Structural Characterization and Antioxidant Activity of Exopolysaccharide from Soybean Whey Fermented by Lacticaseibacillus plantarum 70810
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Isolation and Purification of EPS
2.3. Analysis of Monosaccharide Composition
2.4. UV and FT-IR Spectra Analysis
2.5. Nuclear Magnetic Resonance (NMR) Analysis
2.6. Scanning Electron Microscopy (SEM) Analysis
2.7. Particle Size and Zeta-Potential
2.8. Antioxidant Activities In Vitro
2.8.1. Scavenging Activity of DPPH Radicals
2.8.2. Scavenging Activity of Superoxide Anion Radical
2.8.3. Chelating Metal Ion Capacity
2.9. Statistical Analysis
3. Results and Discussion
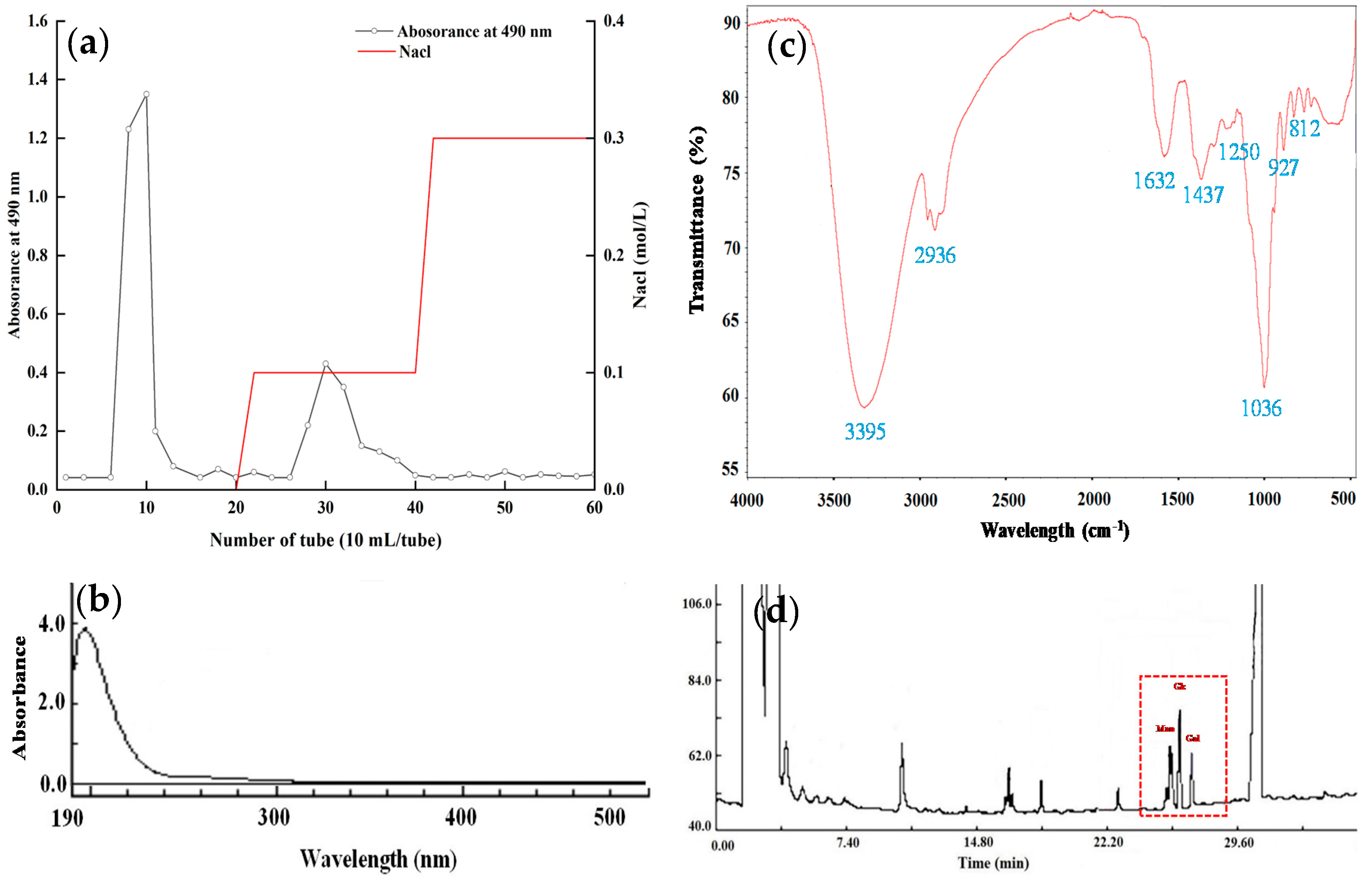
3.1. Isolation and Purification of EPS
3.2. UV and FT-IR Spectra Analysis
3.3. Monosaccharide Composition of LPEPS-1
3.4. NMR Characterization of EPS
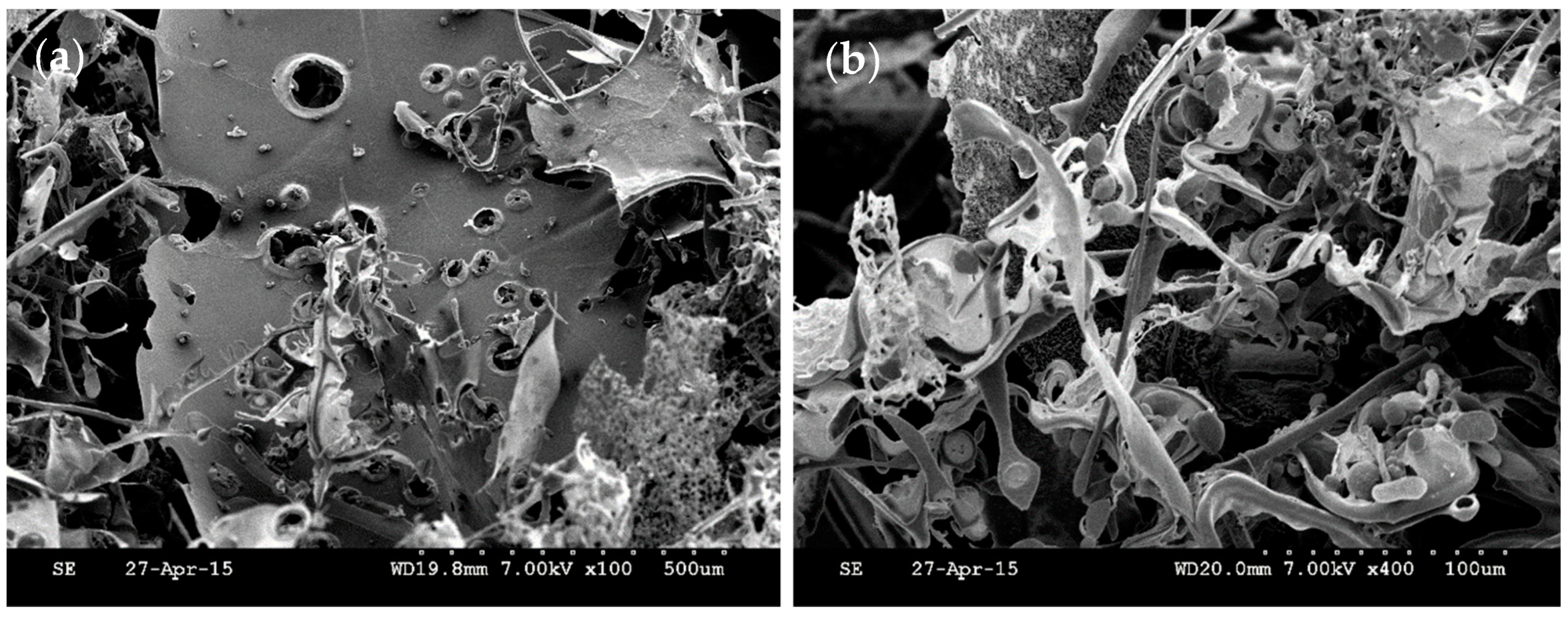
3.5. SEM Analysis
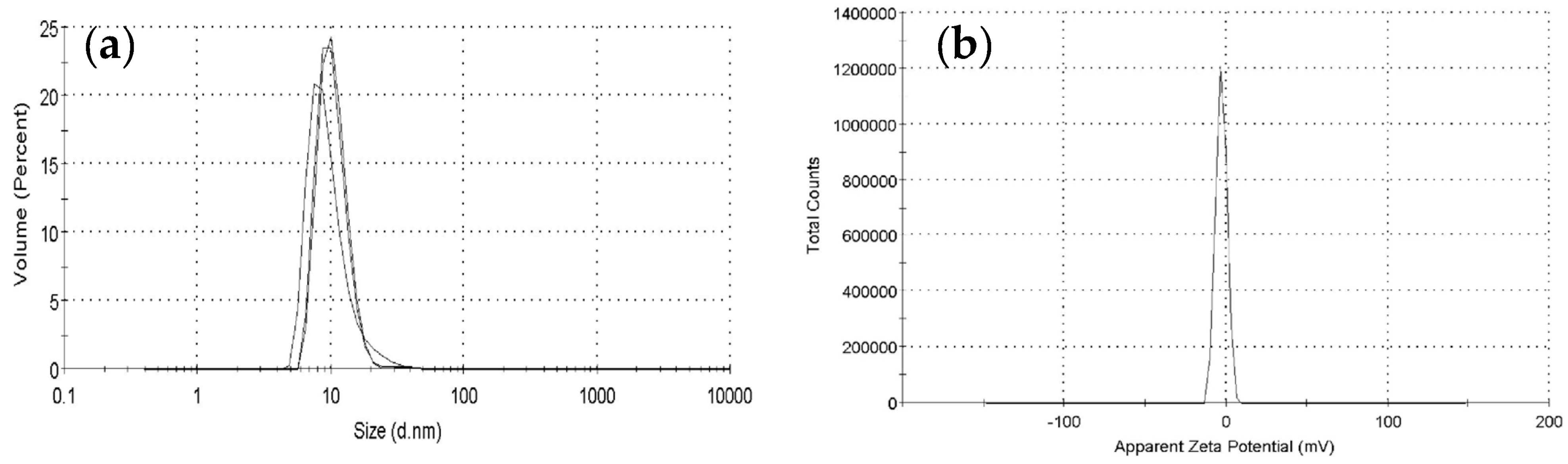
3.6. Particle Size and Zeta-Potential Analysis
3.7. Analysis of Antioxidant Activity In Vitro
3.7.1. DPPH Radical Scavenging Activity
3.7.2. Superoxide Anion Free Radical Scavenging Activity of EPS
3.7.3. Metal Ion-Chelating Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berezhnaya, Y.; Bikaeva, I.; Gachkovskaia, A.; Demidenko, A.; Klimenko, N.; Tyakht, A.; Volokh, O.; Alexeev, D. Temporal dynamics of probiotic Lacticaseibacillus casei and rhamnosus abundance in a fermented dairy product evaluated using a combination of cultivation-dependent and-independent methods. LWT-Food Sci. Technol. 2021, 148, 111750. [Google Scholar] [CrossRef]
- Park, D.W.; Kim, S.H.; Park, J.H. Distribution and characterization of prophages in Lactobacillus plantarum derived from kimchi. Food Microbiol. 2022, 102, 103913. [Google Scholar] [CrossRef]
- Yang, J.; Lu, J.; Zhu, Q.; Tao, Y.; Zhu, Q.; Guo, C.; Fang, Y.; Chen, L.; Koyande, A.; Wang, S.J.; et al. Isolation and characterization of a novel Lactobacillus plantarum MMB-07 from traditional Suanyu for Acanthogobius hasta fermentation. J. Biosci. Bioeng. 2021, 132, 161–166. [Google Scholar] [CrossRef]
