Changes in Physicochemical and Microbiological Properties, Fatty Acid and Volatile Compound Profiles of Apuseni Cheese during Ripening
Abstract
1. Introduction
2. Materials and Methods
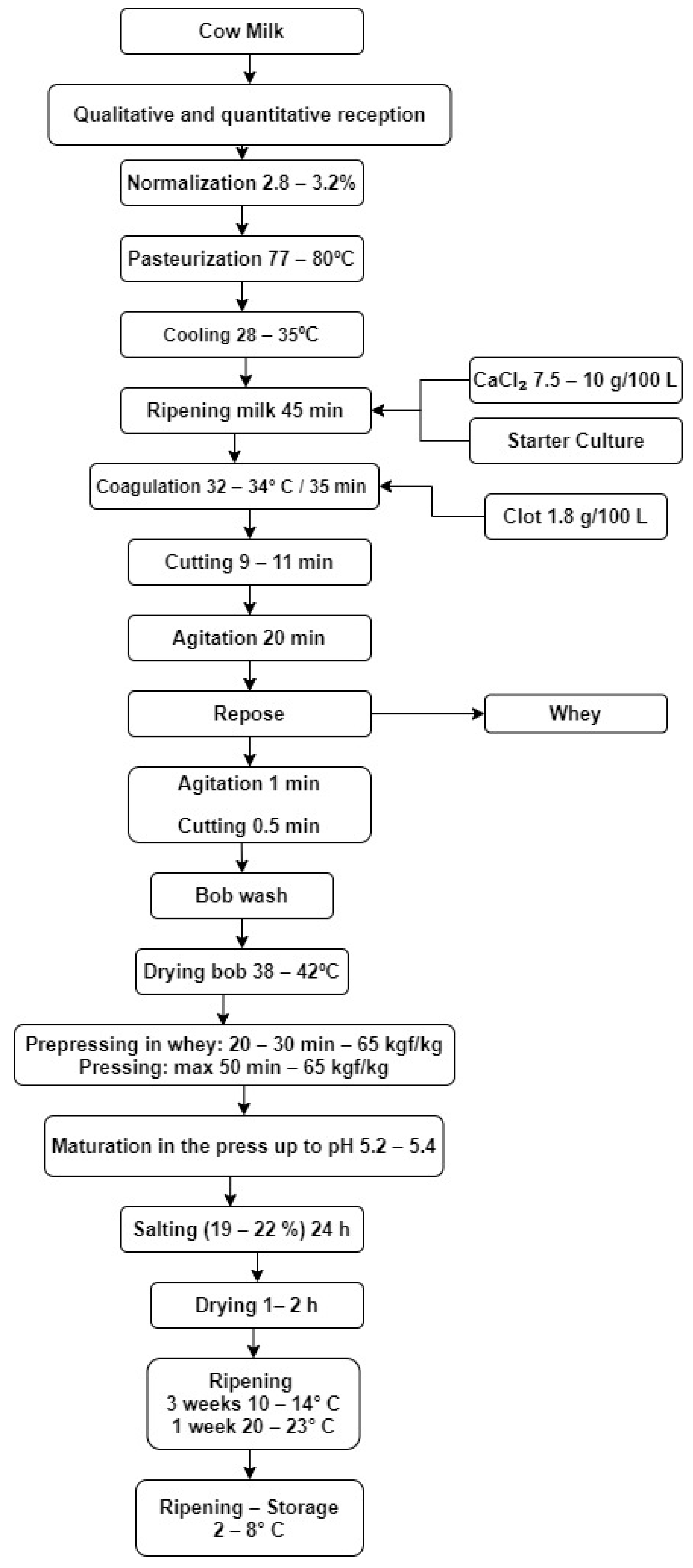
2.1. Materials
2.2. Proximate Composition
2.3. Microbiological Analyses
2.3.1. Determination of Total Combined Yeasts and Molds Count (TYMC)
2.3.2. Determination of Total Viable Count (TVC)
2.3.3. Quantification of Viable Airborne Microorganisms
2.3.4. Identification of Escherichia coli
2.3.5. Identification of Staphylococcus aureus
2.3.6. Detection of Salmonella Species
2.3.7. Determination of Lactic Acid Bacteria (LAB)
2.4. Determination of Fatty Acid Composition in Cheese
2.5. Analysis of Volatile Compounds in Cheese
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physico-Chemical Analyses
3.2. Microbiological Analyses
3.3. Fatty Acid Composition
3.4. ITEX/GCeMS Profile of Volatile Compounds
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| TYMC | total combined yeasts and molds |
| TVC | total viable count |
| LAB | lactic acid bacteria |
| ISO | International Organization for Standardization |
| FFA | free fatty acids |
| FA | fatty acids |
References
- Santiago-López, L.; Aguilar-Toalá, J.E.; Hernández-Mendoza, A.; Vallejo-Cordoba, B.; Liceaga, A.M.; González-Córdova, A.F. Invited review: Bioactive compounds produced during cheese ripening and health effects associated with aged cheese consumption. J. Dairy Sci. 2018, 101, 3742–3757. [Google Scholar] [CrossRef] [PubMed]
- Eurostat. Available online: https://ec.europa.eu/eurostat (accessed on 25 December 2018).
