Abstract
The intake of isoflavones is presumed to be associated with health benefits in humans, but also potential adverse effects of isoflavones are controversially discussed. Isoflavones can be metabolized by gut bacteria leading to modulation of the bioactivity, such as estrogenic effects. Especially bacterial strains of the Eggerthellaceae, a well-known bacterial family of the human gut microbiota, are able to convert the isoflavone daidzein into equol. In addition, metabolization of genistein is also described for strains of the Eggerthellaceae. The aim of this study was to identify and investigate gut bacterial strains of the family Eggerthellaceae as well as the narrowly related family Coriobacteriaceae which are able to metabolize daidzein and genistein. This study provides a comprehensive, polyphasic approach comprising in silico analysis of the equol gene cluster, detection of genes associated with the daidzein, and genistein metabolism via PCR and fermentation of these isoflavones. The in silico search for protein sequences that are associated with daidzein metabolism identified sequences with high similarity values in already well-known equol-producing strains. Furthermore, protein sequences that are presumed to be associated with daidzein and genistein metabolism were detected in the two type strains ‘Hugonella massiliensis’ and Senegalimassilia faecalis which were not yet described to metabolize these isoflavones. An alignment of these protein sequences showed that the equol gene cluster is highly conserved. In addition, PCR amplification supported the presence of genes associated with daidzein and genistein metabolism. Furthermore, the metabolism of daidzein and genistein was investigated in fermentations of pure bacterial cultures under strictly anaerobic conditions and proofed the metabolism of daidzein and genistein by the strains ‘Hugonella massiliensis’ DSM 101782T and Senegalimassilia faecalis KGMB04484T.
Keywords:
Eggerthellaceae; Coriobacteriaceae; anaerobic; isoflavones; daidzein; genistein; equol; microbial; metabolism 1. Introduction
Daidzein and genistein, two isoflavones with a very similar molecular structure, are well-known to be present in soy and soy-based food [1]. Daidzein and genistein belong to the phytoestrogens as they are “biologically active phenolic compounds derived from plants” with “structures similar to the principal mammalian estrogen” [2]. Several health benefits associated with the consumption of isoflavones are discussed, such as the alleviation of menopausal symptoms, prevention of osteoporosis, and improvement of cancer prognosis [3]. Besides beneficial effects, potential adverse effects of isoflavone intake are also controversially discussed [4]. These effects are often associated with their estrogenic activity. For isoflavones the endogenous transformation in humans is well known and they can be metabolized by endogenous phase I and phase II enzymes [4]. Besides that, isoflavones can be metabolized by gut bacteria leading to modulation of their bioactivity. High inter-individual variations in this microbial metabolism are observed in humans. For example, only about one-third of the human population can convert daidzein to equol, an important metabolite showing the highest affinity to estrogen receptors (ER) among all known isoflavones [4]. Especially strains of the family Coriobacteriaceae are able to convert food polyphenols like daidzein [5]. However, there is still limited knowledge of bacteria that are involved in this conversion [6]. In the intestine, daidzein can be reduced by bacterial metabolism either to equol via dihydrodaidzein (DH-daidzein) and tetrahydrodaidzein (TH-daidzein) and/or to O-desmethylangolensin (O-DMA) also via DH-daidzein [6,7] (Figure 1). Similar to the metabolization of daidzein, genistein can be converted by bacterial action to 6′-hydroxy-O-desmethylangolensin (6′-OH-O-DMA) [4]. The formation of 5-hydroxyl-equol (5-OH-equol) from genistein is described for the type strains of Adlercreutzia mucosicola, Slackia equolifaciens and Slackia isoflavoniconvertens [8,9,10]. However, to the best of our knowledge, 5-OH-equol has not yet been detected in human biofluids, so evidence for its formation in vivo is still lacking. It is well-known that not every bacterial strain that is able to metabolize e.g., daidzein can conduct each single metabolization step from daidzein to equol, which is probably the end product of the metabolic transformation. Some strains only convert daidzein to intermediate metabolites or are able to further metabolize intermediate metabolites [6].
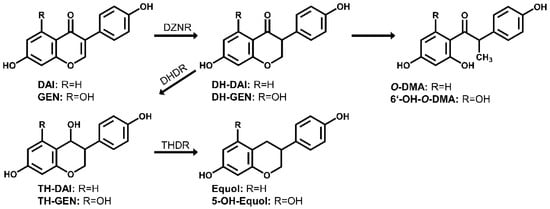
Figure 1.
