antioxidants (from barley husks) that retard lipid damage in frozen Atlantic salmon (Salmo salar L.). Food Res. Int. 2010, 43, 1277–1282. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Natural food additives: Quo vadis? Trends Food Sci. Technol. 2015, 45, 284–295. [Google Scholar] [CrossRef]
- Sanches-Silva, A.; Costa, D.; Albuquerque, T.G.; Buonocore, G.G.; Ramos, F.; Castilho, M.C.; Machado, A.V.; Costa, H.S. Trends in the use of natural antioxidants in active food packaging: A review. Food Addit. Contam. Part A 2014, 31, 374–395. [Google Scholar]
- Andrade, M.A.; de Oliveira Torres, L.R.; Silva, A.S.; Barbosa, C.H.; Vilarinho, F.; Ramos, F.; de Quirós, A.R.B.; Khwaldia, K.; Sendón, R. Industrial multi-fruits juices by-products: Total antioxidant capacity and phenolics profile by LC–MS/MS to ascertain their reuse potential. Eur. Food Res. Technol. 2020, 1, 3. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- The European Parliament and the Council of The European Union. Directive 2009/32/EC of the European Parliament and of the Council of 23 April 2009 on the approximation of the laws of the Member States on extraction solvents used in the production of foodstuffs and food ingredients. Off. J. Eur. Union 2009, L141, 3–10. [Google Scholar]
- Moure, A.; Franco, D.; Sineiro, J.; Domínguez, H.; Núñez, M.J.; Lema, J.M. Antioxidant activity of extracts from Gevuina avellana and Rosa rubiginosa defatted seeds. Food Res. Int. 2001, 34, 103–109. [Google Scholar] [CrossRef]
- Miller, H.E. A simplified method for the evaluation of antioxidants. J. Am. Oil Chem. Soc. 1971, 48, 91. [Google Scholar] [CrossRef]
- Erkan, N.; Ayranci, G.; Ayranci, E. Antioxidant activities of rosemary (Rosmarinus Officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem. 2008, 110, 76–82. [Google Scholar] [CrossRef]
- Yoo, K.M.; Lee, C.H.; Lee, H.; Moon, B.; Lee, C.Y. Relative antioxidant and cytoprotective activities of common herbs. Food Chem. 2008, 106, 929–936. [Google Scholar] [CrossRef]
- de Albuquerque, M.A.C.; Levit, R.; Beres, C.; Bedani, R.; de Moreno de LeBlanc, A.; Saad, S.M.I.; LeBlanc, J.G. Tropical fruit by-products water extracts of tropical fruit by-products as sources of soluble fibres and phenolic compounds with potential antioxidant, anti-inflammatory, and functional properties. J. Funct. Foods 2019, 52, 724–733. [Google Scholar] [CrossRef]
- Guimarães, R.; Barros, L.; Barreira, J.C.M.; Sousa, M.J.; Carvalho, A.M.; Ferreira, I.C.F.R. Targeting excessive free radicals with peels and juices of citrus fruits: Grapefruit, lemon, lime and orange. Food Chem. Toxicol. 2010, 48, 99–106. [Google Scholar] [CrossRef]
- Espinosa-Pardo, F.A.; Nakajima, V.M.; Macedo, G.A.; Macedo, J.A.; Martínez, J. Extraction of phenolic compounds from dry and fermented orange pomace using supercritical CO2 and cosolvents. Food Bioprod. Process. 2017, 101, 1–10. [Google Scholar] [CrossRef]