- Pei, J.J.; Jin, W.G.; Abd El-Aty, A.M.; Baranenko, D.A.; Gou, X.Y.; Zhang, H.X.; Geng, J.Z.; Jiang, L.; Chen, D.J.; Yue, T.L. Isolation, purification, and structural identification of a new bacteriocin made by Lactobacillus plantarum found in conventional kombucha. Food Control 2020, 110, 106923. [Google Scholar] [CrossRef]
- Abdelazez, A.; Abdelmotaal, H.; Zhu, Z.T.; Fang, J.F.; Sami, R.; Zhang, L.J.; Al-Tawaha, A.; Meng, X.C. Potential benefits of Lactobacillus plantarum as probiotic and its advantages in human health and industrial applications: A review. Adv. Environ. Biol. 2018, 12, 16–27. [Google Scholar] [CrossRef]
- Silva, L.A.; Neto, J.H.P.L.; Cardarelli, H.R. Exopolysaccharides produced by Lactobacillus plantarum: Technological properties, biological activity, and potential application in the food industry. Ann. Microbiol. 2019, 69, 321–328. [Google Scholar] [CrossRef]
- Jiang, B.; Tian, L.; Huang, X.; Liu, Z.; Jia, K.; Wei, H.; Tao, X. Characterization and antitumor activity of novel exopolysaccharide APS of Lactobacillus plantarum WLPL09 from human breast milk. Int. J. Biol. Macromol. 2020, 163, 985–995. [Google Scholar] [CrossRef]
- Bomfim, V.B.; Neto, J.H.P.L.; Leite, K.S.; de Andrade Vieira, É.; Iacomini, M.; Silva, C.M.; Olbrich dos Santos, K.M.; Cardarelli, H.R. Partial characterization and antioxidant activity of exopolysaccharides produced by Lactobacillus plantarum CNPC003. LWT-Food Sci. Technol. 2020, 127, 109349. [Google Scholar] [CrossRef]
- Rajoka, M.S.R.; Wu, Y.G.; Mehwish, H.M.; Bansal, M.; Zhao, L.Q. Lactobacillus exopolysaccharides: New perspectives on engineering strategies, physiochemical functions, and immunomodulatory effects on host health. Trends Food Sci. Technol. 2020, 103, 36–48. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure–function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, L.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Enhancement of the antioxidant capacity of soy whey by fermentation with Lactobacillus plantarum B1-6. J. Funct. Foods. 2015, 12, 33–44. [Google Scholar] [CrossRef]
