Brown Algae Potential as a Functional Food against Hypercholesterolemia: Review
Abstract
1. Introduction
2. Hypercholesterolemia and the Actual Treatment
3. The Anti-Hypercholesterolemic Effect of Brown Algae
3.1. Carotenoids—Fucoxanthin
3.2. Polysaccharides
3.3. Phlorotannins
3.4. Proteins/Peptides
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Tanna, B.; Mishra, A. Nutraceutical Potential of Seaweed Polysaccharides: Structure, Bioactivity, Safety, and Toxicity. Compr. Rev. Food Sci. Food Saf. 2019, 18, 817–831. [Google Scholar] [CrossRef]
- Ferdouse, F.; Holdt, S.L.; Smith, R.; Murúa, P.; Yang, Z. The Global Satus of Seaweed Production, Trade and Utilization; FAO: Rome, Italy, 2018; Volume 124. [Google Scholar]
- El Gamal, A.A. Biological importance of marine algae. Saudi Pharm. J. 2010, 18, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Abbott, I.A. Edible Seaweeds of China and Their Place in the Chinese Diet. Econ. Bot. 1987, 41, 341–353. [Google Scholar]
- Mac Monagail, M.; Cornish, L.; Morrison, L.; Araújo, R.; Critchley, A.T. Sustainable harvesting of wild seaweed resources. Eur. J. Phycol. 2017, 52, 371–390. [Google Scholar] [CrossRef]
- Ścieszka, S.; Klewicka, E. Algae in food: A general review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3538–3547. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of the World Fisheries and Aquaculture 2014; FAO: Rome, Italy, 2014. [Google Scholar]
- Lorenzo, J.; Agregán, R.; Munekata, P.; Franco, D.; Carballo, J.; Şahin, S.; Lacomba, R.; Barba, F. Proximate Composition and Nutritional Value of Three Macroalgae: Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata. Mar. Drugs 2017, 15, 360. [Google Scholar] [CrossRef]
- Edible seaweed and microalgae—Regulatory status in France and Europe—2019 update. Available online: https://www.ceva-algues.com/en/document/edible-algae-regulatory-update/ (accessed on 28 July 2020).
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed nutraceuticals and their therapeutic role in disease prevention. Food Sci. Hum. Wellness 2019, 8, 252–263. [Google Scholar] [CrossRef]
- Catarino, M.; Silva, A.; Cardoso, S. Phycochemical Constituents and Biological Activities of Fucus spp. Mar. Drugs 2018, 16, 249. [Google Scholar] [CrossRef]
- Yoon, N.Y.; Kim, H.R.; Chung, H.Y.; Choi, J.S. Anti-hyperlipidemic effect of an edible brown algae, Ecklonia stolonifera, and its constituents on poloxamer 407-induced hyperlipidemic and cholesterol-fed rats. Arch. Pharm. Res. 2008, 31, 1564–1571. [Google Scholar] [CrossRef]
- Neto, R.; Marçal, C.; Queirós, A.; Abreu, H.; Silva, A.; Cardoso, S. Screening of Ulva rigida, Gracilaria sp., Fucus vesiculosus and Saccharina latissima as Functional Ingredients. Int. J. Mol. Sci. 2018, 19, 2987. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Yamanashi, Y.; Takada, T.; Mu, S.; Tanaka, Y.; Komine, T.; Suzuki, H. Hepatic expression of Niemann-Pick C1-like 1, a cholesterol reabsorber from bile, exacerbates western diet-induced atherosclerosis in LDL receptor mutant mice S. Mol. Pharmacol. 2019, 96, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, Y.; Shi, J.; Yu, Y.; Lu, H.; Yu, L.; Liu, Y.; Zhang, F. Diosgenin regulates cholesterol metabolism in hypercholesterolemic rats by inhibiting NPC1L1 and enhancing ABCG5 and ABCG8. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2019, 1864, 1124–1133. [Google Scholar] [CrossRef]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Bairey Merz, C.N.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults. Circulation 2014, 129, S1–S45. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.K.; Park, S.H.; Ha, K.C.; Noh, S.O.; Jung, S.J.; Chae, H.J.; Chae, S.W.; Park, T.S. Clinical trial of the hypolipidemic effects of a brown alga Ecklonia cava extract in patients with hypercholesterolemia. Int. J. Pharmacol. 2015, 11, 798–805. [Google Scholar] [CrossRef]
- Sahoo, D.; Seckbach, J. (Eds.) The Algae World, 1st ed.; Springer: Dordrecht, The Netherlands, 2015; ISBN 9789401773201. [Google Scholar]
- Ge, L.; Wang, J.; Qi, W.; Miao, H.-H.; Cao, J.; Qu, Y.-X.; Li, B.-L.; Song, B.-L. The Cholesterol Absorption Inhibitor Ezetimibe Acts by Blocking the Sterol-Induced Internalization of NPC1L1. Cell Metab. 2008, 7, 508–519. [Google Scholar] [CrossRef]
- Axmann, M.; Strobl, W.M.; Plochberger, B.; Stangl, H. Cholesterol transfer at the plasma membrane. Atherosclerosis 2019, 290, 111–117. [Google Scholar] [CrossRef]
- Altmann, S.W.; Davis, H.R.; Zhu, L.J.; Yao, X.; Hoos, L.M.; Tetzloff, G.; Iyer, S.P.N.; Maguire, M.; Golovko, A.; Zeng, M.; et al. Niemann-Pick C1 Like 1 Protein Is Critical for Intestinal Cholesterol Absorption. Science 2004, 303, 1201–1204. [Google Scholar] [CrossRef]
- Espinheira, M.C.; Vasconcelos, C.; Medeiros, A.M.; Alves, A.C.; Bourbon, M.; Guerra, A. Hipercolesterolemia—Uma patologia com expressão desde a idade pediátrica. Rev. Port. Cardiol. 2013, 32, 379–386. [Google Scholar] [CrossRef]