- Li, B.B.; Smith, B.; Hossain, M.M. Extraction of phenolics from citrus peels: II. Enzyme-assisted extraction method. Sep. Purif. Technol. 2006, 48, 189–196. [Google Scholar] [CrossRef]
- Diñeiro García, Y.; Valles, B.S.; Picinelli Lobo, A. Phenolic and antioxidant composition of by-products from the cider industry: Apple pomace. Food Chem. 2009, 117, 731–738. [Google Scholar] [CrossRef]
- Anagnostopoulou, M.A.; Kefalas, P.; Papageorgiou, V.P.; Assimopoulou, A.N.; Boskou, D. Radical scavenging activity of various extracts and fractions of sweet orange peel (Citrus sinensis). Food Chem. 2006, 94, 19–25. [Google Scholar] [CrossRef]
- De Moraes Barros, H.R.; De Castro Ferreira, T.A.P.; Genovese, M.I. Antioxidant capacity and mineral content of pulp and peel from commercial cultivars of citrus from Brazil. Food Chem. 2012, 134, 1892–1898. [Google Scholar] [CrossRef]
- Garau, M.C.; Simal, S.; Rosselló, C.; Femenia, A. Effect of air-drying temperature on physico-chemical properties of dietary fibre and antioxidant capacity of orange (Citrus aurantium v. Canoneta) by-products. Food Chem. 2007, 104, 1014–1024. [Google Scholar] [CrossRef]
- Vieira, F.G.K.; Borges, G.D.S.C.; Copetti, C.; Di Pietro, P.F.; Nunes, E.D.C.; Fett, R. Phenolic compounds and antioxidant activity of the apple flesh and peel of eleven cultivars grown in Brazil. Sci. Hortic. 2011, 128, 261–266. [Google Scholar] [CrossRef]
- Suleria, H.A.R.; Barrow, C.J.; Dunshea, F.R. Screening and Characterization of Phenolic Compounds and Their Antioxidant Capacity in Different Fruit Peels. Foods 2020, 9, 1206. [Google Scholar] [CrossRef]
- Lee, J.; Chan, B.L.S.; Mitchell, A.E. Identification/quantification of free and bound phenolic acids in peel and pulp of apples (Malus domestica) using high resolution mass spectrometry (HRMS). Food Chem. 2017, 215, 301–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Poojary, M.M.; Choudhary, A.; Rai, D.K.; Tiwari, B.K. Comparison of selected clean and green extraction technologies for biomolecules from apple pomace. Electrophoresis 2018, 39, 1934–1945. [Google Scholar] [CrossRef] [PubMed]
- De Ancos, B.; Cilla, A.; Barberá, R.; Sánchez-Moreno, C.; Cano, M.P. Influence of orange cultivar and mandarin postharvest storage on polyphenols, ascorbic acid and antioxidant activity during gastrointestinal digestion. Food Chem. 2017, 225, 114–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molina-Calle, M.; Priego-Capote, F.; Luque de Castro, M.D. Development and application of a quantitative method for determination of flavonoids in orange peel: Influence of sample pretreatment on composition. Talanta 2015, 144, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Citrus peels waste as a source of value-added compounds: Extraction and quantification of bioactive polyphenols. Food Chem. 2019, 295, 289–299. [Google Scholar] [PubMed]
- Sommella, E.; Pepe, G.; Pagano, F.; Ostacolo, C.; Tenore, G.C.; Russo, M.T.; Novellino, E.; Manfra, M.; Campiglia, P. Detailed polyphenolic profiling of Annurca apple (M. pumila Miller cv Annurca) by a combination of RP-UHPLC and HILIC, both hyphenated to IT-TOF mass spectrometry. Food Res. Int. 2015, 76, 466–477. [Google Scholar] [PubMed]
