Influence of Drying Techniques on the Physicochemical, Nutritional, and Morphological Properties of Bighead Carp (Hypophthalmichthys nobilis) Fillets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Chemical Composition Analysis
2.4. Mineral Determination
2.5. Amino Acid Profile Determination
2.6. Determination of Volatile Components
2.7. Fatty Acid (FA) Composition
2.8. Vitamin Analysis
2.9. Scanning Electron Microscopy (SEM) Analysis
2.10. Thermal Characterization Analysis
2.11. Color Measurement
2.12. Statistical Analysis
3. Results and Discussion
3.1. Proximate Chemical Composition
3.2. Mineral Composition
3.3. Amino Acid Composition
3.4. Volatile Organic Compounds Analysis
3.5. Fatty Acid Analysis
3.5.1. Saturated Fatty Acids (SFA)
3.5.2. Monounsaturated Fatty Acids (MUFA)
3.5.3. Polyunsaturated Fatty Acid Composition
3.6. Vitamin Analysis
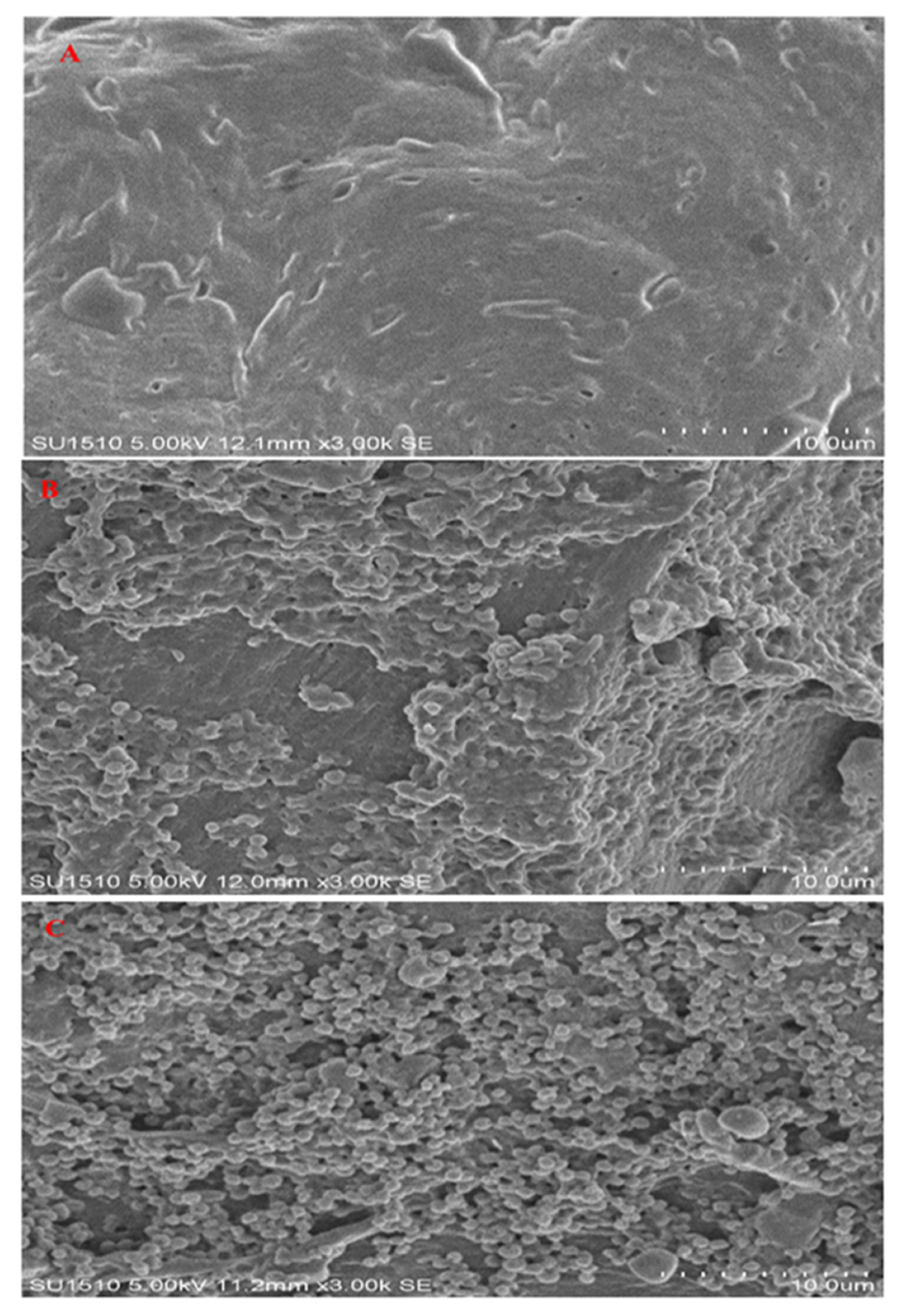
3.7. Scanning Electron Microscopy (SEM) Analysis
3.8. Thermal Characterization (DSC) Analysis
3.9. Color Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carlander, K.D. Handbook of Freshwater Fishery Biology; Iowa State University Press: Ames, IA, USA, 1997; Volume 3. [Google Scholar]
- Deters, J.E.; Chapman, D.C.; McElroy, B. Location and timing of Asian carp spawning in the lower Missouri River. Environ. Biol. Fishes 2013, 96, 617–629. [Google Scholar] [CrossRef]
- Larson, J.H.; Knights, B.C.; McCalla, S.G.; Monroe, E.; Tuttle-Lau, M.; Chapman, D.C.; George, A.E.; Vallazza, J.M.; Amberg, J. Evidence of Asian carp spawning upstream of a key choke point in the Mississippi River. N. Am. J. Fish. Manag. 2017, 37, 903–919. [Google Scholar] [CrossRef]
- Engle, C.R.; Brown, D. Growth, yield, dressout, and net returns of bighead carp Hypophthalmichthys nobilis stocked at three densities in catfish Ictalurus punctatus ponds. J. World Aquac. Soc. 1998, 29, 414–421. [Google Scholar] [CrossRef]
- Bellagha, S.; Amami, E.; Farhat, A.; Kechaou, N. Drying kinetics and characteristic drying curve of lightly salted sardine (Sardinella aurita). Dry. Technol. 2002, 20, 1527–1538. [Google Scholar] [CrossRef]
- Duan, Z.; Zhang, M.; Tang, J. Thin layer hot-air drying of bighead carp. Fish. Sci. 2004, 23, 29–32. [Google Scholar]
- Jain, D. Determination of convective heat and mass transfer coefficients for solar drying of fish. Biosyst. Eng. 2006, 94, 429–435. [Google Scholar] [CrossRef]