- White, C.R.; Anantharamaiah, G.M.; Datta, G. HDL mimetic peptides: Novel therapeutic strategies for the treatment of inflammatory vascular disease. In The HDL Handbook; Elsevier Inc.: Philadelphia, PA, USA, 2010; pp. 179–197. ISBN 9780123821713. [Google Scholar]
- Marques, L.R.; Diniz, T.A.; Antunes, B.M.; Rossi, F.E.; Caperuto, E.C.; Lira, F.S.; Gonçalves, D.C. Reverse cholesterol transport: Molecular mechanisms and the non-medical approach to enhance HDL cholesterol. Front. Physiol. 2018, 9, 526. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Bobryshev, Y.V.; Orekhov, A.N. Macrophage-mediated cholesterol handling in atherosclerosis. J. Cell. Mol. Med. 2016, 20, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wang, J.; Li, F.; Yang, Z.; Yang, X.; Sun, W.; Xia, B.; Li, T.; Song, W.; Guo, S. The fucoidan from the brown seaweed: Ascophyllum nodosum ameliorates atherosclerosis in apolipoprotein E-deficient mice. Food Funct. 2019, 10, 5124–5139. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Zong, C.; Liu, Q.; Si, Y.; Liu, J.; Li, W.; Zhu, P.; Qin, S. SR-BI associates with ABCG1 and inhibits ABCG1-mediated cholesterol efflux from cells to high-density lipoprotein 3. Lipids Health Dis. 2012, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Dikkers, A.; Tietge, U.J.F. Biliary cholesterol secretion: More than a simple ABC. World J. Gastroenterol. 2010, 16, 5936–5945. [Google Scholar] [CrossRef] [PubMed]
- Post, S.M.; De Crom, R.; Van Haperen, R.; Van Tol, A.; Princen, H.M.G. Increased fecal bile acid excretion in transgenic mice with elevated expression of human phospholipid transfer protein. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 892–897. [Google Scholar] [CrossRef]
- Bosner, M.S.; Lange, L.G.; Stenson, W.F.; Ostlund, R.E. Percent cholesterol absorption in normal women and men quantified with dual stable isotopic tracers and negative ion mass spectrometry. J. Lipid Res. 1999, 40, 302–308. [Google Scholar] [CrossRef]
- Betters, J.L.; Yu, L. NPC1L1 and cholesterol transport. FEBS Lett. 2010, 584, 2740–2747. [Google Scholar] [CrossRef]
- Jia, L.; Betters, J.L.; Yu, L. Niemann-Pick C1-Like 1 (NPC1L1) Protein in Intestinal and Hepatic Cholesterol Transport. Annu. Rev. Physiol. 2011, 73, 239–259. [Google Scholar] [CrossRef]
- Yassine, H.N.; Belopolskaya, A.; Schall, C.; Stump, C.S.; Lau, S.S.; Reaven, P.D. Enhanced cholesterol efflux to HDL through the ABCA1 transporter in hypertriglyceridemia of type 2 diabetes. Metabolism 2014, 63, 727–734. [Google Scholar] [CrossRef]
- Hui, D.Y.; Howles, P.N. Molecular mechanisms of cholesterol absorption and transport in the intestine. Semin. Cell Dev. Biol. 2005, 16, 183–192. [Google Scholar] [CrossRef]
- Istvan, E. Statin inhibition of HMG-CoA reductase: A 3-dimensional view. Atheroscler. Suppl. 2003, 4, 3–8. [Google Scholar] [CrossRef]
- Feingold, K.R. Cholesterol Lowering Drugs. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., Dungan, K., Grossman, A., Hershman, J.M., Kaltsas, G., Koch, C., Kopp, P., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2020. [Google Scholar]
- Ono, K. Current concept of reverse cholesterol transport and novel strategy for atheroprotection. J. Cardiol. 2012, 60, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M. Are the effects of statins on HDL-cholesterol clinically relevant? Eur. Hear. J. Suppl. 2004, 6, C58–C63. [Google Scholar] [CrossRef]
- Maejima, T.; Yamazaki, H.; Aoki, T.; Tamaki, T.; Sato, F.; Kitahara, M.; Saito, Y. Effect of pitavastatin on apolipoprotein A-I production in HepG2 cell. Biochem. Biophys. Res. Commun. 2004, 324, 835–839. [Google Scholar] [CrossRef]
- Xie, P.; Zhu, H.; Jia, L.; Ma, Y.; Tang, W.; Wang, Y.; Xue, B.; Shi, H.; Yu, L. Genetic demonstration of intestinal NPC1L1 as a major determinant of hepatic cholesterol and blood atherogenic lipoprotein levels. Atherosclerosis 2014, 237, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.; Xia, P.; Tang, M.; Liu, F.; Shu, M.; Wu, X. Ezetimibe for the prevention of cardiovascular disease and all-cause mortality events. Cochrane Database Syst. Rev. 2017, 2017. [Google Scholar] [CrossRef]
- Jaiganesh, R.; Sampath Kumar, N.S. Marine Bacterial Sources of Bioactive Compounds. Adv. Food Nutr. Res. 2012, 65, 389–408. [Google Scholar] [CrossRef]
- Ji, S.Q.; Wang, B.; Lu, M.; Li, F.L. Direct bioconversion of brown algae into ethanol by thermophilic bacterium Defluviitalea phaphyphila. Biotechnol. Biofuels 2016, 9, 1–10. [Google Scholar] [CrossRef]
- Wehr, J.D. Chapter 19—Brown Algae. In Freshwater Algae of North America; Wehr, J.D., Sheath, R.G., Kociolek, J.P., Eds.; Elsevier: Boston, MA, USA, 2015; pp. 851–871. ISBN 978-0-12-385876-4. [Google Scholar]
- Petruzzello, M. Brown Algae. Available online: https://www.britannica.com/science/brown-algae (accessed on 28 July 2020).