- Chua, J.Y.; Liu, S.Q. Soy whey: More than just wastewater from tofu and soy protein isolate industry. Trends Food Sci. Technol. 2019, 91, 24–32. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Z.; Zhang, L. Optimization of lactic acid fermentation conditions for fermented tofu whey beverage with high-isoflavone aglycones. LWT-Food Sci. Technol. 2019, 111, 211–217. [Google Scholar] [CrossRef]
- Tenorio, M.D.; Espinosa-Martos, I.; Préstamo, G.; Rupérez, P. Soybean whey enhance mineral balance and caecal fermentation in rats. Eur. J. Nutr. 2010, 49, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Azi, F.; Huang, J.; Xu, X.; Xing, G.; Dong, M. Quality and metagenomic evaluation of a novel functional beverage produced from soy whey using water kefir grains. LWT-Food Sci. Technol. 2019, 113, 108258. [Google Scholar] [CrossRef]
- Chua, J.Y.; Liu, S.Q. Effect of single amino acid addition on growth kinetics and flavor modulation by Torulaspora delbrueckii in soy (tofu) whey alcoholic beverage fermentation. Food Res. Int. 2020, 135, 109283. [Google Scholar] [CrossRef]
- Wang, K.; Li, W.; Rui, X.; Chen, X.; Jiang, M.; Dong, M. Characterization of a novel exopolysaccharide with antitumor activity from Lactobacillus plantarum 70810. Int. J. Biol. Macromol. 2014, 63, 133–139. [Google Scholar] [CrossRef]
- Wang, K.; Li, W.; Rui, X.; Chen, X.; Jiang, M.; Dong, M. Structural characterization and bioactivity of released exopolysaccharides from Lactobacillus plantarum 70810. Int. J. Biol. Macromol. 2014, 67, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Chaisuwan, W.; Jantanasakulwong, K.; Wangtueai, S.; Phimolsiripol, Y.; Chaiyaso, T.; Techapun, C.; Phongthai, S.; You, S.; Regenstein, J.; Seesuriyachan, P. Microbial exopolysaccharides for immune enhancement: Fermentation, modifications and bioactivities. Food Biosci. 2020, 35, 100564. [Google Scholar] [CrossRef]