Metabolism of daidzein and genistein by human gut bacteria. Modified based on [6,7]. Daidzein (DAI); genistein (GEN); dihydrodaidzein (DH-DAI); dihydrogenistein (DH-GEN), tetrahydrodaidzein (TH-DAI); tetrahydrogenistein (TH-GEN); 5-hydroxyl-equol (5-OH-equol); O-desmethylangolensin (O-DMA); 6′-hydroxy-O-desmethylangolensin (6′-OH-O-DMA); daidzein reductase (DZNR), dihydrodaidzein reductase (DHDR); tetrahydrodaidzein reductase (THDR).
Since especially strains of the Coriobacteriaceae and Eggerthellaceae are associated with the metabolization of daidzein and genistein, it has to be noted that taxonomic changes within the parent class Coriobacteriia occurred [11,12]. The following Eggerthellaceae strains are described to metabolize daidzein and/or genistein: Adlercreutzia equolifaciens subsp. celatus DSM 18785T [13], A. equolifaciens subsp. equolifaciens DSM 19450T [14], Adlercreutzia mucosicola DSM 19490T [15], Eggerthella sp. YY7918 [16], Slackia equolifaciens DSM 24851T [17], Slackia isoflavoniconvertens DSM 22006T [8], Slackia sp. AUH-JLC159 [18], and Slackia sp. NATTS [19]. The metabolization of daidzein to O-DMA is described for Slackia exigua DSM 15923T [8,14]. The enzymes that are involved in daidzein and genistein metabolism were identified and characterized in five bacterial strains, i.e., Lactococcus garvieae 20–92 [20,21,22], S. isoflavoniconvertens DSM 22006T [23], Slackia sp. NATTS [24], A. equolifaciens DSM 19450T [25] and Eggerthella sp. YY7918 [16]. The following three genes are described to be necessary for equol production: I) daidzein reductase (DZNR), II) dihydrodaidzein reductase (DHDR), and III) tetrahydrodaidzein reductase (THDR). A fourth enzyme (dihydrodaidzein racemase, DDRC) is described to increase equol production [22,23]. The corresponding genes that are associated with the metabolism of daidzein and genistein are located in one cluster [6,25], named equol gene cluster throughout the manuscript. DH-daidzein and equol occur in two enantiomeric forms; S-equol is the exclusive enantiomeric form that is produced by human intestinal bacteria [22,26]. DDRC may catalyze the conversion of R- into S-DH-daidzein and may explain the exclusive S-equol production [22,26].
Due to the similar molecule structure, it is presumed that daidzein converting enzymes also metabolize genistein which is already confirmed for the daidzein reductase of S. isoflavoniconvertens DSM 22006T [23]. However, strain Eggerthella sp. YY7918 converts daidzein, but not genistein [27]. The metabolization of genistein seems to be less investigated than the metabolization of daidzein. However, genistein metabolization is described, for e.g., A. mucosicola DSM 19490T [15] and S. isoflavoniconvertens DSM 22006T [8].
The aim of this study was to investigate the metabolism of daidzein and genistein in gut-related bacterial strains of the families Eggerthellaceae and Coriobacteriaceae. Firstly, an in silico approach detecting genes of the equol gene cluster in recently annotated draft genome sequences of strains of these two families was conducted. In addition, a comprehensive PCR approach of 29 strains was performed to search for the presence of genes of the equol gene cluster. Conclusively, the metabolism of daidzein and genistein was investigated by fermentation of pure cultures of these 29 Eggerthellaceae and Coriobacteriaceae strains under strictly anaerobic conditions.
2. Materials and Methods
Chemicals. The purity of all analytes was determined by LC-DAD (220–600 nm). Daidzein (purity of 99.6%), (R, S)-equol (99.9%), and genistein (99.7%) were purchased from LC Laboratories (Woburn, MA, USA). Dihydrodaidzein (99.7%) and O-DMA (95.9%) were purchased from Toronto Research Chemicals (Toronto, ON, Canada) and Plantech UK (Reading, UK), respectively. DH-genistein (97.8%) and 6′-OH-O-DMA (97.0%) were purchased from APIN Chemicals (Abingdon, UK). All other chemicals used were of analytical grade. Deionized water was taken from the in-house ultrapure water system LaboStar (Siemens) with a conductivity of 0.055 μS/cm.