- Fox, P.F.; Guinee, T.; Cogan, T.M.; McSweeney, P.L.H. Microbiology of Cheese Ripening. Fundam. Cheese Sci. 2016, 333–390. [Google Scholar] [CrossRef]
- Diana, M.; Rafecas, M.; Arco, C.; Quílez, J. Free amino acid profile of Spanish artisanal cheeses: Importance of gamma-aminobutyric acid (GABA) and ornithine content. J. Food Compos. Anal. 2014, 35, 94–100. [Google Scholar] [CrossRef]
- D’Incecco, P.; Limbo, S.; Hogenboom, J.A.; Rosi, V.; Gobbi, S.; Pellegrino, L. Impact of Extending Hard-Cheese Ripening: A Multiparameter Characterization of Parmigiano Reggiano Cheese Ripened up to 50 Months. Foods 2020, 9, 268. [Google Scholar] [CrossRef] [PubMed]
- A Fenelon, M.; Guinee, T.P. Primary proteolysis and textural changes during ripening in Cheddar cheeses manufactured to different fat contents. Int. Dairy J. 2000, 10, 151–158. [Google Scholar] [CrossRef]
- Carvalho, G.P.; Santos, R.; Fino, A.; Ferreira, P.; Rodrigues, F.M.; Dias, J. Evolution during Three Ripening Stages of Évora Cheese. Foods 2020, 9, 1140. [Google Scholar] [CrossRef]
- Pinho, O.; Mendes, E.; Alves, M.; Ferreira, I.M. Chemical, Physical, and Sensorial Characteristics of “Terrincho” Ewe Cheese: Changes During Ripening and Intravarietal Comparison. J. Dairy Sci. 2004, 87, 249–257. [Google Scholar] [CrossRef]
- Seratlić, S.V.; Miloradovic, Z.; Radulović, Z.T.; Maćej, O.D. The effect of two types of mould inoculants on the microbiological composition, physicochemical properties and protein hydrolysis in two Gorgonzola-type cheese varieties during ripening. Int. J. Dairy Technol. 2011, 64, 408–416. [Google Scholar] [CrossRef]
- Cakmakci, S.; Dagdemir, E.; Hayaloglu, A.A.; Gurses, M.; Cetin, B.; Tahmas-Kahyaoglu, D. Effect of Penicillium roqueforti and incorporation of whey cheese on volatile profiles and sensory characteristics of mould-ripened Civil cheese. Int. J. Dairy Technol. 2013, 66, 512–526. [Google Scholar]
- Diezhandino, I.; Fernández, D.; González, L.; McSweeney, P.; Fresno, J. Microbiological, physico-chemical and proteolytic changes in a Spanish blue cheese during ripening (Valdeón cheese). Food Chem. 2015, 168, 134–141. [Google Scholar] [CrossRef]
- Albenzio, M.; Corbo, M.R.; Rehman, S.U.; Fox, P.F.; De Angelis, M.; Corsetti, A.; Sevi, A.; Gobbetti, M. Microbiological and biochemical characteristics of Canestrato Pugliese cheese made from raw milk, pasteurized milk or by heating the curd in hot whey. Int. J. Food Microbiol. 2001, 67, 35–48. [Google Scholar] [CrossRef]
- McSweeney, P.L.H. Biochemistry of cheese ripening. Int. J. Dairy Technol. 2004, 57, 127–144. [Google Scholar] [CrossRef]
- Cuchillo-Hilario, M.; Puga, C.D.; Wrage, N.R. Feeding goats on scrubby Mexican rangeland and pasteurization: Influences on milk and artisan cheese quality. Trop. Anim. Heal. Prod. 2010, 42, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Claeys, W.; Cardoen, S.; Daube, G.; De Block, J.; Dewettinck, K.; Dierick, K.; De Zutter, L.; Huyghebaert, A.; Imberechts, H.; Thiange, P.; et al. Raw or heated cow milk consumption: Review of risks and benefits. Food Control. 2013, 31, 251–262. [Google Scholar] [CrossRef]
- Semeniuc, C.A.; Mandrioli, M.; Rodriguez-Estrada, M.T.; Muste, S.; Lercker, G. Thiobarbituric acid reactive substances in flavored phytosterol-enriched drinking yogurts during storage: Formation and matrix interferences. Eur. Food Res. Technol. 2016, 242, 431–439. [Google Scholar] [CrossRef]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L.H. Fundamentals of Cheese Science; Aspen Publishers Inc.: Gaithersburg, MD, USA, 2000. [Google Scholar]
- ISO 3433:2008. Cheese—Determination of Fat Content—Van Gulik Method. Available online: https://www.iso.org/standard/46336.html (accessed on 11 May 2020).