- Nunes, M.L.; Bandarra, N.; Oliveira, L.; Batista, I.; Calhau, M. Composition and nutritional value of fishery products consumed in Portugal. In Sea Food Research from Fish to Dish: Quality, Safety and Processing of Wild and Farmed Fish; Luten, J.B., Jacobsen, C., Bekaert, K., Sæbø, A., Oehlenschläger, J., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2006; pp. 477–487. [Google Scholar]
- Bhuiyan, A.A.; Ratnayake, W.; Ackman, R. Nutritional composition of raw and smoked Atlantic mackerel (Scomber scombrus): Oil-and water-soluble vitamins. J. Food Compos. Anal. 1993, 6, 172–184. [Google Scholar] [CrossRef]
- Mustapha, M.K.; Ajibola, T.B.; Salako, A.F.; Ademola, S.K. Solar drying and organoleptic characteristics of two tropical A frican fish species using improved low-cost solar driers. Food Sci. Nutr. 2014, 2, 244–250. [Google Scholar] [CrossRef]
- Jain, D.; Pathare, P.B. Study the drying kinetics of open sun drying of fish. J. Food Eng. 2007, 78, 1315–1319. [Google Scholar] [CrossRef]
- Selmi, S.; Bouriga, N.; Cherif, M.; Toujani, M.; Trabelsi, M. Effects of drying process on biochemical and microbiological quality of silverside (fish) Atherina lagunae. Int. J. Food Sci. Technol. 2010, 45, 1161–1168. [Google Scholar] [CrossRef]
- Guiné, R. The drying of foods and its effect on the physical-chemical, sensorial and nutritional properties. Int. J. Food Eng. 2018, 2, 93–100. [Google Scholar] [CrossRef]
- Pu, H.; Li, Z.; Hui, J.; Raghavan, G.V. Effect of relative humidity on microwave drying of carrot. J. Food Eng. 2016, 190, 167–175. [Google Scholar] [CrossRef]
- Wu, T.; Mao, L. Influences of hot air drying and microwave drying on nutritional and odorous properties of grass carp (Ctenopharyngodon idellus) fillets. Food Chem. 2008, 110, 647–653. [Google Scholar] [CrossRef]
- Association of Official Agricultural Chemists. Official Methods of Analysis; Association of Official Agricultural Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Shahidi, F.; Chavan, U.; Bal, A.; McKenzie, D. Chemical composition of beach pea (Lathyrus maritimus L.) plant parts. Food Chem. 1999, 64, 39–44. [Google Scholar] [CrossRef]
- Kumar, K.A.; Sharvanee, S.; Patel, J.; Choudhary, R.K. Chemical composition and antimicrobial activity of the essential oil of Desmostachya bipinnata linn. Int. J. Phytomed. 2010, 2, 436–439. [Google Scholar]
