Selection of Vegetable Oils and Frying Cycles Influencing Acrylamide Formation in the Intermittently Fried Beef Nuggets
Abstract
1. Introduction
2. Materials and Methods
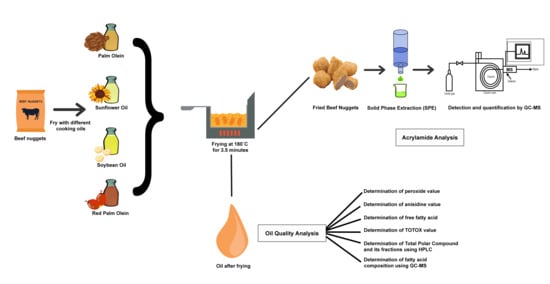
2.1. Sample Preparation
2.2. Frying Experiment
2.3. Analysis Methods
2.3.1. Chemicals and Reagents
2.3.2. Oil Oxidative Rancidity
Determination of Peroxide Value (PV)
Determination of p-Anisidine Value (p-AV)
2.3.3. Determination of Free Fatty Acid (FFA)
2.3.4. Determination of Total Polar Compounds (TPC) and Polar Compound Fractions
2.3.5. Determination of Fatty Acid Composition (FAC)
2.3.6. Acrylamide Analysis
2.3.7. Statistical Analysis
3. Results and Discussion
3.1. Changes in Oxidative Rancidity
3.2. Change in FFA
3.3. Changes in TPC and Polar Fractions
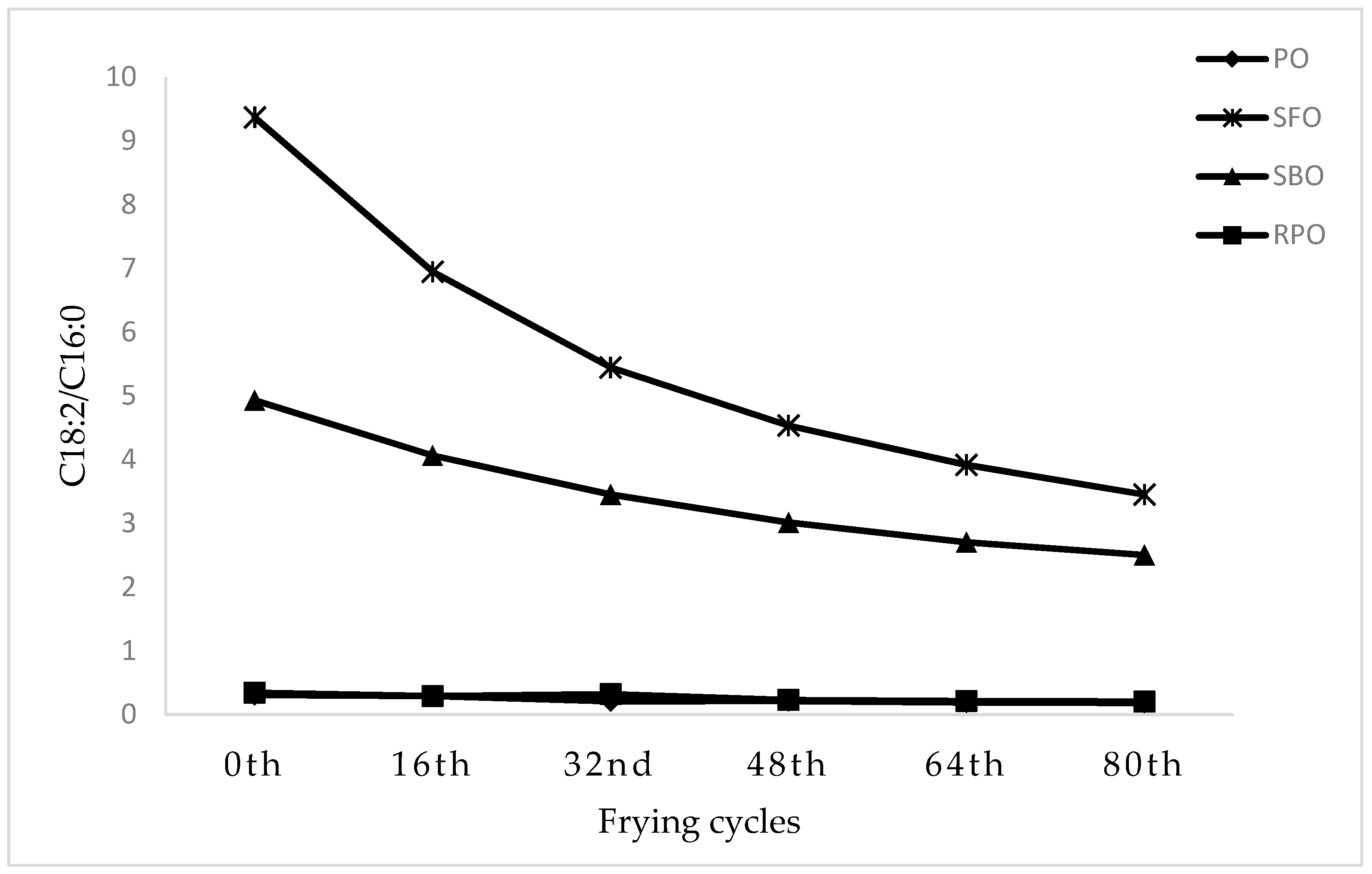
3.4. Changes in the Fatty Acid Composition
3.5. Changes in Acrylamide Concentration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pankaj, S.K.; Keener, K.M. A review and research trends in alternate frying technologies. Curr. Opin. Food Sci. 2017, 16, 74–79. [Google Scholar] [CrossRef]
- Vieira, S.A.; Zhang, G.; Decker, E.A. Biological Implications of Lipid Oxidation Products. J. Am. Oil Chem. Soc. 2017, 94, 339–351. [Google Scholar] [CrossRef]
- Ghasemian, S.; Rezaei, K.; Abedini, R.; Poorazarang, H.; Ghaziani, F. Investigation of different parameters on acrylamide production in the fried beef burger using Taguchi experimental design. J. Food Sci. Technol. 2014, 51, 440–448. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Törnqvist, M. Analysis of Acrylamide, a Carcinogen Formed in Heated Foodstuffs. J. Agric. Food Chem. 2002, 50, 4998–5006. [Google Scholar] [CrossRef] [PubMed]
- IARC. Some Industrial Chemicals. Available online: https://monographs.iarc.fr/wp-content/uploads/2018/06/mono60.pdf (accessed on 1 October 2020).
- EFSA. Scientific opinion on acrylamide in food. EFSA J. 2015, 13, 4104. [Google Scholar]