- ISO 8968-1:2014. Milk and Milk Products—Determination of Nitrogen Content—Part 1: Kjeldahl Principle and Crude Protein Calculation. Available online: https://www.iso.org/standard/61020.html (accessed on 11 May 2020).
- ISO 21527-1:2008. Microbiology of food and animal feeding stuffs. Horizontal Method for the Enumeration of Yeasts and Moulds. Part 1: Colony Count Technique in Products with Water Activity Greater than 0.95. Available online: https://www.iso.org/standard/38275.html (accessed on 16 November 2020).
- ISO 4833:2003. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Microorganisms—Colony-Count Technique at 30 Degrees C. Available online: https://www.iso.org/standard/34524.html (accessed on 11 May 2020).
- ISO 4833-2:2013. Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 2: Colony Count at 30 °C by the Surface Plating Technique. Available online: https://www.iso.org/standard/59509.html (accessed on 11 May 2020).
- ISO 21527-2:2008. Microbiology of Food and Animal Feeding stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 2: Colony Count Technique in Products with Water Activity Less than or Equal to 0,95. Available online: https://www.iso.org/standard/38276.html (accessed on 11 May 2020).
- ISO 16649-2:2001. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia Coli—Part 2: Colony-Count Technique at 44 Degrees C Using 5-Bromo-4-Chloro-3-Indolyl Beta-D-Glucuronide. Available online: https://www.iso.org/standard/29824.html (accessed on 11 May 2020).
- ISO/FDIS 6888-2. Microbiology of the Food Chain—Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus Aureus and Other Species) — Part 2: Method Using Rabbit Plasma Fibrinogen Agar Medium. Available online: https://www.iso.org/standard/76673.html (accessed on 11 May 2020).
- ISO 6579:2002. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Detection of Salmonella spp. Available online: https://www.iso.org/standard/29315.html (accessed on 11 May 2020).
- Rotar, A.M.; Vodnar, D.-C.; Bunghez, F.; Cătunescu, G.M.; Pop, C.R.; Jimborean, M.; Semeniuc, C.A. Effect of Goji Berries and Honey on Lactic Acid Bacteria Viability and Shelf Life Stability of Yoghurt. Not. Bot. Horti Agrobot. Cluj Napoca 2015, 43, 196–203. [Google Scholar] [CrossRef]
- Szabo, K.; Dulf, F.V.; Diaconeasa, Z.; Vodnar, D.-C. Antimicrobial and antioxidant properties of tomato processing byproducts and their correlation with the biochemical composition. LWT 2019, 116, 108558. [Google Scholar] [CrossRef]
- Gan, H.H.; Yan, B.; Linforth, R.S.; Fisk, I.D. Development and validation of an APCI-MS/GC–MS approach for the classification and prediction of Cheddar cheese maturity. Food Chem. 2016, 190, 442–447. [Google Scholar] [CrossRef]
- Manzo, N.; Souto, E.B.; Pizzolongo, F.; Aiello, A.; Marrazzo, A.; Meca, G.; Durazzo, A.; Lucarini, M.; Romano, R. Influence of Ripening on Chemical Characteristics of a Traditional Italian Cheese: Provolone del Monaco. Sustainability 2019, 11, 2520. [Google Scholar] [CrossRef]
- Brickley, C.A.; Auty, M.A.E.; Piraino, P.; McSweeney, P.L.H. The Effect of Natural Cheddar Cheese Ripening on the Functional and Textural Properties of the Processed Cheese Manufactured Therefrom. J. Food Sci. 2007, 72, C483–C490. [Google Scholar] [CrossRef]
- Cichoscki, A.J.; Valduga, E.; Valduga, A.T.; Tornadijo, M.E.; Fresno, J. Characterization of Prato cheese, a Brazilian semi-hard cow variety: Evolution of physico-chemical parameters and mineral composition during ripening. Food Control. 2002, 13, 329–336. [Google Scholar] [CrossRef]
- Møller, K.; Rattray, F.; Bredie, W.; Høier, E.; Ardö, Y. Physicochemical and sensory characterization of Cheddar cheese with variable NaCl levels and equal moisture content. J. Dairy Sci. 2013, 96, 1953–1971. [Google Scholar] [CrossRef] [PubMed]
- Order of the Ministry of Health -976/1998. Available online: http://legislatie.just.ro/Public/DetaliiDocument/18424 (accessed on 16 May 2020).