- Chalamaiah, M.; Jyothirmayi, T.; Bhaskarachary, K.; Vajreswari, A.; Hemalatha, R.; Kumar, B.D. Chemical composition, molecular mass distribution and antioxidant capacity of rohu (Labeo rohita) roe (egg) protein hydrolysates prepared by gastrointestinal proteases. Food Res. Int. 2013, 52, 221–229. [Google Scholar] [CrossRef]
- Erbaş, M.; Certel, M.; Uslu, M. Some chemical properties of white lupin seeds (Lupinus albus L.). Food Chem. 2005, 89, 341–345. [Google Scholar] [CrossRef]
- Horvli, O.; Lie, Ø. Determination of vitamin D3 in fish meals by HPLC. Fisk. Skr. Ser. Ernæring 1994, 6, 163–175. [Google Scholar]
- Mohammed, J.K.; Mahdi, A.A.; Ahmed, M.I.; Abraha, B.; Admassu, H.; Wang, H. Phenolic compounds and the physicochemical, nutritional, antioxidant, and functional characteristics of peel, flesh, and kernel of Medemia argun (argun palm) fruit. J. Food Meas. Charact. 2019, 13, 2275–2287. [Google Scholar] [CrossRef]
- Abdalhai, M.H.; Bashari, M.; Lagnika, C.; He, Q.; Sun, X. Effect of ultrasound treatment prior to vacuum and modified atmosphere packaging on microbial and physical characteristics of fresh beef. J. Food Nutr. Res. 2014, 2, 312–320. [Google Scholar] [CrossRef] [Green Version]
- Duan, Z.-h.; Jiang, L.-n.; Wang, J.-l.; Yu, X.-y.; Wang, T. Drying and quality characteristics of tilapia fish fillets dried with hot air-microwave heating. Food Bioprod. Process. 2011, 89, 472–476. [Google Scholar] [CrossRef]
- Kaur, M.; Kaushal, P.; Sandhu, K.S. Studies on physicochemical and pasting properties of Taro (Colocasia esculenta L.) flour in comparison with a cereal, tuber and legume flour. J. Food Sci. Technol. 2013, 50, 94–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puwastien, P.; Judprasong, K.; Kettwan, E.; Vasanachitt, K.; Nakngamanong, Y.; Bhattacharjee, L. Proximate composition of raw and cooked Thai freshwater and marine fish. J. Food Compos. Anal. 1999, 12, 9–16. [Google Scholar] [CrossRef]
- Akinneye, J.; Amoo, I.; Bakare, O. Effect of drying methods on the chemical composition of three species of fish (Bonga spp., Sardinella spp. and Heterotis niloticus). Afr. J. Biotechnol. 2010, 9, 4369–4373. [Google Scholar]
- Karrar, E.; Sheth, S.; Wei, W.; Wang, X. Gurum (Citrullus lanatus var. Colocynthoide) seed: Lipid, amino acid, mineral, proximate, volatile compound, sugar, vitamin composition and functional properties. J. Food Meas. Charact. 2019, 13, 2357–2366. [Google Scholar] [CrossRef]