- Bertuzzi, T.; Martinelli, E.; Mulazzi, A.; Rastelli, S. Acrylamide determination during an industrial roasting process of coffee and the influence of asparagine and low molecular weight sugars. Food Chem. 2020, 303, 125372. [Google Scholar] [CrossRef]
- Jin, C.; Wu, X.; Zhang, Y. Relationship between antioxidants and acrylamide formation: A review. Food Res. Int. 2013, 51, 611–620. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. Contribution of Lipid Oxidation Products to Acrylamide Formation in Model Systems. J. Agric. Food Chem. 2008, 56, 6075–6080. [Google Scholar] [CrossRef]
- Capuano, E.; Oliviero, T.; Açar, Ö.Ç.; Gökmen, V.; Fogliano, V. Lipid oxidation promotes acrylamide formation in fat-rich model systems. Food Res. Int. 2010, 43, 1021–1026. [Google Scholar] [CrossRef]
- Yasuhara, A.; Tanaka, Y.; Hengel, M.; Shibamoto, T. Gas chromatographic investigation of acrylamide formation in browning model systems. J. Agric. Food Chem. 2003, 51, 3999. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H.; Cheng, L.; Wang, L.; Qian, H. Influence of deep-frying using various commercial oils on acrylamide formation in French fries. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2015, 32, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Mestdagh, F.; De Meulenaer, B.; Van Peteghem, C. Influence of oil degradation on the amounts of acrylamide generated in a model system and in French fries. Food Chem. 2007, 100, 1153–1159. [Google Scholar] [CrossRef]
- Dorni, C.; Sharma, P.; Saikia, G.; Longvah, T. Fatty acid profile of edible oils and fats consumed in India. Food Chem. 2018, 238, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Joe, P. Global Edible Vegetable Oil Market Trends. Biomed. J. Sci. Tech. Res. 2018, 2. [Google Scholar] [CrossRef]
- Kamal-Eldin, A. Antioxidative Activity of Vitamin E. In Vitamin E in Human Health; Humana Press: Totova, NJ, USA, 2019; pp. 19–30. [Google Scholar] [CrossRef]
- Cassiday, L. Red Palm Oil. Available online: https://www.aocs.org/stay-informed/inform-magazine/featured-articles/red-palm-oil-february-2017?SSO=True (accessed on 1 October 2020).