- Hayaloglu, A. Cheese: Microbiology of Cheese. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Akın, N.; Aydemir, S.; Koçak, C.; Yıldız, M.A.; Akin, N. Changes of free fatty acid contents and sensory properties of white pickled cheese during ripening. Food Chem. 2003, 80, 77–83. [Google Scholar] [CrossRef]
- Białek, A.; Białek, A.; Lepionka, T.; Czerwonka, M.; Czauderna, M. Chemometric Analysis of Fatty Acids Profile of Ripening Chesses. Molecules 2020, 25, 1814. [Google Scholar] [CrossRef]
- Collins, Y.F.; McSweeney, P.L.; Wilkinson, M.G. Lipolysis and free fatty acid catabolism in cheese: A review of current knowledge. Int. Dairy J. 2003, 13, 841–866. [Google Scholar] [CrossRef]
- Chávarri, F.; Bustamante, M.A.; Santisteban, A.; Virto, M.; Barron, L.J.R.; De Renobales, M. Changes in Free Fatty Acids During Ripening of Idiazabal Cheese Manufactured at Different Times of the Year. J. Dairy Sci. 1999, 82, 885–890. [Google Scholar] [CrossRef]
- Malacarne, M.; Summer, A.; Franceschi, P.; Formaggioni, P.; Pecorari, M.; Panari, G.; Mariani, P. Free fatty acid profile of Parmigiano–Reggiano cheese throughout ripening: Comparison between the inner and outer regions of the wheel. Int. Dairy J. 2009, 19, 637–641. [Google Scholar] [CrossRef]
- Fuente, M.A.; Fontecha, J.; Juárez, M. Fatty acid composition of the triglyceride and free fatty acid fractions in different cows-, ewes- and goats-milk cheeses. Eur. Food Res. Technol. 1993, 196, 155–158. [Google Scholar] [CrossRef]
- Woo, A.H.; Lindsay, R.C. Concentrations of Major Free Fatty Acids and Flavor Development in Italian Cheese Varieties. J. Dairy Sci. 1984, 67, 960–968. [Google Scholar] [CrossRef]
- McNeill, G.P.; Connolly, J.F. A Method for the Quantification of Individual Free Fatty Acids in Cheese: Applica-tion to Ripening of Cheddar-Type Cheeses. Ir. J. Food Sci. Technol. 1989, 13, 119–128. [Google Scholar]
- Gatzias, I.; Karabagias, I.; Kontominas, M.; Badeka, A.V. Geographical differentiation of feta cheese from northern Greece based on physicochemical parameters, volatile compounds and fatty acids. LWT 2020, 131, 109615. [Google Scholar] [CrossRef]
- Berard, J.; Bianchi, F.; Careri, M.; Chatel, A.; Mangia, A.; Musci, M. Characterization of the volatile fraction and of free fatty acids of “Fontina Valle d’Aosta”, a protected designation of origin Italian cheese. Food Chem. 2007, 105, 293–300. [Google Scholar] [CrossRef]
- Kilcawley, K.N.; Wilkinson, M.; Fox, P. A Survey of Lipolytic and Glycolytic End-Products in Commercial Cheddar Enzyme-Modified Cheese. J. Dairy Sci. 2001, 84, 66–73. [Google Scholar] [CrossRef]
- Bills, D.; Day, E. Determination of the Major Free Fatty Acids of Cheddar Cheese. J. Dairy Sci. 1964, 47, 733–738. [Google Scholar] [CrossRef]
- McSweeney, P.; Sousa, M. Biochemical pathways for the production of flavour compounds in cheeses during ripening: A review. Lait INRA Ed. 2000, 80, 293–324. [Google Scholar] [CrossRef]