- Al-Farga, A.; Zhang, H.; Siddeeg, A.; Shamoon, M.; Chamba, M.V.; Al-Hajj, N. Proximate composition, functional properties, amino acid, mineral and vitamin contents of a novel food: Alhydwan (Boerhavia elegana Choisy) seed flour. Food Chem. 2016, 211, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Joint, F.; World Health Organization. Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Liu, J.-K.; Zhao, S.-M.; Xiong, S.-B.; Zhang, S.-H. Influence of recooking on volatile and non-volatile compounds found in silver carp Hypophthalmichthys molitrix. Fish. Sci. 2009, 75, 1067–1075. [Google Scholar] [CrossRef]
- Odukoya, J.O.; Kayitesi, E.; Mphahlele, M.P.; Tata, C.M.; Njinkoue, J.M.; Gouado, I.; Ikhile, M.I.; Ndinteh, D.T. Effect of processing methods on the volatile components of Ethmalosa fimbriata using a two-dimensional gas chromatography-time-of-flight mass spectrometry (GC× GC-TOF-MS) technique. J. Food Process. Preserv. 2020, 45, e15110. [Google Scholar] [CrossRef]
- Vujković, G.; Karlović, Đ.; Vujković, I.; Vörösbaranyi, I.; Jovanović, B. Composition of muscle tissue lipids of silver carp and bighead carp. J. Am. Oil Chem. Soc. 1999, 76, 475–480. [Google Scholar] [CrossRef]
- Calder, P.; Grimble, R. Polyunsaturated fatty acids, inflammation and immunity. Eur. J. Clin. Nutr. 2002, 56, S14–S19. [Google Scholar] [CrossRef] [Green Version]
- De Pablo, M.A.; De Cienfuegos, G.Á. Modulatory effects of dietary lipids on immune system functions. Immunol. Cell Biol. 2000, 78, 31–39. [Google Scholar] [CrossRef]
- Chang, N.W.; Huang, P.C. Effects of the ratio of polyunsaturated and monounsaturated fatty acid to saturated fatty acid on rat plasma and liver lipid concentrations. Lipids 1998, 33, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.; Das, S.; Girj, S.; Mohanty, S. Water soluble Vitamins in Aquaculture Nutrition. Fish. Chimes 2010, 30, 13–16. [Google Scholar]
- Ersoy, B.; Özeren, A. The effect of cooking methods on mineral and vitamin contents of African catfish. Food Chem. 2009, 115, 419–422. [Google Scholar] [CrossRef]
- Golgolipour, S.; Khodanazary, A.; Ghanemi, K. Effects of different cooking methods on minerals, vitamins and nutritional quality indices of grass carp (Ctenopharyngodon idella). Iran. J. Fish. Sci. 2019, 18, 110–123. [Google Scholar]
- Bakar, J.; Moradi, Y.; Man, Y.C. Fat uptake evaluation in fried fish fillet by using Scanning Electron Microscopy (SEM). Iranian J. Fish. Sci. 2010, 9, 327–336. [Google Scholar]