- El-Hadad, N.; Abou-Gharbia, H.A.; Abd El-Aal, M.H.; Youssef, M.M. Red Palm Olein: Characterization and Utilization in Formulating Novel Functional Biscuits. J. Am. Oil Chem. Soc. 2009, 87, 295–304. [Google Scholar] [CrossRef]
- Wu, X.; Wu, S.; Ji, M.; Yoong, J.H. Influence of red palm oil on the physicochemical and sensory qualities of flavouring oil gravy for instant noodles. RSC Adv. 2018, 8, 1148–1158. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Chemistry of deep-fat frying oils. J. Food Sci. 2007, 72, R77–R86. [Google Scholar] [CrossRef]
- Castelo-Branco, V.N.; Santana, I.; Di-Sarli, V.O.; Freitas, S.P.; Torres, A.G. Antioxidant capacity is a surrogate measure of the quality and stability of vegetable oils. Eur. J. Lipid Sci. Technol. 2016, 118, 224–235. [Google Scholar] [CrossRef]
- Lim, P.K.; Jinap, S.; Sanny, M.; Tan, C.P.; Khatib, A. The influence of deep frying using various vegetable oils on acrylamide formation in sweet potato (Ipomoea batatas L. Lam) chips. J. Food Sci. 2014, 79, T115–T121. [Google Scholar] [CrossRef]
- Daniali, G.; Jinap, S.; Hajeb, P.; Sanny, M.; Tan, C.P. Acrylamide formation in vegetable oils and animal fats during heat treatment. Food Chem. 2016, 212, 244–249. [Google Scholar] [CrossRef]
- Gökmen, V. Acrylamide in Food: Analysis, Content and Potential Health Effects; Elsevier Science: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Arslan, F.N.; Şapçı, A.N.; Duru, F.; Kara, H. A study on monitoring of frying performance and oxidative stability of cottonseed and palm oil blends in comparison with original oils. Int. J. Food Prop. 2016, 20, 704–717. [Google Scholar] [CrossRef]
- Gertz, C. Optimising the baking and frying process using oil-improving agents. Eur. J. Lipid Sci. Technol. 2004, 106, 736–745. [Google Scholar] [CrossRef]
- Matthäus, B.; Haase, N.U.; Vosmann, K. Factors affecting the concentration of acrylamide during deep-fat frying of potatoes. Eur. J. Lipid Sci. Technol. 2004, 106, 793–801. [Google Scholar] [CrossRef]
- Williams, J. Influence of variety and processing conditions on acrylamide levels in fried potato crisps. Food Chem. 2005, 90, 875–881. [Google Scholar] [CrossRef]
- Barbut, S. Frying—Effect of coating on crust microstructure, color, and texture of lean meat portions. Meat Sci. 2013, 93, 269–274. [Google Scholar] [CrossRef]
- Bansal, G.; Zhou, W.; Barlow, P.J.; Lo, H.-L.; Neo, F.-L. Performance of palm olein in repeated deep frying and controlled heating processes. Food Chem. 2010, 121, 338–347. [Google Scholar] [CrossRef]
- Enríquez-Fernández, B.E.; Álvarez de la Cadena y Yañez, L.; Sosa-Morales, M.E. Comparison of the stability of palm olein and a palm olein/canola oil blend during deep-fat frying of chicken nuggets and French fries. Int. J. Food Sci. Technol. 2011, 46, 1231–1237. [Google Scholar] [CrossRef]
- Park, J.M.; Kim, J.M. Monitoring of Used Frying Oils and Frying Times for Frying Chicken Nuggets Using Peroxide Value and Acid Value. Korean J. Food Sci. Anim. Resour. 2016, 36, 612–616. [Google Scholar] [CrossRef]
- Soncu, E.D.; Kolsarici, N. Microwave thawing and green tea extract efficiency for the formation of acrylamide throughout the production process of chicken burgers and chicken nuggets. J. Sci. Food Agric. 2017, 97, 1790–1797. [Google Scholar] [CrossRef]
- FDA. Survey Data on Acrylamide in Food: Total Diet Study Results. Available online: https://www.fda.gov/food/chemicals/survey-data-acrylamide-food-total-diet-study-results#table4 (accessed on 1 October 2020).