- Garaffo, M.A.; Vassallo-Agius, R.; Nengas, Y.; Lembo, E.; Rando, R.; Maisano, R.; Dugo, G.; Giuffrida, D. Fatty Acids Profile, Atherogenic (IA) and Thrombogenic (IT) Health Lipid Indices, of Raw Roe of Blue Fin Tuna (Thunnus thynnus L.) and Their Salted Product “Bottarga”. Food Nutr. Sci. 2011, 2, 736–743. [Google Scholar] [CrossRef]
- Perotti, M.; Bernal, S.; Meinardi, C.; Zalazar, C. Free fatty acid profiles of Reggianito Argentino cheese produced with different starters. Int. Dairy J. 2005, 15, 1150–1155. [Google Scholar] [CrossRef]
- Elgersma, A. Grazing increases the unsaturated fatty acid concentration of milk from grass-fed cows: A review of the contributing factors, challenges and future perspectives. Eur. J. Lipid Sci. Technol. 2015, 117, 1345–1369. [Google Scholar] [CrossRef]
- Mitani, T.; Kobayashi, K.; Ueda, K.; Kondo, S. Discrimination of “grazing milk” using milk fatty acid profile in the grassland dairy area in Hokkaido. Anim. Sci. J. 2016, 87, 233–241. [Google Scholar] [CrossRef][Green Version]
- Summer, A.; Formaggioni, P.; Franceschi, P.; Di Frangia, F.; Righi, F.; Malacarne, M. Cheese as Functional Food: The Example of Parmigiano Reggiano and Grana Padano. Food Technol. Biotechnol. 2017, 55, 277–289. [Google Scholar] [CrossRef]
- Carbonell, M.; Nuñez, M.; Fernández-García, E. Evolution of the volatile components of ewe raw milk La Serena cheese during ripening. Correlation with flavour characteristics. Lait 2002, 82, 683–698. [Google Scholar] [CrossRef]
- Bertuzzi, A.S.; McSweeney, P.L.H.; Rea, M.C.; Kilcawley, K.N. Detection of Volatile Compounds of Cheese and Their Contribution to the Flavor Profile of Surface-Ripened Cheese. Compr. Rev. Food Sci. Food Saf. 2018, 17, 371–390. [Google Scholar] [CrossRef]
- Karoui, R.; De Baerdemaeker, J. A review of the analytical methods coupled with chemometric tools for the determination of the quality and identity of dairy products. Food Chem. 2007, 102, 621–640. [Google Scholar] [CrossRef]
- Faulkner, H.; O’Callaghan, T.F.; McAuliffe, S.; Hennessy, D.; Stanton, C.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Effect of different forage types on the volatile and sensory properties of bovine milk. J. Dairy Sci. 2018, 101, 1034–1047. [Google Scholar] [CrossRef] [PubMed]
- Belviso, S.; Giordano, M.; Dolci, P.; Zeppa, G. Degradation and biosynthesis of terpenoids by lactic acid bacteria isolated from cheese: First evidence. Dairy Sci. Technol. 2011, 91, 227–236. [Google Scholar] [CrossRef][Green Version]
- Kilic-Akyilmaz, M.; Lindsay, R. Distribution of Conjugates of Alkylphenols in Milk from Different Ruminant Species. J. Dairy Sci. 2005, 88, 7–12. [Google Scholar] [CrossRef]