- Asami, A.; Nakamura, M.; Takeuchi, M.; Nakayama, A.; Nakamura, H.; Yoshida, T.; Nagasawa, S.; Hiraoka, B.Y.; Ito, M.; Udagawa, N. Effects of heat treatment of hydroxyapatite on osteoblast differentiation. J. Hard Tissue Biol. 2008, 17, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Latip, L.D.; Zzaman, W.; Abedin, M.Z.; Yang, T.A. Optimization of spray drying process in commercial hydrolyzed fish scale collagen and characterization by scanning electron microscope and Fourier transform infrared spectroscopy. J. Food Process. Preserv. 2015, 39, 1754–1761. [Google Scholar] [CrossRef]
- HÄGERDAL, B.; MARTENS, H. Influence of water content on the stability of myoglobin to heat treatment. J. Food Sci. 1976, 41, 933–937. [Google Scholar]
- Uddin, M.; Ahmad, M.U.; Jahan, P.; Sanguandeekul, R. Differential scanning calorimetry of fish and shellfish meat. Asian J. Chem. 2001, 13, 965–968. [Google Scholar]
- Kim, B.-S.; Oh, B.-J.; Lee, J.-H.; Yoon, Y.S.; Lee, H.-I. Effects of various drying methods on physicochemical characteristics and textural features of yellow croaker (Larimichthys Polyactis). Foods 2020, 9, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sablani, S.; Rahman, M.; Haffar, I.; Mahgoub, O.; Al-Marzouki, A.; Al-Ruzeiqi, M.; Al-Habsi, N.; Al-Belushi, R. Drying rates and quality parameters of fish sardines processed using solar dryers. Agric. Mar. Sci. 2003, 8, 79–86. [Google Scholar]
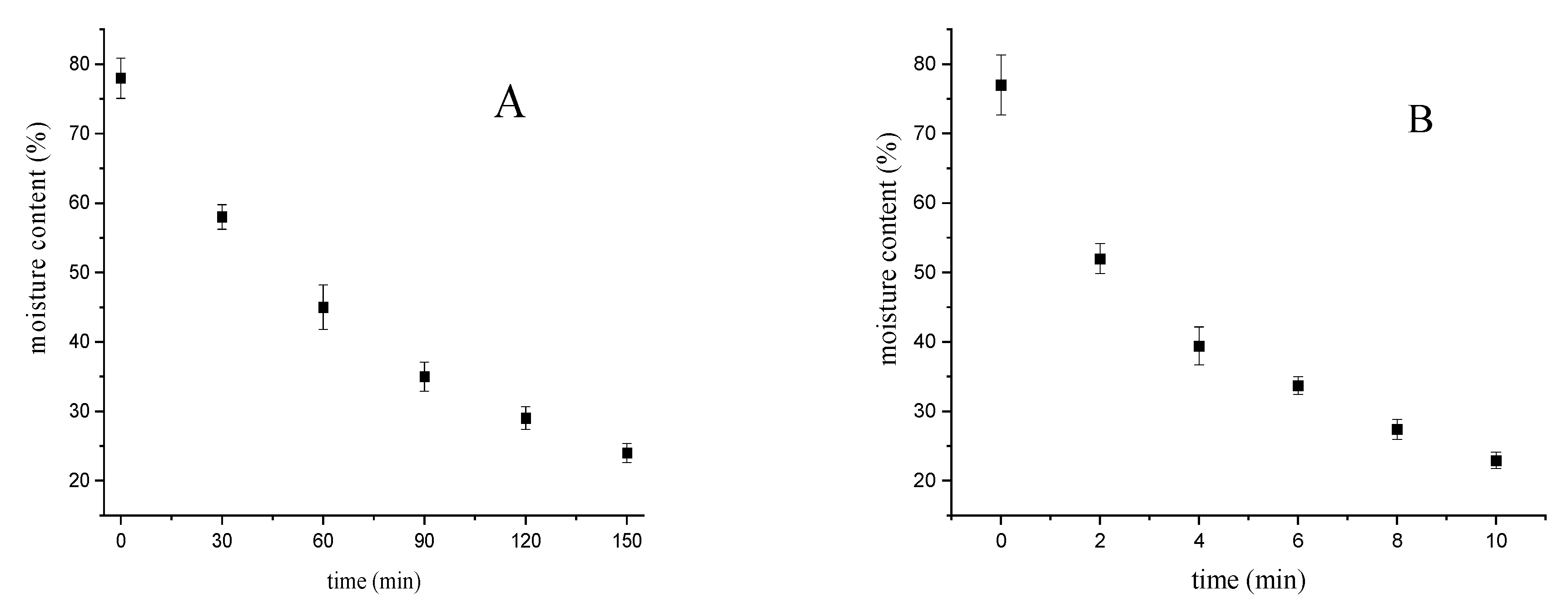

| Samples | Raw | Oven Drying | Microwave Drying |
|---|---|---|---|
| Moisture (%) | 77.69 ± 0.88 a | 24.19 ± 0.91 b | 23.92 ± 0.86 b |
| Ash 1 | 1.06 ± 0.05 c | 3.48 ± 0.06 b | 3.62 ± 0.07 a |
| Fat 1 | 1.07 ± 0.12 b | 4.10 ± 0.16 a | 4.45 ± 0.15 a |
| Protein 1 | 17.72 ± 0.25 b | 64.57 ± 1.47 a | 64.89 ± 0.58 a |
| Ash 2 | 4.36 ± 0.07 b | 4.83 ± 0.06 a | 4.79 ± 0.16 a |
| Fat 2 | 5.97 ± 0.82 a | 5.69 ± 0.22 a, b | 5.88 ± 0.16 a |
| Protein 2 | 82.66 ± 1.02 b | 84.79 ± 0.39 a | 85.27 ± 0.53 a |
| Sodium (Na) 2 | 648.57 ± 4.09 a | 647.63 ± 2.35 a | 647.85 ± 2.61 a |
| Magnesium (Mg) 2 | 251.13 ± 3.47 a | 250.94 ± 4.17 a | 249.46 ± 1.82 a |
| Potassium (K) 2 | 793.54 ± 5.07 a | 787.93 ± 3.08 b | 789.28 ± 2.36 b |
| Calcium (Ca) 2 | 981.62 ± 3.25 a | 978.17 ± 1.73 b | 976.87 ± 4.07 b |
| Iron (Fe) 2 | 21.17 ± 0.18 a | 20.89 ± 1.36 b | 20.67 ± 0.56 b |