- Zhang, H.; Li, W.; Rui, X.; Sun, X.; Dong, M. Lactobacillus plantarum 70810 from Chinese paocai as a potential source of β-galactosidase for prebiotic galactooligosaccharides synthesis. Eur. Food Res. Technol. 2013, 236, 817–826. [Google Scholar] [CrossRef]
- Li, W.; Mutuvulla, M.; Chen, X.; Jiang, M.; Dong, M. Isolation and identification of high viscosity-producing lactic acid bacteria from a traditional fermented milk in Xinjiang and its role in fermentation process. Eur. Food Res. Technol. 2012, 235, 497–505. [Google Scholar] [CrossRef]
- Tang, W.; Dong, M.; Wang, W.; Han, S.; Rui, X.; Chen, X.; Jiang, M.; Zhang, Q.; Wu, J.; Li, W. Structural characterization and antioxidant property of released exopolysaccharides from Lactobacillus delbrueckii ssp. bulgaricus SRFM-1. Carbohydr. Polym. 2017, 173, 654–664. [Google Scholar] [CrossRef]
- Qiao, D.; Ke, C.; Hu, B.; Luo, J.; Ye, H.; Sun, Y.; Yan, X.; Zeng, X. Antioxidant activities of polysaccharides from Hyriopsis cumingii. Carbohydr. Polym. 2009, 78, 199–204. [Google Scholar] [CrossRef]
- Qi, H.; Zhang, Q.; Zhao, T.; Hu, R.; Zhang, K.; Li, Z. In vitro antioxidant activity of acetylated and benzoylated derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta). Bioorg. Med. Chem. Lett. 2006, 16, 2441–2445. [Google Scholar] [CrossRef]
- Liu, C.H.; Wang, C.H.; Xu, Z.L.; Wang, Y. Isolation, chemical characterization and antioxidant activities of two polysaccharides from the gel and the skin of Aloe barbadensis Miller irrigated with sea water. Process Biochem. 2007, 42, 961–970. [Google Scholar] [CrossRef]
- Wu, L.; Sun, H.; Hao, Y.; Zheng, X.; Song, Q.; Dai, S.; Zhu, Z. Chemical structure and inhibition on α-glucosidase of the polysaccharides from Cordyceps militaris with different developmental stages. Int. J. Biol. Macromol. 2020, 148, 722–736. [Google Scholar] [CrossRef]
- Ji, X.; Hou, C.; Yan, Y.; Shi, M.; Liu, Y. Comparison of structural characterization and antioxidant activity of polysaccharides from jujube (Ziziphus jujuba Mill.) fruit. Int. J. Biol. Macromol. 2020, 149, 1008–1018. [Google Scholar] [CrossRef]