- Salum, P.; Govce, G.; Kendirci, P.; Baş, D.; Erbay, Z. Composition, proteolysis, lipolysis, volatile compound profile and sensory characteristics of ripened white cheeses manufactured in different geographical regions of Turkey. Int. Dairy J. 2018, 87, 26–36. [Google Scholar] [CrossRef]
- Khattab, A.R.; Guirguis, H.A.; Tawfik, S.M.; Farag, M.A. Cheese ripening: A review on modern technologies towards flavor enhancement, process acceleration and improved quality assessment. Trends Food Sci. Technol. 2019, 88, 343–360. [Google Scholar] [CrossRef]
- Van Der Spek, R.; Kreier, F.; Fliers, E.; Kalsbeek, A. Circadian rhythms in white adipose tissue. Cholinergic Mech. Mol. Biol. Clin. Signif. 2012, 183–201. [Google Scholar] [CrossRef]

| Samples | Moisture (%) | Fat (%) | Fat in Dry Matter (%) | Ash (%) | Total Protein (%) | NaCl (%) | pH |
|---|---|---|---|---|---|---|---|
| D1 | 54.66 ± 0.49 a | 24.40 ± 0.23 d | 43.79 ± 0.28 d | 4.04 ± 0.12 c | 25.00 ± 0.17 d | 1.81 ± 0.08 d | 5.44 ± 0.02 a |
| D2 | 52.91 ± 0.36 a | 27.55 ± 0.48 c | 57.55 ± 0.45 c | 4.32 ± 0.10 bc | 26.71 ± 0.36 c | 1.93 ± 0.04 cd | 5.37 ± 0.03 ab |
| D3 | 50.04 ± 0.59 b | 29.11 ± 0.33 b | 58.46 ± 0.46 bc | 4.56 ± 0.09 ab | 27.25 ± 0.20 bc | 2.12 ± 0.05 bc | 5.32 ± 0.03 bc |
| D4 | 47.80 ± 0.40 c | 31.56 ± 0.19 a | 59.77 ± 0.28 ab | 4.74 ± 0.07 a | 28.21 ± 0.32 ab | 2.30 ± 0.06 ab | 5.23 ± 0.02 cd |
| D5 | 46.69 ± 0.39 c | 32.16 ± 0.23 a | 60.32 ± 0.27 a | 4.91 ± 0.06 a | 29.47 ± 0.54 a | 2.43 ± 0.06 a | 5.19 ± 0.02 d |
| Samples | D1 | D2 | D3 | D4 | D5 |
|---|---|---|---|---|---|
| TYMC (cfu/m3 × 102) | 0.31 ± 0.03 e | 0.83 ± 0.02 d | 1.55 ± 0.06 c | 2.45 ± 0.07 b | 2.85 ± 0.08 a |
| TVC (cfu/m3 × 102) | 2.05 ± 0.06 c | 2.10 ± 0.11 c | 3.40 ± 0.11 b | 5.50 ± 0.07 a | 5.82 ± 0.04 a |
| Samples | TYMC (cfu/g × 103) | TVC (cfu/g × 102) | Escherichia coli (cfu/g × 102) | Staphylococcus aureus (cfu/g × 102) | Salmonella (cfu/25 g) | LAB (cfu/g × 108) |
|---|---|---|---|---|---|---|
| D1 | 0.12 ± 0.01 e | 1.25 ± 0.06 e | 5.02 ± 0.05 | 0.90 ± 0.03 d | n.d. | 0.55 ± 0.07 a |
| D2 | 2.68 ± 0.08 d | 4.50 ± 0.14 d | not detected | 1.07 ± 0.06 d | n.d. | 0.64 ± 0.08 a |
| D3 | 8.70 ± 0.13 c | 6.20 ± 0.13 c | not detected | 2.35 ± 0.15 c | n.d. | 0.73 ± 0.18 a |
| D4 | 12.4 ± 0.14 a | 10.50 ± 0.08 b | not detected | 4.37 ± 0.22 b | n.d. | 0.84 ± 0.17 a |
| D5 | 16.52 ± 0.11 a | 18.40 ± 0.07 a | not detected | 7.80 ± 0.14 a | n.d. | 0.90 ± 0.13 a |
| No | Fatty Acids (% of Total Fatty Acids) | D1 | D2 | D3 | D4 | D5 |
|---|---|---|---|---|---|---|
| 1 | C4:0 | 3.035 ± 0.021 a | 2.680 ± 0.014 b | 2.580 ± 0.028 c | 2.200 ± 0.014 d | 1.555 ± 0.021 e |
| 2 | C6:0 | 3.270 ± 0.014 a | 2.795 ± 0.021 b | 2.695 ± 0.021 c | 2.320 ± 0.028 d | 2.085 ± 0.021 e |
| 3 | C8:0 | 1.689 ± 0.001 c | 1.700 ± 0.001 b | 1.712 ± 0.001 a | 1.722 ± 0.002 a | 1.117 ± 0.005 d |
| 4 | C10:0 | 3.380 ± 0.001 d | 3.780 ± 0.001 b | 3.812 ± 0.002 a | 2.931 ± 0.002 e | 3.510 ± 0.002 c |