| Copper (Cu) 2 | 0.87 ± 0.02 b | 0.93 ± 0.03 a | 0.91 ± 0.02 a |
| Zinc (Zn) 2 | 1.41 ± 0.12 b | 1.44 ± 0.09 a | 1.43 ± 0.34 a |
| Ascorbic acid (C) 2 | 1.11 ± 0.00 a | 1.07 ± 0.07 a | 1.05 ± 0.02 a |
| Thiamine (B1) 2 | 0.13 ± 0.01 a | 0.10 ± 0.01 a | 0.14 ± 0.02 a |
| Riboflavin (B2) 2 | 0.22 ± 0.00 b | 0.24 ± 0.00 a,b | 0.27 ± 0.00 a |
| Pyridoxine (B6) 2 | 0.68 ± 0.01 a | 0.59 ± 0.02 b | 0.55 ± 0.01 b |
| Cyanocobalamin (B12) 2 | 0.02 ± 0.00 a | 0.01 ± 0.00 b | 0.01 ± 0.00 b |
| Vitamin (D3) 2 | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.01 a |
| Color parameters | |||
| L* | 77.90 ± 0.22 a | 72.02 ± 0.43 b | 69.54 ± 0.27 c |
| a* | 2.84 ± 0.09 c | 4.19 ± 0.19 b | 5.28 ± 0.02 a |
| b* | 16.15 ± 0.50 c | 19.24 ± 0.12 b | 20.89 ± 0.04 a |
| ΔE | 4.16 ± 0.28 b | 6.57 ± 0.18 a | 5.91 ± 0.15 a |
| Samples | Raw | Oven Drying | Microwave Drying |
|---|---|---|---|
| Essential amino acids (EAAs) | |||
| Histidine | 1.98 ± 0.11 b | 2.31 ± 0.14 a | 2.16 ± 0.21 a, b |
| Threonine | 3.43 ± 0.03 a | 3.32 ± 0.04 b | 3.24 ± 0.06 b |
| Valine | 5.46 ± 0.04 a | 5.29 ± 0.08 b | 5.41 ± 0.02 a |
| Methionine | 3.38 ± 0.03 c | 3.65 ± 0.06 a | 3.52 ± 0.04 b |
| Phenylalanine | 4.71 ± 0.05 a | 4.42 ± 0.06 b | 4.64 ± 0.03 a |
| Isoleucine | 4.67 ± 0.04 a | 4.39 ± 0.04 c | 4.55 ± 0.05 b |
| Leucine | 7.62 ± 0.02 b | 7.51 ± 0.03 c | 7.68 ± 0.02 a |
| Lysine | 8.34 ± 0.05 c | 8.48 ± 0.03 b | 8.63 ± 0.02 a |
| Non-essential amino acids (NAAs) | |||
| Tyrosine | 2.72 ± 0.07 a | 2.54 ± 0.02 b | 2.48 ± 0.01 b |
| Cystenie | 0.34 ± 0.01 c | 0.47 ± 0.02 a | 0.41 ± 0.02 b |
| Aspartic acid | 9.61 ± 0.06 b | 9.76 ± 0.04 a | 9.85 ± 0.06 a |
| Glutamic acid | 16.07 ± 0.03 b | 16.36 ± 0.05 a | 15.80 ± 0.04 c |
| Serine | 3.59 ± 0.04 a | 3.38 ± 0.03 b | 3.28 ± 0.03 c |
| Glycine | 3.77 ± 0.03 c | 4.07 ± 0.04 b | 4.19 ± 0.03 a |
| Arginine | 5.30 ± 0.03 a | 5.07 ± 0.04 b | 4.98 ± 0.07 b |
| Proline | 2.69 ± 0.06 b | 2.49 ± 0.06 c | 2.86 ± 0.08 a |
| Alanine | 5.13 ± 0.04 b | 5.27 ± 0.03 a | 5.06 ± 0.04 b |
| TEAA | 39.65 ± 0.04 b | 39.71 ± 0.16 a, b | 39.83 ± 0.24 a |
| TNAA | 49.25 ± 0.06 b | 49.41 ± 0.10 a | 49.16 ± 0.07 c |
| TAA | 88.90 ± 0.08 b | 89.11 ± 0.20 a | 88.99 ± 0.16 b |
| Samples | Raw (%) | Oven Drying (%) | Microwave Drying (%) |
|---|---|---|---|
| Methylamine, N, N-dimethyl- | ND | ND | ND |
| Oxirane, 2,3-diethyl- | ND | ND | ND |
| Hexanal | 0.15 | 1.01 | 1.13 |
| Undecane | 1.55 | 0.86 | 0.68 |