- Wang, B. Chemical characterization and ameliorating effect of polysaccharide from Chinese jujube on intestine oxidative injury by ischemia and reperfusion. Int. J. Biol. Macromol. 2011, 48, 386–391. [Google Scholar] [CrossRef]
- Ismail, B.; Nampoothiri, K.M. Production, purification and structural characterization of an exopolysaccharide produced by a probiotic Lactobacillus plantarum MTCC 9510. Arch. Microbiol. 2010, 192, 1049–1057. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Qiu, L.; Zhang, F.; Xu, X.; Wei, H.; Tao, X. Characterization and bioactivities of the exopolysaccharide from a probiotic strain of Lactobacillus plantarum WLPL04. J. Dairy Sci. 2017, 100, 6895–6905. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.; Qin, P.; Shi, Z.; Zhang, W.; Yang, X.; Yao, Y.; Ren, G. Structural characterization and antioxidant activity of alkali-extracted polysaccharides from quinoa. Food Hydrocolloid. 2021, 113, 106392. [Google Scholar] [CrossRef]
- Jing, Y.; Li, J.; Zhang, Y.; Zhang, R.; Zheng, Y.; Hu, B.; Wu, L.; Zhang, D. Structural characterization and biological activities of a novel polysaccharide from Glehnia littoralis and its application in preparation of nano-silver. Int. J. Biol. Macromol. 2021, 183, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Zhong, R.F.; Chen, J.; Luo, Z.G. Structural characterization, anticancer, hypoglycemia and immune activities of polysaccharides from Russula virescens. Int. J. Biol. Macromol. 2021, 184, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Han, Y.; Yang, L.; Hu, L.; Duan, X.; Yang, X.; Huang, R. Structural characterization and immunostimulatory activity of a novel polysaccharide from green alga Caulerpa racemosa var peltata. Int. J. Biol. Macromol. 2019, 134, 891–900. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, M.; Hao, H.; Deng, J.; Li, M.; Sun, Y.; Yang, R.; Wang, H.; Huang, R. Structure characterization of a novel polysaccharide from Chinese wild fruits (Passiflora foetida) and its immune-enhancing activity. Int. J. Biol. Macromol. 2019, 136, 324–331. [Google Scholar] [CrossRef]