| 5 | C12:0 | 3.660 ± 0.028 b | 3.360 ± 0.042 c | 4.200 ± 0.014 a | 3.685 ± 0.021 b | 3.760 ± 0.014 b |
| 6 | C14:0 | 13.615 ± 0.021 c | 13.640 ± 0.042 c | 13.870 ± 0.028 b | 14.090 ± 0.071 a | 12.215 ± 0.021 d |
| 7 | C15:0 | 1.281 ± 0.004 a | 1.140 ± 0.001 b | 1.101 ± 0.002 c | 1.000 ± 0.001 d | 0.920 ± 0.003 e |
| 8 | C16:0 | 38.425 ± 0.049 a | 38.250 ± 0.028 a | 36.985 ± 0.078 b | 36.670 ± 0.057 c | 35.603 ± 0.053 d |
| 9 | C16:1 n-9 | 0.901 ± 0.001 a | 0.681 ± 0.003 b | 0.420 ± 0.001 c | 0.260 ± 0.001 d | 0.250 ± 0.001 e |
| 10 | C18:0 | 9.570 ± 0.028 a | 7.940 ± 0.035 c | 7.567 ± 0.045 d | 8.195 ± 0.021 b | 7.640 ± 0.049 d |
| 11 | C18:1 n-9 | 19.860 ± 0.014 e | 22.390 ± 0.085 d | 23.718 ± 0.067 c | 24.588 ± 0.039 b | 28.113 ± 0.032 a |
| 12 | C18:1 n-7 | 0.059 ± 0.001 e | 0.130 ± 0.004 d | 0.272 ± 0.009 c | 0.500 ± 0.004 b | 0.620 ± 0.003 a |
| 13 | C18:2 n-6 | 1.540 ± 0.001 a | 1.260 ± 0.002 b | 0.950 ± 0.001 c | 0.870 ± 0.001 d | 0.761 ± 0.002 e |
| 14 | C18:3 n-3 | 0.181 ± 0.005 c | 0.300 ± 0.007 b | 0.311 ± 0.001 b | 0.341 ± 0.008 a | 0.351 ± 0.004 a |
| ƩSFAs | 77.930 ± 0.028 a | 75.290 ± 0.014 b | 74.515 ± 0.049 c | 72.828 ± 0.025 d | 68.423 ± 0.018 e | |
| ƩMUFAs | 20.820 ± 0.014 e | 23.200 ± 0.014 d | 24.415 ± 0.021 c | 25.340 ± 0.014 b | 28.975 ± 0.021 a | |
| ƩPUFAs | 1.722 ± 0.006 a | 1.560 ± 0.014 b | 1.259 ± 0.005 c | 1.210 ± 0.014 d | 1.109 ± 0.005 e | |
| n-6/n-3 | 8.545 ± 0.021 a | 4.203 ± 0.018 b | 3.060 ± 0.014 c | 2.550 ± 0.014 d | 2.170 ± 0.042 e | |
| Compound Name | RI (Retention Indices) | D1 | D2 | D3 | D4 | D5 | Odor Characteristic Descriptors |
|---|---|---|---|---|---|---|---|
| Alcohols | |||||||
| 2,3-Butanediol | 771 | n.d. | 0.96 ± 0.03 | n.d. | n.d. | n.d. | fruity |
| Phenol | 981 | n.d. | 3.85 ± 0.07 | n.d. | n.d. | n.d. | phenolic |
| Aldehydes | |||||||
| Benzaldehyde | 958 | 10.52 ± 0.04 a | 1.41 ± 0.02 e | 1.97 ± 0.04 d | 2.84 ± 0.02 b | 2.57 ± 0.05 c | almond, burnt sugar |
| Ketones | |||||||
| 2-Heptanone | 896 | n.d. | 11.99 ± 0.19 c | 24.53 ± 0.11 b | 12.25 ± 0.11 c | 54.97 ± 0.26 a | sulfur, pungent, green, fruity |
| Acetophenone | 1042 | 68.66 ± 0.39 a | 8.78 ± 0.12 c | 10.52 ± 0.10 b | 7.88 ± 0.16 d | 4.90 ± 0.13 e | sweet, flower, almond |
| 2-Nonanone | 1094 | n.d. | 2.96 ± 0.11 b | n.d. | n.d. | 5.20 ± 0.21 a | fruity, floral |
| Benzophenone | 1605 | n.d. | n.d. | 1.18 ± 0.10 b | 1.97 ± 0.08 a | 1.45 ± 0.16 b | faint, sweet rose, light herbal |
| Ethanone, 1-(4-methylphenyl)- | 1182 | n.d. | 7.14 ± 0.18 | n.d. | n.d. | n.d. | almond, sweet, floral, hay |
| Terpene hydrocarbons and oxygenated derivatives | |||||||
| p-Cymene | 1028 | n.d. | 1.37 ± 0.05 | n.d. | n.d. | n.d. | citrus |
| Caryophyllene | 1468 | n.d. | 3.22 ± 0.18 | n.d. | n.d. | n.d. | woody, spicy, fruity, sweet |
| α-Caryophyllene | 1454 | n.d. | 3.95 ± 0.14 | n.d. | n.d. | n.d. | fruity, woody |