- Gholami, F.; Rahman, A.; Mostaghim, T. Effects of rosemary and thyme extracts on acrylamide formation in fried beef. Int. J. Sci. Res. Sci. Technol. 2017, 3, 352–360. [Google Scholar]
- Ahmad Tarmizi, A.H.; Abd Razak, R.A.; Abdul Hammid, A.N.; Kuntom, A. Effect of Anti-Clouding Agent on the Fate of 3-Monochloropropane-1,2-Diol Esters and Glycidyl Esters in Palm Olein during Repeated Frying. Molecules 2019, 24, 2332. [Google Scholar] [CrossRef] [PubMed]
- AOCS. Official Method Cd 8b-90. Peroxide Value Acetic Acid–Isooctane Method; American Oil Chemists’ Society: Urbana, IL, USA, 2011; p. 3. [Google Scholar]
- AOCS. Official Method Cd 18-90 p-Anisidine Value; American Oil Chemists’ Society: Urbana, IL, USA, 2011; p. 2. [Google Scholar]
- AOCS. Official Method Ca 5a-40 Free Fatty Acid; American Oil Chemists’ Society: Urbana, IL, USA, 2012; p. 2. [Google Scholar]
- Dobarganes, M.; Velasco, J.; Dieffenbacher, A. Determination of polar compounds, polymerized and oxidized triacylglycerols, and diacylglycerols in oils and fats - Results of collaborative studies and the standardized method (Technical Report). Pure Appl. Chem. 2000, 72, 1563–1575. [Google Scholar] [CrossRef]
- AOCS. Official Method Ce 1i–07, Fatty acid Composition of Marine Oils and Other Oils Containing Long Chain Polyunsaturated Fatty Acids by GLC; American Oil Chemists’ Society: Champaign, IL, USA, 2009. [Google Scholar]
- Sanny, M.; Jinap, S.; Bakker, E.J.; van Boekel, M.A.; Luning, P.A. Possible causes of variation in acrylamide concentration in French fries prepared in food service establishments: An observational study. Food Chem. 2012, 132, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Wawire, M.; Mathooko, F.M. Impact of frying practices and frying conditions on the quality and safety of frying oils used by street vendors and restaurants in Nairobi, Kenya. J. Food Compost Anal. 2017, 62, 239–244. [Google Scholar] [CrossRef]
- Sebastian, A.; Ghazani, S.M.; Marangoni, A.G. Quality and safety of frying oils used in restaurants. Food Res. Int. 2014, 64, 420–423. [Google Scholar] [CrossRef]
- Ali, M.; Daud, A.; Latip, R.; Othman, N.; Islam, M. Impact of chicken nugget presence on the degradation of canola oil during frying. Int. Food Res J. 2014, 21, 1083. [Google Scholar]
- Ahmad Tarmizi, A.H. Effect of Frying on the Palm Oil Quality Attributes—A Review—Review Article. J. Oil Palm Res. 2016, 28, 143–153. [Google Scholar] [CrossRef]
- Loganathan, R.; Tarmizi, A.H.A.; Vethakkan, S.R.; Teng, K.T. Retention of Carotenes and Vitamin E, and Physico-chemical Changes Occurring upon Heating Red Palm Olein Using Deep-fat Fryer, Microwave Oven and Conventional Oven. J. Oleo Sci. 2020, 69, 167–183. [Google Scholar] [CrossRef]