- Vinogradov, E.; Valence, F.; Maes, E.; Jebava, I.; Chuat, V.; Lortal, S.; Grard, T.; Guerardel, Y.; Sadovskaya, I. Structural studies of the cell wall polysaccharides from three strains of Lactobacillus helveticus with different autolytic properties: DPC4571, BROI, and LH1. Carbohydr. Res. 2013, 379, 7–12. [Google Scholar] [CrossRef]
- Ktari, N.; Bkhairia, I.; Nasri, M.; Salah, R.B. Structure and biological activities of polysaccharide purified from Senegrain seed. Int. J. Biol. Macromol. 2020, 144, 190–197. [Google Scholar] [CrossRef]
- Xu, Y.; Cui, Y.; Wang, X.; Yue, F.; Shan, Y.; Liu, B.; Zhou, Y.; Yi, Y.; Lü, X. Purification, characterization and bioactivity of exopolysaccharides produced by Lactobacillus plantarum KX041. Int. J. Biol. Macromol. 2019, 128, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Feng, S.; Jia, X.; Li, Q.; Zhou, Y.; Ding, C. Structural characterization and antioxidant activities of polysaccharides extracted from Epimedium acuminatum. Carbohydr. Polym. 2013, 92, 63–68. [Google Scholar] [CrossRef]
- Kanamarlapudi, S.L.R.K.; Muddada, S. Characterization of exopolysaccharide produced by Streptococcus thermophilus CC30. BioMed Res. Int. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, L. Effect of extraction method on structure and antioxidant activity of Hohenbuehelia serotina polysaccharides. Int. J. Biol. Macromol. 2016, 83, 270–276. [Google Scholar] [CrossRef]
- Han, Q.; Yu, Q.Y.; Shi, J.; Xiong, C.Y.; Ling, Z.J.; He, P.M. Molecular characterization and hypoglycemic activity of a novel water-soluble polysaccharide from tea (Camellia sinensis) flower. Carbohydr. Polym. 2011, 86, 797–805. [Google Scholar] [CrossRef]