- Sheath, R.G.; Wehr, J.D. Introduction to the Freshwater Algae. In Freshwater Algae of North America; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–11. ISBN 9780123858771. [Google Scholar]
- Díaz-Rubio, M.E.; Pérez-Jiménez, J.; Saura-Calixto, F. Dietary fiber and antioxidant capacity in Fucus vesiculosus products. Int. J. Food Sci. Nutr. 2009, 60, 23–34. [Google Scholar] [CrossRef]
- Scarpini, E.; Scheltens, P.; Feldman, H. Treatment of Alzheimer’s disease: Current status and new perspectives. Lancet Neurol. 2003, 2, 539–547. [Google Scholar] [CrossRef]
- Rajauria, G.; Foley, B.; Abu-Ghannam, N. Identification and characterization of phenolic antioxidant compounds from brown Irish seaweed Himanthalia elongata using LC-DAD–ESI-MS/MS. Innov. Food Sci. Emerg. Technol. 2016, 37, 261–268. [Google Scholar] [CrossRef]
- Afonso, N.C.; Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Brown Macroalgae as Valuable Food Ingredients. Antioxidants 2019, 8, 365. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Park, M.Y.; Shim, B.J.; Youn, H.J.; Hwang, H.J.; Shin, H.C.; Jeon, H.K. Effects of Ecklonia cava Polyphenol in Individuals with Hypercholesterolemia: A Pilot Study. J. Med. Food 2012, 15, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Ara, J.; Sultana, V.; Qasim, R.; Ahmad, V.U. Hypolipidaemic activity of seaweed from Karachi coast. Phyther. Res. 2002, 16, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Bañuelos, T.; Gutiérrez-Rodríguez, A.; Méndez-Bellido, R.; Tovar-Miranda, R.; Arroyo-Helguera, O.; Juárez-Portilla, C.; Meza-Menchaca, T.; Aguilar-Rosas, L.; Hernández-Kelly, L.; Ortega, A.; et al. Brown Seaweed Egregia menziesii’s Cytotoxic Activity against Brain Cancer Cell Lines. Molecules 2019, 24, 260. [Google Scholar] [CrossRef]
- Miyashita, K.; Beppu, F.; Hosokawa, M.; Liu, X.; Wang, S. Nutraceutical characteristics of the brown seaweed carotenoid fucoxanthin. Arch. Biochem. Biophys. 2020, 686, 108364. [Google Scholar] [CrossRef]
- Lin, H.-T.; Tsou, Y.-C.; Chen, Y.-T.; Lu, W.-J.; Hwang, P.-A. Effects of Low-Molecular-Weight Fucoidan and High Stability Fucoxanthin on Glucose Homeostasis, Lipid Metabolism, and Liver Function in a Mouse Model of Type II Diabetes. Mar. Drugs 2017, 15, 113. [Google Scholar] [CrossRef]
- Gunathilaka, T.L.; Samarakoon, K.; Ranasinghe, P.; Peiris, L.D.C. Antidiabetic Potential of Marine Brown Algae—A Mini Review. J. Diabetes Res. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Krylova, N.V.; Ermakova, S.P.; Lavrov, V.F.; Leneva, I.A.; Kompanets, G.G.; Iunikhina, O.V.; Nosik, M.N.; Ebralidze, L.K.; Falynskova, I.N.; Silchenko, A.S.; et al. The Comparative Analysis of Antiviral Activity of Native and Modified Fucoidans from Brown Algae Fucus evanescens In Vitro and In Vivo. Mar. Drugs 2020, 18, 224. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, J.; Ge, K.; Tian, Q.; Zhao, P.; Guo, Y. Anti-inflammatory effect of low molecular weight fucoidan from Saccharina japonica on atherosclerosis in apoE-knockout mice. Int. J. Biol. Macromol. 2018, 118, 365–374. [Google Scholar] [CrossRef]