| Limonene | 1031 | n.d. | 10.8 ± 0.16 b | 8.46 ± 0.09 c | 12.90 ± 0.13 a | 6.26 ± 0.21 b | citrus, mint |
| 2,6-Octadienal, 3,7-dimethyl-, (E)- | 1255 | 20.82 ± 0.23 | n.d. | n.d. | n.d. | n.d. | lemon |
| Verbenone | 1205 | n.d. | 1.76 ± 0.10 a | 1.72 ± 0.09 a | n.d. | n.d. | spicy |
| Thymol | 1291 | n.d. | 22.53 ± 0.37 a | 20.42 ± 0.26 b | 9.16 ± 0.19 c | 3.66 ± 0.09 d | herbal, thyme, earthy |
| Carvacrol | 1299 | n.d. | 2.46 ± 0.35 a | 3.23 ± 0.11 a | 0.84 ± 0.07 b | n.d. | caraway |
| Esters | |||||||
| Butanoic acid, ethyl ester | 805 | n.d. | 11.60 ± 0.13 c | 13.54 ± 0.21 c | 23.92 ± 0.13 a | 4.91 ± 0.04 d | fruity, sweet |
| Acetic acid, butyl ester | 813 | n.d. | n.d. | n.d. | 14.92 ± 0.16 a | 4.13 ± 0.11 b | fruity, sweet, green, pear-like |
| Butanoic acid, propyl ester | 897 | n.d. | n.d. | n.d. | 4.57 ± 0.13 a | 2.60 ± 0.13 b | pineapple, solvent |
| Butanoic acid, butyl ester | 993 | n.d. | n.d. | n.d. | n.d. | 1.21 ± 0.16 | fresh, sweet, fruity |
| Hexanoic acid, ethyl ester | 1003 | n.d. | 1.54 ± 0.22 c | 3.89 ± 0.14 ab | 4.46 ± 0.14 a | 3.36 ± 0.11 b | fruity, fresh, sweet |
| Others | |||||||
| n.i. | n.d. | 1.55 ± 0.08 | n.d. | n.d. | n.d. | ||
| Benzoic Acid | 1174 | n.d. | n.d. | 10.54 ± 0.22 a | 4.27 ± 0.18 b | 4.79 ± 0.28 b | winey, balsamic |
| n.i. | n.d. | 2.13 ± 0.17 a | 1.72 ± 0.13 a | n.d. | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mureşan, C.C.; Marc, R.A.; Anamaria Semeniuc, C.; Ancuţa Socaci, S.; Fărcaş, A.; Fracisc, D.; Rodica Pop, C.; Rotar, A.; Dodan, A.; Mureşan, V.; et al. Changes in Physicochemical and Microbiological Properties, Fatty Acid and Volatile Compound Profiles of Apuseni Cheese during Ripening. Foods 2021, 10, 258. https://doi.org/10.3390/foods10020258
Mureşan CC, Marc RA, Anamaria Semeniuc C, Ancuţa Socaci S, Fărcaş A, Fracisc D, Rodica Pop C, Rotar A, Dodan A, Mureşan V, et al. Changes in Physicochemical and Microbiological Properties, Fatty Acid and Volatile Compound Profiles of Apuseni Cheese during Ripening. Foods. 2021; 10(2):258. https://doi.org/10.3390/foods10020258
Chicago/Turabian StyleMureşan, Crina Carmen, Romina Alina (Vlaic) Marc, Cristina Anamaria Semeniuc, Sonia Ancuţa Socaci, Anca Fărcaş, Dulf Fracisc, Carmen Rodica Pop, Ancuţa Rotar, Andreea Dodan, Vlad Mureşan, and et al. 2021. "Changes in Physicochemical and Microbiological Properties, Fatty Acid and Volatile Compound Profiles of Apuseni Cheese during Ripening" Foods 10, no. 2: 258. https://doi.org/10.3390/foods10020258
APA StyleMureşan, C. C., Marc, R. A., Anamaria Semeniuc, C., Ancuţa Socaci, S., Fărcaş, A., Fracisc, D., Rodica Pop, C., Rotar, A., Dodan, A., Mureşan, V., & Mureşan, A. E. (2021). Changes in Physicochemical and Microbiological Properties, Fatty Acid and Volatile Compound Profiles of Apuseni Cheese during Ripening. Foods, 10(2), 258. https://doi.org/10.3390/foods10020258





.jpg)