Strains and culture conditions. For comparative analysis, bacterial strains were obtained from the German Collection of Microorganisms and Cell Cultures GmbH (DSMZ), the Japanese Culture Collection (JCM), the Collection de Souches de l’Unité des Rickettsies, Unités des Rickettsies, France (CSUR), the Korean Collection for Type Cultures (Korea Research Institute of Bioscience and Biotechnology) (KGMB) and the own laboratory collection (MRI-F and ResAG): Adlercreutzia equolifaciens subsp. equolifaciens DSM 19450T, Adlercreutzia equolifaciens subsp. celatus DSM 18785T, Adlercreutzia rubneri ResAG-91T, Adlercreutzia caecimuris DSM 21839T, Adlercreutzia mucosicola DSM 19490T, Adlercreutzia muris DSM 29508T, Cryptobacterium curtum DSM 15641T, Denitrobacterium detoxificans DSM 21843T, Eggerthella lenta DSM 2243T, DSM 15644, Eggerthella sinensis DSM 16107T, ‘Eggerthella timonensis’ CSUR P3135T, Ellagibacter isourolithinifaciens DSM 104140T, Enteroscipio rubneri ResAG-96T, ‘Gordonibacter massiliensis’ CSUR P2775T, Gordonibacter pamelaeae DSM 19378T, Gordonibacter urolithinfaciens DSM 27213T, JCM 16058, ‘Hugonella massiliensis’ DSM 101782T, Paraeggerthella hongkongensis DSM 16106T, Parvibacter caecicola DSM 22242T, Rubneribacter badeniensis ResAG-85T, Slackia exigua DSM 15923T, Slackia equolifaciens DSM 24851T, Slackia faecicanis DSM 17537T, Slackia heliotrinireducens DSM 20476T, Slackia isoflavoniconvertens DSM 22006T, Slackia piriformis DSM 22477T, Senegalimassilia anaerobia DSM 25959T and Senegalimassilia faecalis KGMB04484T. Cultivation of all strains was performed at 37 °C under strictly anaerobic conditions either in Hungate tubes flushed with N2/CO2 (80/20) or in an A45 anaerobic workstation (Don Whitley Scientific) under atmospheric conditions of N2/CO2/H2 (80/10/10). All strains were cultured for 48–72 h in modified BHI (5 g L−1 yeast extract, 0.05 g L−1 l-cysteine monohydrochloride, 1 mg L−1 resazurin sodium salt, 2.5 mg L−1 haem solution, 2 μg mL−1 vitamin K1 solution).
In silico identification of the equol gene cluster. The annotated draft genome sequence of S. isoflavoniconvertens DSM 22006T (QIBZ00000000) [28] was used as a reference genome. The equol gene cluster was localized by using the protein sequences (DZNR: AFV15453; DHDR: AFV15451; THDR: AFV15450; DDRC: AFV15447) of the respective genes. These protein sequences were used to perform a protein BLASTp search. Clustering of the protein sequences was performed using BioNumerics (version 8.0, Applied Maths). Genome segments and annotations of the respective strains were visualized, and analyzed by the use of the CLC Sequence Viewer software (version 8, Qiagen).
DNA Isolation, PCR, agarose gel electrophoresis, and clustering. Bacterial cells (10 mL) were harvested by centrifugation (10 min, 4 °C, 6540× g) and genomic DNA was isolated using the blood and tissue kit (Qiagen) for Gram-positive bacteria according to the manufacturer’s instructions. DNA was quantified with the double-stranded DNA (dsDNA) high-sensitivity (HS) assay kit on a Qubit version 2.0 fluorometer (Thermo Fischer Scientific) and was adjusted to a concentration of 10 ng µL−1. Gene-specific primers for dzr (dzr.qPCR-F: 5′-GAA GCT TGA TAT GGA CGA CT-3′; dzr.qPCR-R: 5′-GGA ATA TGC ACC TGT TCC T-3′), ddr (ddr.qPCR-F: 5′-CTC GAY CTS GTS TAC AAC GT-3′; ddr.qPCR-R: 5′-GAR TTG CAG CGR ATK CCG AA-3′) and tdr (tdr.qPCR-F: 5′-RTY AAC GGC RAY ATG CAG GT-3′; tdr.qPCR-R: 5′-GGM AYY TCC ATG TTG TAG GA-3′) developed by [29] were used. Each PCR reaction consisted of 5 µL of DNA template, 1.25 µL of each of the respective primer (10 pmol/µL; synthesized by biomers.net), 12.5 µL of ALLin Hot Start Taq Mastermix 2× (HighQu), and 5 µL PCR Water (HighQu). PCR was performed in a peqSTAR 96 Universal Thermocycler (PeqLab) with the following conditions: Initial degradation at 94 °C for 4 min, followed by 30 cycles (94 °C 45 s, 60 °C 45 s, 72 °C 30 s) and a final elongation at 72 °C for 6 min. Genomic DNA of S. isoflavoniconvertens DSM 22006T was used as positive control. PCR products were subjected to electrophoresis on a 3% agarose gel containing ethidium bromide (120 min, 100 V). PCR products were visualized using the gel documentation system Gel Doc XR+ (BioRad).