- Dong, X.; Bai, Y.; Xu, Z.; Shi, Y.; Sun, Y.; Janaswamy, S.; Yu, C.; Qi, H. Phlorotannins from Undaria pinnatifida Sporophyll: Extraction, Antioxidant, and Anti-Inflammatory Activities. Mar. Drugs 2019, 17, 434. [Google Scholar] [CrossRef] [PubMed]
- Ara, J.; Sultana, V.; Qasim, R.; Ehteshamul-Haque, S.; Ahmad, V.U. Biological activity of Spatoglossum asperum: A brown alga. Phyther. Res. 2005, 19, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Noda, H.; Amano, H.; Nishino, T.; Nishizawa, K. Study on Antihypertensive and Antihyperlipidemic Effects of Marine Algae. Fish. Sci. 1994, 60, 83–88. [Google Scholar] [CrossRef]
- Matanjun, P.; Mohamed, S.; Muhammad, K.; Mustapha, N.M. Comparison of Cardiovascular Protective Effects of Tropical Seaweeds, Kappaphycus alvarezii, Caulerpa lentillifera, and Sargassum polycystum, on High-Cholesterol/High-Fat Diet in Rats. J. Med. Food 2010, 13, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yoshie, Y.; Suzuki, T. Effect of small particle size of seaweeds on digestibility and lipid metabolism in rats. Nippon Suisan Gakkaishi 2002, 68, 172–179. [Google Scholar] [CrossRef][Green Version]
- Zha, X.-Q.; Xiao, J.-J.; Zhang, H.-N.; Wang, J.-H.; Pan, L.-H.; Yang, X.-F.; Luo, J.-P. Polysaccharides in Laminaria japonica (LP): Extraction, physicochemical properties and their hypolipidemic activities in diet-induced mouse model of atherosclerosis. Food Chem. 2012, 134, 244–252. [Google Scholar] [CrossRef]
- Peng, J.; Yuan, J.-P.; Wu, C.-F.; Wang, J.-H. Fucoxanthin, a Marine Carotenoid Present in Brown Seaweeds and Diatoms: Metabolism and Bioactivities Relevant to Human Health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, Y.; Zhang, Y.; Zhang, S.; Qu, J.; Wang, X.; Kong, R.; Han, C.; Liu, Z. Fucoxanthin: A Promising Medicinal and Nutritional Ingredient. Evid.-Based Complement. Altern. Med. 2015, 2015, 723515. [Google Scholar] [CrossRef]
- Ha, A.W.; Kim, W.K. The effect of fucoxanthin rich power on the lipid metabolism in rats with a high fat diet. Nutr. Res. Pract. 2013, 7, 287–293. [Google Scholar] [CrossRef]
- Woo, M.-N.; Jeon, S.-M.; Kim, H.-J.; Lee, M.-K.; Shin, S.-K.; Shin, Y.C.; Park, Y.-B.; Choi, M.-S. Fucoxanthin supplementation improves plasma and hepatic lipid metabolism and blood glucose concentration in high-fat fed C57BL/6N mice. Chem. Biol. Interact. 2010, 186, 316–322. [Google Scholar] [CrossRef]