- Nayak, P.K.; Dash, U.; Rayaguru, K.; Krishnan, K.R. Physio-Chemical Changes During Repeated Frying of Cooked Oil: A Review. J. Food Biochem. 2016, 40, 371–390. [Google Scholar] [CrossRef]
- Aladedunye, F.A.; Przybylski, R. Protecting oil during frying: A comparative study. Eur. J. Lipid Sci. Technol. 2009, 111, 893–901. [Google Scholar] [CrossRef]
- Tyagi, V.; Vasishtha, A. Changes in the characteristics and composition of oils during deep-fat frying. J. Am. Oil Chem. Soc. 1996, 73, 499–506. [Google Scholar] [CrossRef]
- Abdulkarim, S.; Long, K.; Lai, O.M.; Muhammad, S.; Ghazali, H. Frying quality and stability of high-oleic Moringa oleifera seed oil in comparison with other vegetable oils. Food Chem. 2007, 105, 1382–1389. [Google Scholar] [CrossRef]
- Flores-Alvarez Mdel, C.; Molina-Hernandez, E.F.; Hernandez-Raya, J.C.; Sosa-Morales, M.E. The effect of food type (fish nuggets or French fries) on oil blend degradation during repeated frying. J. Food Sci. 2012, 77, C1136–C1143. [Google Scholar] [CrossRef]
- Ahmad Tarmizi, A.H.; Hishamuddin, E.; Abd Razak, R.A. Impartial assessment of oil degradation through partitioning of polar compounds in vegetable oils under simulated frying practice of fast food restaurants. Food Control 2019, 96, 445–455. [Google Scholar] [CrossRef]
- Feng, H.X.; Sam, R.; Jiang, L.Z.; Li, Y.; Cao, W.M. High-performance size-exclusion chromatography studies on the formation and distribution of polar compounds in camellia seed oil during heating. J. Zhejiang Univ. Sci. B. 2016, 17, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Aladedunye, F.A.; Przybylski, R. Minor components in oils and their effects on frying performance. Lipid Technol. 2013, 25, 87–90. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Wang, Y.; Cao, P.; Liu, Y. Effects of frying oils’ fatty acids profile on the formation of polar lipids components and their retention in French fries over deep-frying process. Food Chem. 2017, 237, 98–105. [Google Scholar] [CrossRef]
- Serjouie, A.; Ping Tan, C.; Mirhossein, H.; Bin Che Ma, Y. Effect of Vegetable-Based Oil Blends on Physicochemical Properties of Oils During Deep-Fat Frying. Am. J. Food Technol. 2010, 5, 310–323. [Google Scholar] [CrossRef]
- Kuek, S.L.; Ahmad Tarmizi, A.H.; Abd Razak, R.A.; Jinap, S.; Norliza, S.; Sanny, M. Contribution of lipid towards acrylamide formation during intermittent frying of French fries. Food Control 2020, 118. [Google Scholar] [CrossRef]
- Kamarudin, S.A.; Jinap, S.; Sukor, R.; Foo, S.P.; Sanny, M. Effect of Fat-Soluble Anti-oxidants in Vegetable Oils on Acrylamide Concentrations During Deep-Fat Frying of French Fries. Malays. J. Med. Sci. 2018, 25, 128–139. [Google Scholar] [CrossRef]