| 2-Hydroxymandelic acid, ethylester, di-TMS | ND | 1.46 | 1.07 |
| 1-Penten-3-ol | 0.93 | ND | 0.69 |
| Cyclohexene, 1-methyl-4-(1-methylethenyl)-,(S)- | 0.97 | ND | 0.57 |
| Tetradecane | ND | 0.98 | 0.63 |
| 1-Pentanol | 0.79 | ND | ND |
| Acetoin | 0.68 | ND | 1.92 |
| 1-Hexanol | 2.94 | ND | ND |
| Nonanal | ND | ND | 0.71 |
| 1-Octen-3-ol | ND | 6.7 | ND |
| Pentasiloxane, dodecamethyl- | ND | ND | 2.97 |
| 2,3-Butanediol | ND | ND | ND |
| Propanoic acid, 2-methyl- | ND | 8.97 | 5.24 |
| Cyclohexanol,5-methyl-2-(1 methylethyl)-,(1à,2á,5à)-(ñ)- | 8.47 | 5.39 | 4.62 |
| Nonadecane | 14.37 | 5.75 | 5.02 |
| Nonanoic acid | ND | 1.73 | 0.91 |
| 1,4,7,10,13,16-Hexaoxacyclooctadecane | ND | ND | ND |
| 2-Butanol, 3-methyl- | 5.92 | 3.21 | 7.26 |
| Diazene, dimethyl- | ND | 1.42 | ND |
| 2-Butanone | ND | 4.03 | ND |
| 2,7-Octadiene-1,6-diol, 2,6-dimethyl-, (E)- | ND | 1.37 | ND |
| 3-Heptanone, 6-methyl- | ND | 2.6 | ND |
| Nonanone | ND | 2.21 | 3.08 |
| 2-Oxo-4-phenyl-6-(4-chlorophenyl)-1,2-dihydropyrimidine | 8.12 | 4.87 | 1.08 |
| Acetonitrile | ND | 0.35 | ND |
| Butanoic acid, 3-methyl- | ND | 15.08 | 7.38 |
| Butanoic acid, 2-(aminooxy)- | 0.47 | 4.14 | 0.9 |
| Acetic acid, [(aminocarbonyl)]amino]oxo | ND | ND | 0.89 |
| Vinyl butyrate | ND | ND | 1.7 |
| Acetic acid | ND | 1.05 | 4.73 |
| Heptadecane | ND | ND | 21.61 |
| Hexaethylene glycol | ND | ND | 5.3 |
| Ethyne, fluoro- | 0.96 | 2.08 | ND |
| p-Trimethylsilyloxyphenyl-bis(trimethylsilyloxy) ethane | 3.40 | ND | ND |
| 1,3,6-Octatriene,3,7-dimethyl-, (Z)- | 0.5 | ND | ND |
| Cetene | 0.44 | 0.9 | ND |
| Acetaldehyde, tetramer | 3.35 | ND | ND |
| 2-Butenoic acid, 2 methoxy-, methyl esters, (Z)- | 1.25 | 0.19 | ND |
| Samples | Raw | Oven Drying | Microwave Drying |
|---|---|---|---|
| Saturated fatty acids (SFAs) | |||
| C12:0 | 0.32 ± 0.02 a | 0.24 ± 0.01 b | 0.19 ± 0.01 c |
| C14:0 | 3.48 ± 0.03 a | 3.02 ± 0.07 b | 2.34 ± 0.03 c |
| C15:0 | 1.00 ± 0.04 a | 0.96 ± 0.03 a | 0.67 ± 0.03 b |
| C16:0 | 18.74 ± 0.09 b | 18.50 ± 0.18 b | 19.16 ± 0.08 a |
| C17:0 | 1.12 ± 0.06 b | 1.14 ± 0.08 b | 2.33 ± 0.07 a |
| C18:0 | 6.44 ± 0.10 a | 4.60 ± 0.07 c | 4.91 ± 0.05 b |
| C20:0 | 0.22 ± 0.03 c | 0.31 ± 0.02 b | 0.38 ± 0.03 a |
| C22:0 | 0.13 ± 0.01 b | 0.11 ± 0.00 b, c | 0.16 ± 0.01 a |
| C24:0 | 1.02 ± 0.06 a | 0.81 ± 0.03 b | 0.72 ± 0.03 c |
| Total SFAs | 32.48 ± 0.13 a | 29.35 ± 0.04 a | 30.69 ± 0.06 a |