- Xu, S.Y.; Huang, X.; Cheong, K.L. Recent advances in marine algae polysaccharides: Isolation, structure, and activities. Mar. Drugs 2017, 15, 388. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.P.; Le, V.; Sligar, A.D.; Mei, L.; Chavarria, D.; Yang, E.Y.; Baker, A.B. Algal Polysaccharides as Therapeutic Agents for Atherosclerosis. Front. Cardiovasc. Med. 2018, 5, 153. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Abu-Ghannam, N. Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci. Technol. 2011, 22, 315–326. [Google Scholar] [CrossRef]
- Ben Gara, A.; Ben Abdallah Kolsi, R.; Chaaben, R.; Hammami, N.; Kammoun, M.; Paolo Patti, F.; El Feki, A.; Fki, L.; Belghith, H.; Belghith, K. Inhibition of key digestive enzymes related to hyperlipidemia and protection of liver-kidney functions by Cystoseira crinita sulphated polysaccharide in high-fat diet-fed rats. Biomed. Pharmacother. 2017, 85, 517–526. [Google Scholar] [CrossRef]
- Raghavendran, H.R.B.; Sathivel, A.; Devaki, T. Effect of Sargassum polycystum (Phaeophyceae)-sulphated polysaccharide extract against acetaminophen-induced hyperlipidemia during toxic hepatitis in experimental rats. Mol. Cell. Biochem. 2005, 276, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Freire, M.J.; Lamela, M.; Calleja, J.M. Hypolipidaemic Activity of a Polysaccharide Extract from Fucus vesiculosus L. Phyther. Res. 1996, 10, 647–650. [Google Scholar] [CrossRef]
- Kimura, Y.; Watanabe, K.; Okuda, H. Effects of soluble sodium alginate on cholesterol excretion and glucose tolerance in rats. J. Ethnopharmacol. 1996, 54, 47–54. [Google Scholar] [CrossRef]
- Li, Z.; Anbuchezhian, R.; Karuppiah, V. Prospect of Marine Algae for Production of Industrially Important Chemicals. In Algal Biorefinery: An Integrated Approach; Springer International Publishing: Cham, Switzerland, 2016; pp. 195–217. ISBN 9783319228136. [Google Scholar]
- Kuda, T.; Taniguchi, E.; Nishizawa, M.; Araki, Y. Fate of water-soluble polysaccharides in dried Chorda filum a brown alga during water washing. J. Food Compos. Anal. 2002, 15, 3–9. [Google Scholar] [CrossRef]
- Yokota, T.; Nomura, K.; Nagashima, M.; Kamimura, N. Fucoidan alleviates high-fat diet-induced dyslipidemia and atherosclerosis in ApoEshl mice deficient in apolipoprotein E expression. J. Nutr. Biochem. 2016, 32, 46–54. [Google Scholar] [CrossRef]
- Huang, L.; Wen, K.; Gao, X.; Liu, Y. Hypolipidemic effect of fucoidan from Laminaria japonica in hyperlipidemic rats. Pharm. Biol. 2010, 48, 422–426. [Google Scholar] [CrossRef]
- Park, J.; Yeom, M.; Hahm, D.H. Fucoidan improves serum lipid levels and atherosclerosis through hepatic SREBP-2-mediated regulation. J. Pharmacol. Sci. 2016, 131, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Cuong, H.D.; Thuy, T.T.T.; Huong, T.T.; Ly, B.M.; Van, T.T.T. Structure and hypolipidaemic activity of fucoidan extracted from brown seaweed Sargassum henslowianum. Nat. Prod. Res. 2015, 29, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Mišurcová, L.; Škrovánková, S.; Samek, D.; Ambrožová, J.; Machů, L. Health Benefits of Algal Polysaccharides in Human Nutrition. In Advances in Food and Nutrition Research; Academic Press Inc.: New York, NY, USA, 2012; Volume 66, pp. 75–145. [Google Scholar]