- Daniali, G.; Jinap, S.; Sanny, M.; Tan, C.P. Effect of amino acids and frequency of reuse frying oils at different temperature on acrylamide formation in palm olein and soy bean oils via modeling system. Food Chem. 2018, 245, 1–6. [Google Scholar] [CrossRef]
- Sundram, K.; Sambanthamurthi, R.; Tan, Y.-A. Palm fruit chemistry and nutrition. Asia Pac. J. Clin. Nutr. 2003, 12, 355–362. [Google Scholar] [PubMed]
- Edem, D.O. Palm oil: Biochemical, physiological, nutritional, hematological and toxicological aspects: A review. Plant Foods Hum. Nutr. 2002, 57, 319–341. [Google Scholar] [CrossRef] [PubMed]
- Becalski, A.; Lau, B.P.-Y.; Lewis, D.; Seaman, S.W. Acrylamide in foods: Occurrence, sources, and modeling. J. Agric. Food Chem. 2003, 51, 802–808. [Google Scholar] [CrossRef]
- Ehling, S.; Hengel, M.; Shibamoto, T. Formation of acrylamide from lipids. In Chemistry and Safety of Acrylamide in Food; Springer: Berlin/Heidelberg, Germany, 2005; pp. 223–233. [Google Scholar]
- Mestdagh, F.J.; De Meulenaer, B.; Van Poucke, C.; Detavernier, C.; Cromphout, C.; Van Peteghem, C. Influence of oil type on the amounts of acrylamide generated in a model system and in French fries. J Agric. Food Chem. 2005, 53, 6170–6174. [Google Scholar] [CrossRef]
- Bouchon, P.; Aguilera, J.; Pyle, D. Structure Oil-Absorption Relationships During Deep-Fat Frying. J. Food Sci. 2003, 68, 2711–2716. [Google Scholar] [CrossRef]
| Type of Oils | |||||
|---|---|---|---|---|---|
| Parameters | Frying Cycles | PO | SFO | SBO | RPO |
| PV (meq O2/kg) | 0th | 2.10 ± 0.04 Bb | 3.74 ± 0.22 Da | 4.43 ± 0.04 Ca | 1.45 ± 0.15 Bb |
| 16th | 7.13 ± 0.09 Ab | 16.4 ± 0.14 Aa | 6.70 ± 0.61 ABb | 8.39 ± 0.56 Ab | |
| 32nd | 7.39 ± 1.15 Aa | 8.87 ± 0.68 Ba | 7.16 ± 0.21 Aa | 8.14 ± 0.33 Aa | |
| 48th | 10.2 ± 0.61 Aa | 5.83 ± 0.04 Cb | 5.58 ± 0.06 BCb | 7.45 ± 0.57 Ab | |
| 64th | 7.49 ± 0.15 Aa | 5.79 ± 0.09 Cb | 6.15 ± 0.17 ABb | 7.48 ± 0.16 Aa | |
| 80th | 7.99 ± 0.55 Aa | 5.51 ± 0.25 CDb | 5.60 ± 0.05 BCb | 7.76 ± 0.11 Aa | |
| p-AV (unit) | 0th | 2.02 ± 0.05E c | 6.72 ± 0.23 Da | 4.45 ± 0.28 Eb | 5.41 ± 0.33 Dab |
| 16th | 58.2 ± 0.47 Ab | 63.1 ± 1.63 Aab | 64.1 ± 0.61 Aa | 41.4 ± 0.21 Cc | |
| 32nd | 59.8 ± 0.32 Aa | 61.2 ± 1.23 Aba | 58.8 ± 0.78 Ba | 56.2 ± 1.24 Aa | |
| 48th | 49.3 ± 0.12 Bc | 60.2 ± 0.91 Aba | 53.2 ± 0.57 Cb | 51.2 ± 0.27 Bbc | |
| 64th | 46.6 ± 0.61 Cb | 55.7 ± 1.12 BCa | 49.9 ± 0.83 Db | 49.0 ± 0.93 Bb | |
| 80th | 44.6 ± 0.28 Dc | 51.8 ± 0.23 Ca | 47.9 ± 0.37 Da | 44.5 ± 0.17 Cc | |
| TOTOX (unit) | 0th | 6.26 ± 0.04 Cb | 14.2 ± 0.66 Ea | 13.3 ± 0.35 Ca | 8.31 ± 0.63 Eb |
| 16th | 72.4 ± 0.64 Ab | 95.8 ± 1.92 Aa | 77.4 ± 1.81 Ab | 58.2 ± 0.93 Dc | |
| 32nd | 71.3 ± 0.44 Ab | 82.6 ± 1.83 Ba | 73.17 ± 1.21 Ab | 72.5 ± 0.58 Ab | |
| 48th | 69.8 ± 1.09 Aab | 71.9 ± 0.97 Ba | 64.3 ± 0.45 Bb | 65.6 ± 1.41 Bb | |