- Tang, W.; Han, S.; Zhou, J.; Xu, Q.; Dong, M.; Fan, X.; Rui, X.; Zhang, Q.; Chen, X.; Jiang, M.; et al. Selective fermentation of Lactobacillus delbrueckii ssp. Bulgaricus SRFM-1 derived exopolysaccharide by Lactobacillus and Streptococcus strains revealed prebiotic properties. J. Funct. Foods. 2020, 69, 103952. [Google Scholar] [CrossRef]
- Lin, Y.; Zeng, H.; Wang, K.; Lin, H.; Li, P.; Huang, Y.; Zhou, S.; Zhang, W.; Chen, C.; Fan, H. Microwave-assisted aqueous two-phase extraction of diverse polysaccharides from Lentinus edodes: Process optimization, structure characterization and antioxidant activity. Int. J. Biol. Macromol. 2019, 136, 305–315. [Google Scholar] [CrossRef]
- Lee, J.; Koo, N.; Min, D.B. Reactive oxygen species, aging, and antioxidative nutraceuticals. Compr. Rev. Food Sci. Food Saf. 2004, 3, 21–33. [Google Scholar] [CrossRef]
- Yuan, Q.; Xie, Y.; Wang, W.; Yan, Y.; Ye, H.; Jabbar, S.; Zeng, X. Extraction optimization, characterization and antioxidant activity in vitro of polysaccharides from mulberry (Morus alba L.) leaves. Carbohydr. Polym. 2015, 128, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Ghazala, I.; Sila, A.; Frikha, F.; Driss, D.; Ellouz-Chaabouni, S.; Haddar, A. Antioxidant and antimicrobial properties of water soluble polysaccharide extracted from carrot peels by-products. J. Food Sci. Tech. 2015, 52, 6953–6965. [Google Scholar] [CrossRef]
- Li, L.; Thakur, K.; Liao, B.Y.; Zhang, J.G.; Wei, Z.J. Antioxidant and antimicrobial potential of polysaccharides sequentially extracted from Polygonatum cyrtonema Hua. Int. J. Biol. Macromol. 2018, 114, 317–323. [Google Scholar] [CrossRef]
- Sun, Y.X.; Liu, J.C.; Kennedy, J.F. Purification, composition analysis and antioxidant activity of different polysaccharide conjugates (APPs) from the fruiting bodies of Auricularia polytricha. Carbohydr. Polym. 2010, 82, 299–304. [Google Scholar] [CrossRef]
- Lei, N.; Wang, M.; Zhang, L.; Xiao, S.; Fei, C.; Wang, X.; Zhang, K.; Zheng, W.; Wang, C.; Yang, R.; et al. Effects of low molecular weight yeast β-glucan on antioxidant and immunological activities in mice. Int. J. Mol. Sci. 2015, 16, 21575–21590. [Google Scholar] [CrossRef] [Green Version]