- Kim, Y.-M.; Han, C.-K.; Bang, S.-J.; Park, J.-H. Effects of Laminaran from Eisenia bicyclis on Serum Lipids in Rats Fed High Cholesterol Diet. J. Korean Soc. Food Sci. Nutr. 2006, 35, 841–846. [Google Scholar] [CrossRef]
- Qin, Y. Applications of Bioactive Seaweed Substances in Functional Food Products. In Bioactive Seaweeds for Food Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 111–134. ISBN 9780128133125. [Google Scholar]
- Burmaoglu, S.; Yilmaz, A.O.; Taslimi, P.; Algul, O.; Kilic, D.; Gulcin, I. Synthesis and biological evaluation of phloroglucinol derivatives possessing α-glycosidase, acetylcholinesterase, butyrylcholinesterase, carbonic anhydrase inhibitory activity. Arch. Pharm. 2018, 351. [Google Scholar] [CrossRef] [PubMed]
- Machu, L.; Misurcova, L.; Ambrozova, J.V.; Orsavova, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic content and antioxidant capacity in algal food products. Molecules 2015, 20, 1118–1133. [Google Scholar] [CrossRef]
- Puspita, M.; Déniel, M.; Widowati, I.; Radjasa, O.K.; Douzenel, P.; Marty, C.; Vandanjon, L.; Bedoux, G.; Bourgougnon, N. Total phenolic content and biological activities of enzymatic extracts from Sargassum muticum (Yendo) Fensholt. J. Appl. Phycol. 2017, 29, 2521–2537. [Google Scholar] [CrossRef]
- Shin, H.-C.; Kim, S.H.; Park, Y.; Lee, B.H.; Hwang, H.J. Effects of 12-week Oral Supplementation of Ecklonia cava Polyphenols on Anthropometric and Blood Lipid Parameters in Overweight Korean Individuals: A Double-Blind Randomized Clinical Trial. Phyther. Res. 2011, 26. [Google Scholar] [CrossRef]
- André, R.; Guedes, L.; Melo, R.; Ascensão, L.; Pacheco, R.; Vaz, P.D.; Serralheiro, M.L. Effect of Food Preparations on In Vitro Bioactivities and Chemical Components of Fucus vesiculosus. Foods 2020, 9, 955. [Google Scholar] [CrossRef]
- Ressaissi, A.; Attia, N.; Pacheco, R.; Falé, P.L.; Serralheiro, M.L.M. Cholesterol transporter proteins in HepG2 cells can be modulated by phenolic compounds present in Opuntia ficus-indica aqueous solutions. J. Funct. Foods 2020, 64, 103674. [Google Scholar] [CrossRef]
- Arantes, A.A.; Falé, P.L.; Costa, L.C.B.; Pacheco, R.; Ascensão, L.; Serralheiro, M.L. Inhibition of HMG-CoA reductase activity and cholesterol permeation through Caco-2 cells by caffeoylquinic acids from Vernonia condensata leaves. Braz. J. Pharmacogn. 2016, 26, 738–743. [Google Scholar] [CrossRef]
- Kobayashi, S. The Effect of Polyphenols on Hypercholesterolemia through Inhibiting the Transport and Expression of Niemann–Pick C1-Like 1. Int. J. Mol. Sci. 2019, 20, 4939. [Google Scholar] [CrossRef] [PubMed]