- Li, X.; Wu, X.; Bi, J.; Liu, X.; Li, X.; Guo, C. Polyphenols accumulation effects on surface color variation in apple slices hot air drying process. LWT 2019, 108, 421–428. [Google Scholar] [CrossRef]
- Xi, W.; Lu, J.; Qun, J.; Jiao, B. Characterization of phenolic profile and antioxidant capacity of different fruit part from lemon (Citrus limon Burm.) cultivars. J. Food Sci. Technol. 2017, 54, 1108–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mcharek, N.; Hanchi, B. Maturational effects on phenolic constituents, antioxidant activities and LC-MS / MS profiles of lemon (Citrus limon) peels. J. Appl. Bot. Food Qual. 2017, 90, 1–9. [Google Scholar]
| Fruits By-Products | Inhibition Percentage (%) | Trolox Equivalent (mg Trolox/g of Extract) |
|---|---|---|
| Lemon | 51.67 ± 4.61 a | 33.17 ± 2.94 d |
| Orange | 31.20 ± 1.28 b | 20.13 ± 0.43 e |
| Apple | 39.92 ±1.68 c | 25.69 ± 0.56 f |
| β-Carotene Bleaching Assay | TPC (mg GAE/g) | TFC (mg ECE/g) | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Lemon | 67.35 ± 1.96 a | 43.38 ± 0.84 c | 20.76 ± 0.61 f |
| Orange | 237.21 ± 29.78 b | 23.32 ± 0.18 d | 7.29 ± 0.32 g |
| Apple | 107.44 ± 23.81 a | 14.02 ± 0.13 e | 24.63 ± 1.61 h |
| Peak | Rt (min) | λmax | [M-H] (m/z) | Main MS/MS Fragments (m/z) | Molecular Formula | Structural Class | Tentative Identification | By-Product | Confirmation/ Ref. § | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Orange | Lemon | Apple | |||||||||
| 1 | 4.31 | 260, 293 | 153 | 109, 108 | C7H6O4 | Benzoic acid derivates | Protocatechuic acid | ✓ | ✓ | ✓ | [38,39], Std* |
| 2 | 7.09 | 255 | 137 | 93, 65 | C7H6O3 | Benzoic acid derivates | Hydroxybenzoic acid | ✓ | ✓ | ✓ | [38,39,40,41], Std* |
| 3 | 9.59 | 325 | 179 | 134, 135 | C9H8O4 | Hydroxycinnamic acids | Caffeic acid | ✓ | ✓ | ✓ | [39,40,46], Std* |
| 4 | 9.99 | 250, 325 | 353 | 191, 173 | C16H18O9 | Hydroxycinnamic acids | Chlorogenic acid | ✓ | ✓ | [38,39,40], Std* | |
| 5 | 11.42 | 240, 330 | 355 | 193 | C16H20O9 | Hydroxycinnamic acids glycosides | Ferulic acid-O-hexoside | ✓ | ✓ | [41] | |
| 6 | 11.64 | 280 | 289 | 245, 203 | C15H14O6 | Flavan-3-ols | Epicatechin | ✓ | [40,44], Std* | ||
| 7 | 11.8 | 270, 330 | 385 | 223 | C17H22O10 | Hydroxycinnamic acids glycosides | Sinapic acid-O-hexoside | ✓ | ✓ | [41] | |
| 8 | 13.39 | 310 | 163 | 119, 93 | C9H8O3 | Hydroxycinnamic acids | p-Coumaric acid | ✓ | ✓ | ✓ | [38,39,40,46], Std* |
| 9 | 15.26 | 325 | 193 | 134 | C10H10O4 | Hydroxycinnamic acids | Ferulic acid | ✓ | [38,39,40,46], Std* | ||
| 10 | 15.68 | 270, 340 | 593 | 473 | C26H28O14 | Flavone glycosides | Apigenin-6,8-di-C-glucoside (Vicenin-2) | ✓ | ✓ | [41] | |
| 11 | 17.95 | 285, 330 | 595 | 287, 151, 135 | C27H32O15 | Flavanone glycosides | Eriodyctiol-O-rutinoside (Eriocitrin) | ✓ | ✓ | [41,46,47], Std* | |