- Lo, T.C.T.; Chang, C.A.; Chiu, K.H.; Tsay, P.K.; Jen, J.F. Correlation evaluation of antioxidant properties on the monosaccharide components and glycosyl linkages of polysaccharide with different measuring methods. Carbohydr. Polym. 2011, 86, 320–327. [Google Scholar] [CrossRef]
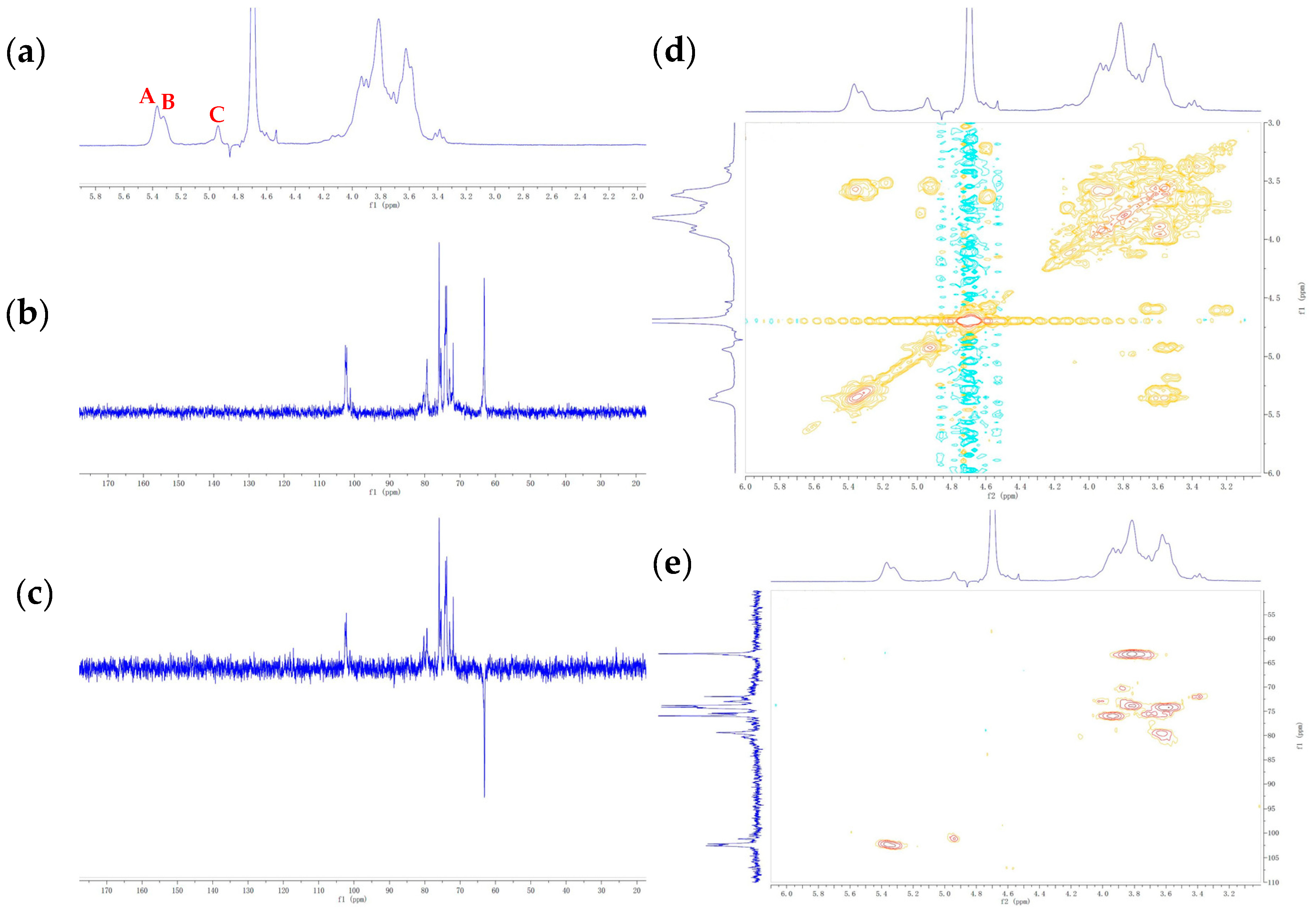

| Residues | Sugar Linkages | H-1/C-1 | H-2/C-2 | H-3/C-3 | H-4/C-4 | H-5/C-5 | H-6/C-6 |
|---|---|---|---|---|---|---|---|
| A | →4)-α-Glcp (1→ | 5.37 102.19 | 3.58 73.88 | 3.93 75.13 | 3.67 79.52 | 3.78 73.88 | 3.82 63.11 |
| B | →2)-α-Manp (1→ | 5.32 102.57 | 3.60 79.24 | 3.90 70.88 | 3.69 71.94 | 3.89 69.53 | 3.74 62.40 |
| C | →3)-α-Glap (1→ | 4.94 101.10 | 3.58 74.38 | 3.97 76.10 | 4.00 72.37 | 3.72 75.50 | 3.80 63.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, J.; Mao, Q.; Dong, M.; Wang, X.; Rui, X.; Zhang, Q.; Chen, X.; Li, W. Structural Characterization and Antioxidant Activity of Exopolysaccharide from Soybean Whey Fermented by Lacticaseibacillus plantarum 70810. Foods 2021, 10, 2780. https://doi.org/10.3390/foods10112780
Tian J, Mao Q, Dong M, Wang X, Rui X, Zhang Q, Chen X, Li W. Structural Characterization and Antioxidant Activity of Exopolysaccharide from Soybean Whey Fermented by Lacticaseibacillus plantarum 70810. Foods. 2021; 10(11):2780. https://doi.org/10.3390/foods10112780
Chicago/Turabian StyleTian, Juanjuan, Qingyan Mao, Mingsheng Dong, Xiaomeng Wang, Xin Rui, Qiuqin Zhang, Xiaohong Chen, and Wei Li. 2021. "Structural Characterization and Antioxidant Activity of Exopolysaccharide from Soybean Whey Fermented by Lacticaseibacillus plantarum 70810" Foods 10, no. 11: 2780. https://doi.org/10.3390/foods10112780
APA StyleTian, J., Mao, Q., Dong, M., Wang, X., Rui, X., Zhang, Q., Chen, X., & Li, W. (2021). Structural Characterization and Antioxidant Activity of Exopolysaccharide from Soybean Whey Fermented by Lacticaseibacillus plantarum 70810. Foods, 10(11), 2780. https://doi.org/10.3390/foods10112780