| Monounsaturated fatty acids (MUFAs) | |||
| C14:1 | 0.90 ± 0.03 a | 0.81 ± 0.02 b | 0.37 ± 0.05 c |
| C16:1 | 7.15 ± 0.08 a | 6.87 ± 0.07 b | 5.65 ± 0.07 c |
| C17:1 | 1.21 ± 0.02 a | 1.18 ± 0.01 a | 0.70 ± 0.10 b |
| C18:1 | 27.08 ± 0.31 a | 25.04 ± 0.28 b | 25.27 ± 0.15 b |
| C20:1 | 3.35 ± 0.09 a | 3.20 ± 0.19 a | 2.75 ± 0.07 b |
| C22:1 | 0.61 ± 0.06 a | 0.49 ± 0.05 b | 0.21 ± 0.01 c |
| C24:1 | 0.12 ± 0.02 a | 0.11 ± 0.01 a | 0.06 ± 0.00 b |
| Total MUFAs | 40.23 ± 0.46 a | 37.74 ± 0.26 b | 34.70 ± 0.22 c |
| Polyunsaturated fatty acids (PUFAs) | |||
| C18:2 n-6 | 7.35 ± 0.09 c | 8.27 ± 0.19 a | 7.70 ± 0.14 b |
| C18:3 n-6 | 0.30 ± 0.01 b | 0.44 ± 0.04 a | 0.41 ± 0.03 a |
| C18:3 n-3 | 5.02 ± 0.10 b | 5.96 ± 0.30 a | 5.30 ± 0.11 b |
| C20:2 n-6 | 0.61 ± 0.05 b | 0.77 ± 0.005 a | 0.81 ± 0.02 a |
| C20:3 n-6 | 0.59 ± 0.02 b | 0.71 ± 0.01 a | 0.72 ± 0.03 a |
| C20:4 n-6 | 2.92 ± 0.04 c | 3.85 ± 0.20 b | 4.27 ± 0.19 a |
| C20:3 n-3 | 0.61 ± 0.04 b | 0.87 ± 0.01 a | 0.91 ± 0.02 a |
| C20:5 n-3 EPA | 3.47 ± 0.09 b | 4.99 ± 0.22 a | 5.29 ± 0.15 a |
| C22:6 n-3 DHA | 5.41 ± 0.14 c | 5.94 ± 0.48 b | 7.57 ± 0.11 a |
| Total PUFAs | 26.73 ± 0.50 c | 31.03 ± 0.27 b | 32.85 ± 0.35 a |
| PUFAs/SFAs | 0.82 | 1.05 | 1.07 |
| MUFAs/SFAs | 1.23 | 1.28 | 1.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alahmad, K.; Xia, W.; Jiang, Q.; Xu, Y. Influence of Drying Techniques on the Physicochemical, Nutritional, and Morphological Properties of Bighead Carp (Hypophthalmichthys nobilis) Fillets. Foods 2021, 10, 2837. https://doi.org/10.3390/foods10112837
Alahmad K, Xia W, Jiang Q, Xu Y. Influence of Drying Techniques on the Physicochemical, Nutritional, and Morphological Properties of Bighead Carp (Hypophthalmichthys nobilis) Fillets. Foods. 2021; 10(11):2837. https://doi.org/10.3390/foods10112837
Chicago/Turabian StyleAlahmad, Kamal, Wenshui Xia, Qixing Jiang, and Yanshun Xu. 2021. "Influence of Drying Techniques on the Physicochemical, Nutritional, and Morphological Properties of Bighead Carp (Hypophthalmichthys nobilis) Fillets" Foods 10, no. 11: 2837. https://doi.org/10.3390/foods10112837
APA StyleAlahmad, K., Xia, W., Jiang, Q., & Xu, Y. (2021). Influence of Drying Techniques on the Physicochemical, Nutritional, and Morphological Properties of Bighead Carp (Hypophthalmichthys nobilis) Fillets. Foods, 10(11), 2837. https://doi.org/10.3390/foods10112837