| 12 | 20.29 | 270, 350 | 567 | 273 | C26H31O14 | Dihydrochalcone glycosides | Phloretin-O-apiofuranosyl-glucopyranoside | ✓ | [40] | ||
| 13 | 20.56 | 290, 330 | 579 | 271 | C27H32O14 | Flavanone glycosides | Naringenin-7-O-rutinoside (Narirutin) | ✓ | ✓ | [40,41,42] | |
| 14 | 21.00 | 356 | 593 | 285 | C27H30O15 | Flavonol glycosides | Kaempferol-3-O-rutinoside | ✓ | ✓ | [40] | |
| 15 | 21.36 | 290, 355 | 609 | 300, 286, 242 | C28H34O15 | Flavanone glycosides | Hesperetin-7-O-rutinoside (Hesperidin) | ✓ | ✓ | [40,41,42,43,46,47], Std* | |
| 16 | 21.37 | 278 | 435 | 273, 167, 123 | C21H24O10 | Dihydrochalcone glycosides | Phloretin-2′-O-beta-glucoside (Phlorizin) | ✓ | [40], Std* | ||
| 17 | 21.49 | 360 | 463 | 301, 271 | C21H20O12 | Flavonol glycosides | Quercetin-3-O-glucoside (Isoquercetin) | ✓ | [38], Std* | ||
| 18 | 21.52 | 290, 355 | 609 | 286 | C28H34O15 | Flavanone glycosides | Hesperetin-7-O-neohesperidoside (Neohesperidin) | ✓ | [42] | ||
| 19 | 21.58 | 255, 360 | 609 | 300, 271 | C27H30O16 | Flavonol glycosides | Quercetin-3-O-rutinoside (Rutin) | ✓ | ✓ | ✓ | [40,41,43,44,46,47], Std* |
| 20 | 22.28 | 270, 350 | 433 | 301, 271 | C21H22O10 | Flavanone glycosides | Naringenin-7-O-glucoside (Prunin) | ✓ | ✓ | ✓ | [40] |
| 21 | 22.49 | 250, 330 | 359 | 197, 161 | C18H16O8 | Hydroxycinnamic acids | Rosmarinic acid | ✓ | ✓ | [39], Std* | |
| 22 | 22.51 | 250, 350 | 447 | 331, 300, 301 | C21H20O11 | Flavonol glycosides | Quercetin-3-O-rhamnoside (Quercitrin) | ✓ | ✓ | ✓ | [38,40,44,45], Std* |
| 23 | 23.19 | 285, 330 | 593 | 285 | C28H34O14 | Flavanone glycosides | Isosakuranetin-7-O-rutinoside (Dydimin) | ✓ | [41] | ||
| 24 | 23.71 | 295 | 271 | 151, 119 | C15H12O5 | Flavanone | Naringenin | ✓ | ✓ | ✓ | [40,41,42,44,46], Std* |
| 25 | 23.84 | 270, 360 | 274 | 70, 88 | C15H10O7 | Flavonols | Quercetin | ✓ | ✓ | ✓ | [21,38,40,41,47], Std* |
| 26 | 24.9 | 280, 360 | 269 | 117 | C15H10O5 | Flavones | Apigenin | ✓ | ✓ | Std* | |
| Phenolic Compound | Detection λmax (nm) | Slope | Intercept | R2 | Concentration Range (µg/mL) | LOD (µg/g By-Product) | LOQ (µg/g By-Product) |
|---|---|---|---|---|---|---|---|
| Protocatechuic acid | 300 | 40.63 | −4.334 | 0.9980 | 0.05–20 | 0.04 | 0.10 |
| Hydroxybenzoic acid | 278 | 210.0 | −14.52 | 0.9990 | 0.10–20 | 0.10 | 0.20 |
| Caffeic acid | 325 | 146.6 | −40.50 | 0.9980 | 0.10–20 | 0.10 | 0.21 |
| Chlorogenic acid | 325 | 61.58 | −14.27 | 0.9989 | 0.10–20 | 0.10 | 0.20 |
| Epicatechin | 278 | 21.66 | −2.006 | 0.9996 | 0.10–20 | 0.10 | 0.20 |
| p-Coumaric acid | 325 | 169.6 | −10.05 | 0.9990 | 0.05–20 | 0.04 | 0.10 |
| Ferulic acid | 325 | 159.9 | 1.128 | 0.9999 | 0.05–20 | 0.05 | 0.20 |
| Eriocitrin | 278 | 19.12 | −1.025 | 0.9999 | 0.05–20 | 0.04 | 0.10 |
| Hesperidin | 278 | 50.42 | −2.975 | 0.9997 | 0.03–20 | 0.01 | 0.05 |
| Phlorizin | 278 | 24.78 | 0.1248 | 0.9999 | 0.05–20 | 0.04 | 0.10 |
| Isoquercitrin | 360 | 52.55 | 5.218 | 0.9994 | 0.05–20 | 0.04 | 0.10 |
| Rutin | 360 | 49.90 | −3.978 | 0.9999 | 0.05–20 | 0.04 | 0.10 |