- Yeo, A.R.; Lee, J.; Tae, I.H.; Park, S.R.; Cho, Y.H.; Lee, B.H.; Cheol Shin, H.; Kim, S.H.; Yoo, Y.C. Anti-hyperlipidemic effect of polyphenol extract (Seapolynol™) and dieckol isolated from Ecklonia cava in in vivo and in vitro models. Prev. Nutr. Food Sci. 2012, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive porteins, peptides and amino acids from Macroalgae. J. Phycol. 2011, 47, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, L. Insights into the regulation of algal proteins and bioactive peptides using proteomic and transcriptomic approaches. Molecules 2019, 24, 1708. [Google Scholar] [CrossRef] [PubMed]
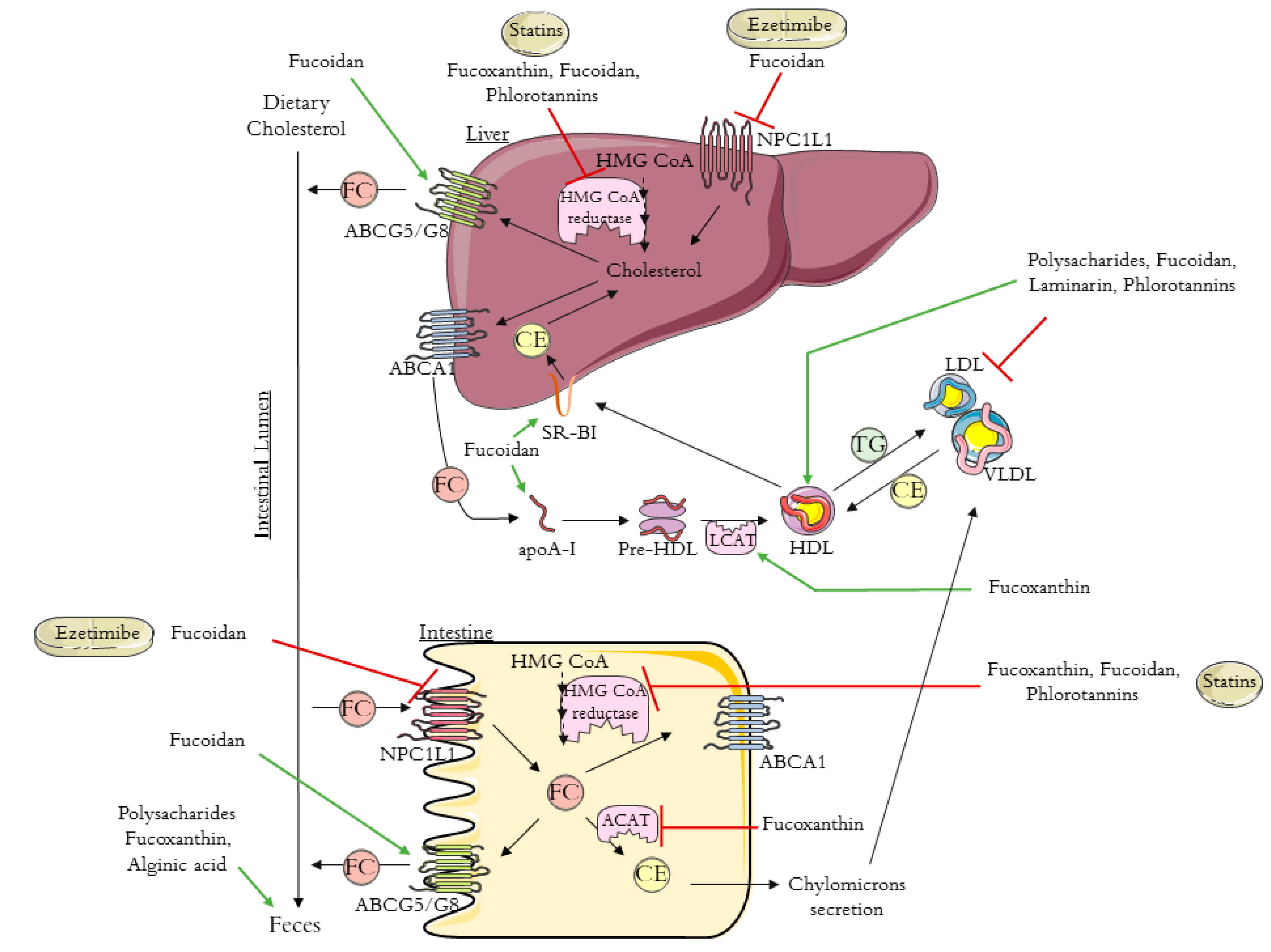

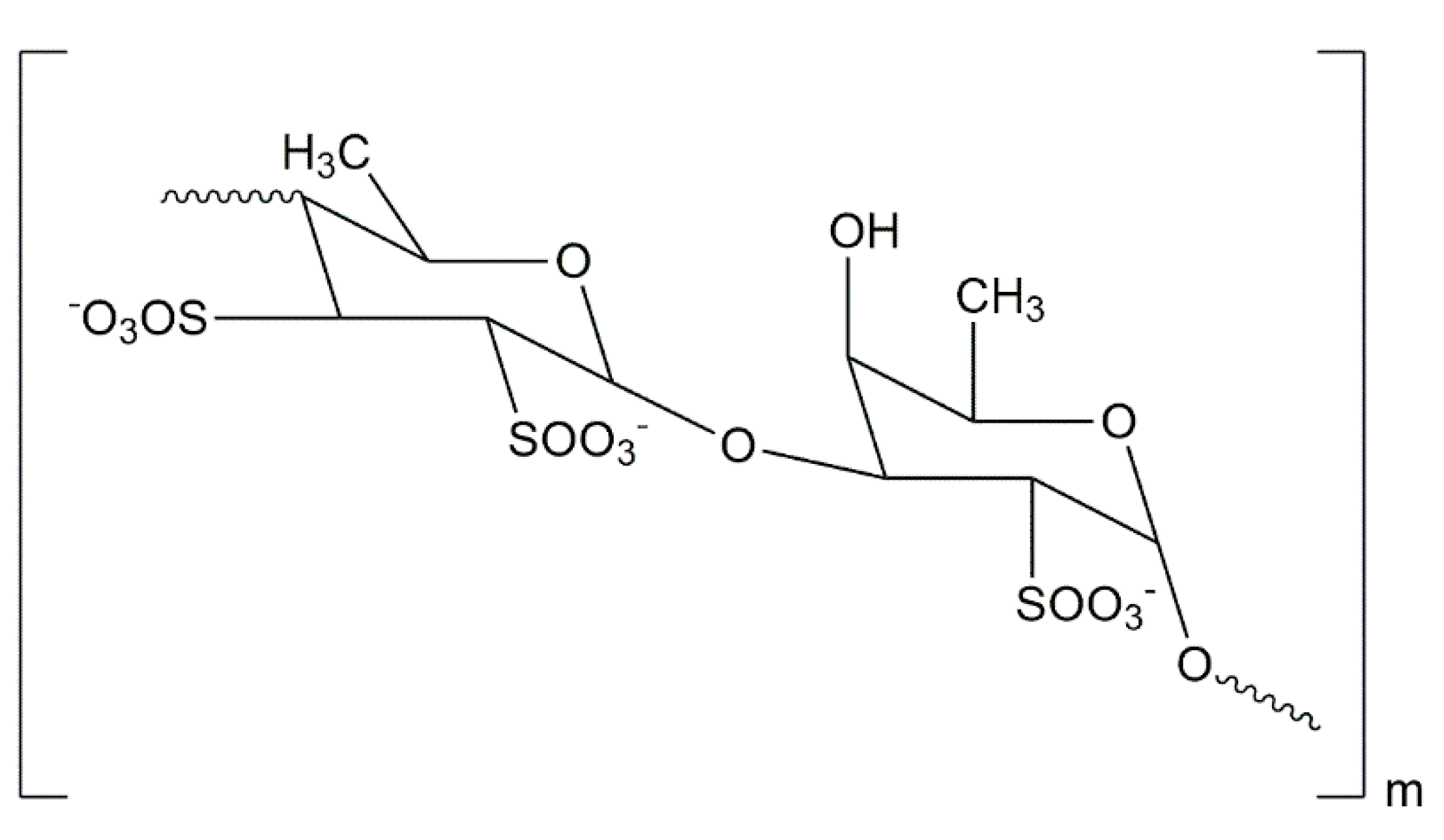
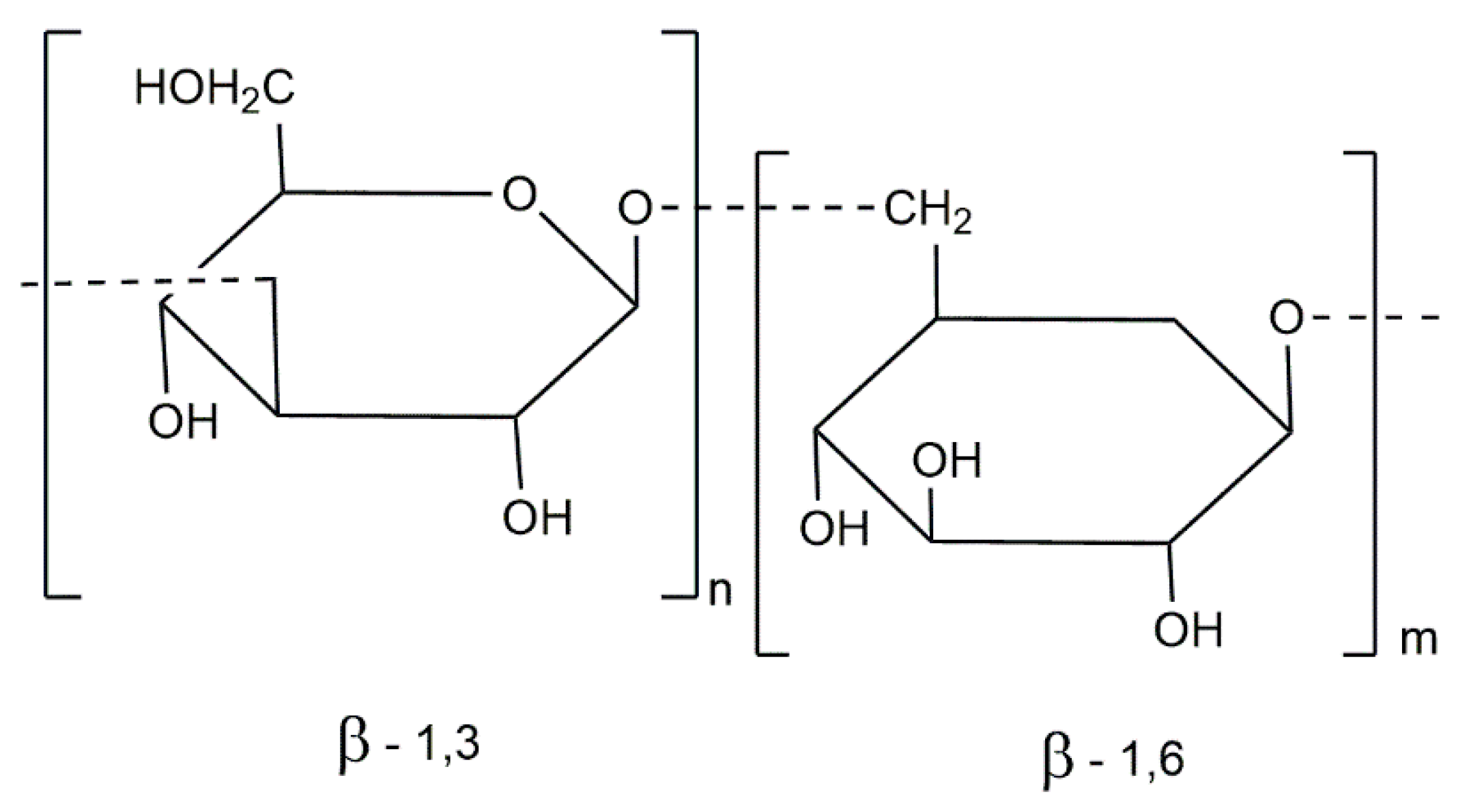

| Brown Algae Species |
|---|
| Ascophyllum nodosum |
| Alaria esculenta |
| Eisenia bicyclis |
| Fucus vesiculosus |
| Fucus serratus |
| Fucus spiralis |
| Himanthalia elongata |
| Laminaria digitata |
| Saccharina japonica |
| Saccharina latissima |
| Saccharina longicruris |
| Sargassum fusiforme |
| Undaria pinnatifida |
| Brown Algae Specie | Algae Preparation and Administration Mode | Effect | Ref. |
|---|---|---|---|
| Ecklonia stolonifera | Oral administration of EtOAc and n-BuOH fractions derived from EtOH seaweed extract at a dose of 100 mg/kg of body weight | ↓ Tc, TGs, LDL-c ↑ HDL-c | [13] |
| Ecklonia cava | Capsules, twice per day (200 mg seaweed powder per tablet) | ↓ Tc, LDL-c | [18] |
| Iyengaria stellata | Oral administration of ethanol extracts suspended in distilled water at 10 mg/200 g of body weight | ↓ Tc, TGs, LDL-c ↑ HDL-c | [53] |
| Colpomenia sinuosa | Oral administration of ethanol extracts suspended in water at 10 mg/200 g of body weight | ↓ Tc, TGs, LDL-c ↑ HDL-c | [53] |
| Spatoglossum asperum | Oral administration of ethanol extracts suspended in distilled water at 10 mg/200 g of body weight | ↓ Tc, TGs, LDL-c ↑ HDL-c | [53] |
| Spatoglossum asperum | Diet supplemented with oily fractions of seaweed at 10 mg/200 g of body weight | ↓ Tc, TGs, LDL-c | [61] |
| Heterochordaria abietina | Diet supplemented with 5% seaweed powder | ↓ Tc, LDL-c | [62] |
| Sargassum micracanthum | Diet supplemented with 5% seaweed powder | ↓ Tc, TGs | [62] |
| Sargassum patens | Diet supplemented with 5% seaweed powder | ↓ Tc, LDL-c | [62] |
| Cystoseira sisymbrioides | Diet supplemented with 5% seaweed powder | ↓ Tc, free cholesterol, LDL-c | [62] |
| Laminaria diabolica | Diet supplemented with 5% seaweed powder | ↑ HDL-c | [62] |
| Sargassum ringgoldianum | Diet supplemented with 5% seaweed powder | ↑ HDL-c | [62] |
| Padina arborescens | Diet supplemented with 5% seaweed powder | ↑ HDL-c | [62] |
| Sargassum polycystum | Diet supplemented with 5% seaweed powder | ↓ Tc, TGs, LDL-c ↑ HDL-c | [63] |
| Undaria pinnatifida | Diet supplemented with 100 g of seaweed powder/kg of body weight | ↓ Tc, TGs, LDL-c ↑ HDL-c | [64] |