| 64th | 61.5 ± 0.31 Bb | 67.3 ± 0.93 CDa | 61.9 ± 1.18 Bab | 63.9 ± 1.26 BCab | |
| 80th | 60.5 ± 1.37 Ba | 62.8 ± 0.28 Da | 59.26 ± 0.47 Ba | 60.1 ± 0.05 CDa | |
| Type of Oils | |||||
|---|---|---|---|---|---|
| Parameter | Frying Cycles | PO | SFO | SBO | RPO |
| FFA (%) | 0th | 0.06 ± 0.001 Fb | 0.05 ± 0.001 Db | 0.10 ± 0.001 Fa | 0.06 ± 0.001 Fb |
| 16th | 0.18 ± 0.001 Eab | 0.14 ± 0.01 Db | 0.20 ± 0.01 Ea | 0.18 ± 0.001 Eab | |
| 32nd | 0.32 ± 0.001 Da | 0.24 ± 0.03 Ca | 0.29 ± 0.01 Da | 0.31 ± 0.01 Da | |
| 48th | 0.49 ± 0.001 Ca | 0.32 ± 0.01 Cc | 0.38 ± 0.01 Cb | 0.48 ± 0.01 Ca | |
| 64th | 0.70 ± 0.001 Ba | 0.43 ± 0.01 Bb | 0.48 ± 0.02 Bb | 0.61 ± 0.01 Ba | |
| 80th | 0.86 ± 0.001 Aa | 0.54 ± 0.01 Ac | 0.56 ± 0.001 Ac | 0.70 ± 0.02 Ab | |
| Type of Oils | |||||
|---|---|---|---|---|---|
| Parameters | Frying Cycles | PO | SFO | SBO | RPO |
| Polymerized TAG (%) | 0th | 0.001 ± 0.001 Cc | 0.40 ± 0.02 Ba | 0.12 ± 0.01 Cb | 0.01 ± 0.01 Cc |
| 16th | 2.81 ± 0.70 BCc | 7.50 ± 0.10 Aa | 5.92 ± 0.30 Bb | 2.51 ± 0.10 Cc | |
| 32nd | 5.78 ± 0.20 ABc | 12.6 ± 0.60 Ab | 14.8 ± 0.20 Aa | 6.21 ± 0.10 Bc | |
| 48th | 8.18 ± 0.30 Ab | 15.3 ± 0.50 Aa | 14.1 ± 0.86 Aa | 10.1 ± 1.52 Aab | |
| 64th | 7.29 ± 2.21 ABb | 17.6 ± 0.85 Aa | 16.3 ± 0.79 Aa | 10.5 ± 0.30 Aab | |
| 80th | 10.3 ± 0.10 Ab | 16.7 ± 0.33 Aa | 16.2 ± 0.71 Aa | 11.1 ± 0.14 Ab | |
| Oxidized TAG (%) | 0th | 0.47 ± 0.01 Db | 1.84 ± 0.01 BCa | 1.81 ± 0.36 Ba | 0.42 ± 0.10 Db |
| 16th | 5.00 ± 0.80 Cab | 7.66 ± 0.70 Ca | 6.59 ± 0.20 ABab | 4.22 ± 0.08 CDb | |
| 32nd | 7.88 ± 0.90 BCa | 8.52 ± 0.30 BCa | 6.54 ± 1.60 Aba | 8.81 ± 0.96 BCa | |
| 48th | 9.72 ± 0.90 ABa | 9.69 ± 0.70 BCa | 8.63 ± 1.40 Aa | 14.3 ± 1.80 Aa | |
| 64th | 11.9 ± 0.07 Ab | 10.5 ± 0.30 ABc | 11.40 ± 0.20 Abc | 13.2 ± 0.10 ABa | |
| 80th | 12.6 ± 0.50 Aa | 9.81 ± 1.70 Aa | 10.9 ± 0.40 Aa | 13.5 ± 1.20 Aa | |
| DAG (%) | 0th | 6.84 ± 0.01 Aa | 1.55 ± 0.04 BCb | 1.00 ± 0.09 Bb | 3.47 ± 0.90 Bb |
| 16th | 8.08 ± 0.20 Aa | 1.06 ± 0.05 Cc | 1.10 ± 0.01 Bc | 5.71 ± 0.20 ABb | |
| 32nd | 7.45 ± 0.20 Aa | 1.41 ± 0.02 BCc | 1.64 ± 0.40 Ac | 5.94 ± 0.20 ABb | |
| 48th | 7.86 ± 0.01 Aa | 1.43 ± 0.03 BCb | 1.88 ± 0.30 Abb | 7.13 ± 0.50 Aa | |
| 64th | 9.54 ± 1.30 Aa | 2.01 ± 0.02 ABb | 2.11 ± 0.09 Abb | 7.42 ± 0.50 Aa | |
| 80th | 8.70 ± 0.01 Aa | 2.47 ± 0.40 Ab | 2.45 ± 0.01 Ab | 8.25 ± 0.70 Aa | |
| Total (%) | 0th | 7.33 ± 0.01 Eb | 4.05 ± 0.08 Bb | 3.09 ± 0.40 Db | 3.96 ± 1.01 Db |
| 16th | 15.9 ± 0.70 Da | 16.3 ± 0.50 BCa | 13.7 ± 0.50 Cab | 12.6 ± 0.30 Cb | |
| 32nd | 21.2 ±0.90 Ca | 22.7 ± 0.90 ABa | 23.2 ± 1.80 Ba | 21.0 ± 0.80 Ba | |
| 48th | 25.8 ±1.20 Ba | 26.6 ± 0.20 ABa | 24.8 ± 0.20 Ba | 31.5 ± 3.60 Ba | |
| 64th | 29.2 ± 0.40 ABa | 30.2 ±1.10 ABa | 29.9 ± 1.10 Aa | 31.2 ± 0.90 Aa | |