| Rosmarinic acid | 325 | 117.5 | −21.47 | 0.9987 | 0.05–20 | 0.04 | 0.10 |
| Quercetrin | 360 | 67.76 | −4.616 | 0.9998 | 0.05–20 | 0.05 | 0.10 |
| Naringenin | 300 | 70.09 | −22.15 | 0.9970 | 0.05–20 | 0.03 | 0.05 |
| Phenolic Compound (µg/g of By-Product) | Fruit By-Product | ||
|---|---|---|---|
| Orange | Lemon | Apple | |
| Benzoic acid derivates | |||
| Protocatechuic acid | 317.3 ± 7.173 | 16.08 ± 1.729 | 2.465 ± 0.0248 |
| Hydroxybenzoic acid | <LOQ | 20.27 ± 1.053 | 1.939 ± 0.0472 |
| Σ | 317.3 ± 7.173 | 36.35 ± 0.7208 | 4.404 ± 0.0682 |
| Hydroxycinnamic acids | |||
| Caffeic acid | 26.19 ± 1.195 | 14.67 ± 0.4107 | <LOQ |
| Chlorogenic acid | n.a. | 386.7 ± 11.80 | 39.41 ± 1.016 |
| p-Coumaric acid | 18.49 ± 0.5547 | 6.424 ± 0.2468 | <LOQ |
| Ferulic acid | 22.88 ± 0.9469 | n.a. | n.a. |
| Rosmarinic acid | 12.97 ± 0.6995 | n.a. | 88.62 ± 3.606 |
| Σ | 80.53 ± 2.915 | 407.8 ± 12.17 | 128.0 ± 4.554 |
| Flavan-3-ols | |||
| Epicatechin | n.a. | n.a. | 31.24 ± 0.7253 |
| Σ | 31.24 ± 0.7253 | ||
| Flavanone glycosides | |||
| Eriocitrin | 24.63 ± 1.409 | 17,493 ± 115.5 | n.a. |
| Hesperidin | 4901 ± 155.4 | 2728 ± 17.32 | n.a. |
| Naringenin | 30.09 ± 0.4647 | 42.12 ± 0.8605 | 16.33 ± 0.834 |
| Σ | 4956 ± 156.9 | 20,263 ± 131.6 | 16.33 ± 0.834 |
| Flavonols glycosides | |||
| Isoquercetin | n.a. | 111.6 ± 0.8220 | n.a. |
| Rutin | 31.43 ± 1.130 | 44.72 ± 0.5788 | 22.44 ± 0.977 |
| Quercetrin | 7.964 ± 0.4449 | 106.0 ± 0.3979 | 150.3 ± 3.769 |
| Σ | 39.40 ± 0.9735 | 262.3 ± 1.793 | 172.7 ± 4.709 |
| Dihydrochalcone glycosides | |||
| Phlorizin | n.a. | n.a. | 542.0 ± 7.882 |
| Σ | 542.0 ± 7.882 | ||
| Total content (µg/g) | 5393 ± 166.1 | 20,969 ± 144.7 | 894.8 ± 16.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, C.H.; Andrade, M.A.; Séndon, R.; Silva, A.S.; Ramos, F.; Vilarinho, F.; Khwaldia, K.; Barbosa-Pereira, L. Industrial Fruits By-Products and Their Antioxidant Profile: Can They Be Exploited for Industrial Food Applications? Foods 2021, 10, 272. https://doi.org/10.3390/foods10020272
Barbosa CH, Andrade MA, Séndon R, Silva AS, Ramos F, Vilarinho F, Khwaldia K, Barbosa-Pereira L. Industrial Fruits By-Products and Their Antioxidant Profile: Can They Be Exploited for Industrial Food Applications? Foods. 2021; 10(2):272. https://doi.org/10.3390/foods10020272
Chicago/Turabian StyleBarbosa, Cássia H., Mariana A. Andrade, Raquel Séndon, Ana Sanches Silva, Fernando Ramos, Fernanda Vilarinho, Khaoula Khwaldia, and Letricia Barbosa-Pereira. 2021. "Industrial Fruits By-Products and Their Antioxidant Profile: Can They Be Exploited for Industrial Food Applications?" Foods 10, no. 2: 272. https://doi.org/10.3390/foods10020272
APA StyleBarbosa, C. H., Andrade, M. A., Séndon, R., Silva, A. S., Ramos, F., Vilarinho, F., Khwaldia, K., & Barbosa-Pereira, L. (2021). Industrial Fruits By-Products and Their Antioxidant Profile: Can They Be Exploited for Industrial Food Applications? Foods, 10(2), 272. https://doi.org/10.3390/foods10020272