| Isolated Compound | Model Used | Effect | Ref. |
|---|---|---|---|
| Sulfated polysaccharides (Cystoseira crinite) | in vivo | ↓ Tc, TGs, LDL-c ↑ HDL-c | [73] |
| Sulfated polysaccharides (Sargassum polycystum) | in vivo | ↓ Tc, TGs, LDL-c ↑ HDL-c | [74] |
| Polysaccharide extract (Fucus vesiculosus) | in vivo | ↓ Tc, TGs ↑ HDL-c | [75] |
| Polysaccharide extract (Laminaria japonica) | in vivo | ↓ Tc, TGs, LDL-c, HDL-c | [65] |
| Sodium alginate | in vivo | ↑ Cholesterol excretion | [76] |
| Fucoidan (Ascophyllum nodosum) | in vivo | ↓ LDL-c, apoB, NPC1L1 ↑ SR-B1, apoA-I, ABCA1, ABCG8 | [27] |
| Fucoidan (Fucus vesiculosus) | in vivo | ↓ Tc, TGs, LDL-c ↑ HDL-c | [81] |
| in vitro | ↓ HMG-CoA mRNA, LDL receptor | ||
| Fucoidan (Saccharina Japonica) | in vivo | ↓ TGs, oxidative-LDL | [59] |
| Fucoidan (Laminaria Japonica) | in vivo | ↓ Tc, TGs, LDL-c ↑ HDL-c, LCAT | [80] |
| Fucoidan (Sargassum Henslowianum) | in vivo | ↓ Tc, TGs, LDL-c | [82] |
| Fucoidan (Cladosiphon okamuranus) | in vivo | ↓ Tc, TGs, LDL-c ↑ HDL-c | [79] |
| Laminarin (Eisenia bicyclis) | in vivo | ↓ Tc, TGs, LDL-c ↑ HDL-c | [84] |
| Isolated Compound | Model Used | Effect | Ref. |
|---|---|---|---|
| Phlorotannin ethyl acetate fraction (Ecklonia stolonifera) | in vivo | ↓ Tc, TGs, LDL-c ↑ HDL-c | [13] |
| Phlorotannin n-butanol fraction (Ecklonia stolonifera) | in vivo | ↓ Tc, TGs, LDL-c ↑ HDL-c | [13] |
| Polyphenol extract (Ecklonia cava) | in vivo | ↓ Tc, LDL-c ↑ HDL-c | [89] |
| Phlorotannin and peptide-rich extract (Fucus vesiculosus) | in vitro | ↓ HMG-CoA reductase activity ↓ Cholesterol absorption | [90] |
| Seapolynol™ (Ecklonia cava) | in vivo | ↓ Tc, TGs, LDL-c | [52,94] |
| in vitro | ↓ HMG-CoA reductase activity | [94] | |
| Eckol | in vivo | ↓ Tc, TGs, LDL-c | [13] |
| Dieckol | in vivo | ↓ Tc, TGs, LDL-c ↑ HDL | [13,94] |
| in vitro | ↓ HMG-CoA reductase activity | [94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
André, R.; Pacheco, R.; Bourbon, M.; Serralheiro, M.L. Brown Algae Potential as a Functional Food against Hypercholesterolemia: Review. Foods 2021, 10, 234. https://doi.org/10.3390/foods10020234
André R, Pacheco R, Bourbon M, Serralheiro ML. Brown Algae Potential as a Functional Food against Hypercholesterolemia: Review. Foods. 2021; 10(2):234. https://doi.org/10.3390/foods10020234
Chicago/Turabian StyleAndré, Rebeca, Rita Pacheco, Mafalda Bourbon, and Maria Luísa Serralheiro. 2021. "Brown Algae Potential as a Functional Food against Hypercholesterolemia: Review" Foods 10, no. 2: 234. https://doi.org/10.3390/foods10020234
APA StyleAndré, R., Pacheco, R., Bourbon, M., & Serralheiro, M. L. (2021). Brown Algae Potential as a Functional Food against Hypercholesterolemia: Review. Foods, 10(2), 234. https://doi.org/10.3390/foods10020234