| 80th | 31.7 ± 0.70 Aa | 29.1 ± 2.40 Aa | 29.6 ± 0.30 Aa | 33.0 ± 1.90 Aa | |
| Type of Oils | ||||
|---|---|---|---|---|
| Parameters | PO | SFO | SBO | RPO |
| C14:0 | 1.07 ± 0.01 a | 0.09 ± 0.01 c | 0.09 ± 0.001 c | 0.98 ± 0.01 b |
| C16:0 | 38.3 ± 0.09 a | 6.14 ± 0.06 d | 10.7 ± 0.001 c | 34.9 ± 0.19 b |
| C18:0 | 3.93 ± 0.01 a | 3.35 ± 0.001 a | 4.36 ± 0.001 a | 3.76 ± 0.02 a |
| C18:1 | 43.7 ± 0.07 b | 31.1 ± 0.03 c | 23.3 ± 0.001 d | 46.2 ±0.25 a |
| C18:2 | 11.8 ± 0.36 c | 57.5 ± 0.06 a | 52.9 ± 0.001 b | 12.1 ± 0.07 c |
| C18:3 | 0.31 ± 0.001 b | 0.30 ± 0.01 b | 7.47 ± 0.001 a | 0.31 ± 0.001 b |
| SFA | 43.9 ± 0.20 a | 10.5 ± 0.07 d | 15.9 ± 0.001 c | 40.5 ± 0.22 b |
| MUFA | 43.8 ± 0.16 b | 31.2 ± 0.03 c | 23.4 ± 0.001 d | 46.4 ± 0.33 a |
| PUFA | 12.1 ± 0.36 c | 57.8 ± 0.05 b | 60.3 ± 0.001 a | 12.4 ± 0.07 c |
| Polyene index (PUFA/SFA) | 0.28 c | 5.52 a | 3.78 b | 0.31 c |
| Type of Oils | |||||
|---|---|---|---|---|---|
| Parameter | Frying Cycles | PO | SFO | SBO | RPO |
| Acrylamide (ng/g) | 1st | 252 ± 22.1 d | 451 ± 31.9 c | 608 ± 31.7 b | 827 ± 17.3 a |
| 16th | 229 ± 29.5 c | 566 ± 10.6 b | 607 ± 27.5 b | 853 ± 44.7 a | |
| 32nd | 317 ± 11.8 c | 554 ± 28.3 b | 602 ± 1.35 b | 821 ± 58.3 a | |
| 48th | 398 ± 47.4 b | 466 ± 14.6 b | 605 ± 13.5 a | 799 ± 15.2 a | |
| 64th | 366 ± 5.19 c | 508 ± 13.0 b | 596 ± 13.8 b | 790 ± 24.9 a | |
| 80th | 395 ± 37.6 b | 579 ± 69.2 ab | 659 ± 53.8 ab | 757 ± 18.4 a | |
| Average | 327 ± 9.16 d | 521 ± 4.89 c | 613 ± 5.22 b | 808 ± 29.8 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, S.N.S.; Tarmizi, A.H.A.; Razak, R.A.A.; Jinap, S.; Norliza, S.; Sulaiman, R.; Sanny, M. Selection of Vegetable Oils and Frying Cycles Influencing Acrylamide Formation in the Intermittently Fried Beef Nuggets. Foods 2021, 10, 257. https://doi.org/10.3390/foods10020257
Ahmad SNS, Tarmizi AHA, Razak RAA, Jinap S, Norliza S, Sulaiman R, Sanny M. Selection of Vegetable Oils and Frying Cycles Influencing Acrylamide Formation in the Intermittently Fried Beef Nuggets. Foods. 2021; 10(2):257. https://doi.org/10.3390/foods10020257
Chicago/Turabian StyleAhmad, Siti Nur Syahirah, Azmil Haizam Ahmad Tarmizi, Raznim Arni Abd Razak, Selamat Jinap, Saparin Norliza, Rabiha Sulaiman, and Maimunah Sanny. 2021. "Selection of Vegetable Oils and Frying Cycles Influencing Acrylamide Formation in the Intermittently Fried Beef Nuggets" Foods 10, no. 2: 257. https://doi.org/10.3390/foods10020257
APA StyleAhmad, S. N. S., Tarmizi, A. H. A., Razak, R. A. A., Jinap, S., Norliza, S., Sulaiman, R., & Sanny, M. (2021). Selection of Vegetable Oils and Frying Cycles Influencing Acrylamide Formation in the Intermittently Fried Beef Nuggets. Foods, 10(2), 257. https://doi.org/10.3390/foods10020257