Metabolization of daidzein and genistein by pure bacterial cultures. The preparation of pure culture experiments was conducted in an anaerobic workstation under strictly anaerobe conditions of N2/CO2/H2 (80/10/10) at 37 °C. Bacterial pure strains were precultured in Hungate tubes flushed with N2/CO2 (80/20) at 37 °C. A volume of 100 µL of bacterial precultures was used to inoculate 10 mL modified BHI (5 g L−1 yeast extract, 0.05 g L−1 l-cysteine monohydrochloride, 1 mg L−1 resazurin sodium salt, 2.5 mg L−1 haem solution, 2 μg mL−1 vitamin K1 solution) in Hungate tubes. Fermentation was performed with 100 µL isoflavone (8 mM daidzein or 8 mM genistein in DMSO; final concentration 78.4 µM) in triplicates. The growth of strains was visually confirmed. In addition, two controls (without bacterial suspension and without isoflavone but 100 µL DMSO) were conducted for each trial. Samples (2 × 500 µL) were taken at 0, 24, 48, and 72 h and bacterial cells were removed by centrifugation (10 min, 4 °C, 15,000× g). The supernatant (2 × 400 µL) was stored at −80 °C until further analysis.
LC-DAD and LC-MS analyses of fermentation samples. Sample clean-up was performed according to [30] with some modifications. Samples were prepared on ice whenever this was possible. Briefly, supernatants from fermentations were thawed on ice and shortly vortexed. A sample volume of 400 µL was extracted three times with each 500 µL extraction solvent (ethyl acetate/isopropanol/1-butanol, 90/5/5, v/v/v). The combined organic layers were dried under a constant stream of nitrogen. Samples were dissolved in 20 µL DSMO followed by the addition of 180 µL dissolvent (0.1% aqueous formic acid/acetonitrile/methanol, 90/5/5, v/v/v). Afterwards, samples were vortexed (5 s) and centrifuged (5 min, 4 °C, 23,100× g). A volume of 180 µL of the supernatant was transferred to a vial and stored at 4 °C until further analysis. Samples were analyzed on a Prominence HPLC system (Shimadzu Europa GmbH, Duisburg, Germany) consisting of a controller (CBM-20A), degasser (DGU-20A3), two pumps (LC-20AD), a column oven (CTO-20AC), and an autosampler (SIL-20AC HT) coupled with an SPD-M20A diode array detector (DAD). Chromatography was carried out on a Cortecs C18 column (3.0 × 150 mm, 2.7 μm particle size; Waters GmbH, Eschborn, Germany) equipped with a pre-column (Cortecs C18, 2.1 × 5 mm, 2.7 μm particle size; Waters GmbH, Eschborn, Germany). Concerning the analysis of daidzein and its metabolites, 0.1% aqueous formic acid and methanol/acetonitrile (1/1, v/v) were used as eluents A and B, respectively. A flow rate of 0.7 mL/min was adjusted and the following gradient elution profile was used: 0.0–0.5 min isocratic with 25.5% B, 0.5–11.5 min from 25.5–42% B, 11.5–12.0 min from 42–99% B, 12.0–14.5 min isocratic with 99% B, 14.5–15.0 min from 99–25.5% B, and 15.0–20.0 min isocratic with initial conditions. Concerning the analysis of genistein and its metabolites, 0.1% aqueous formic acid and acetonitrile were used as eluents A and B, respectively. A flow rate of 0.7 mL/min was adjusted and the following gradient elution profile was used: 0.0–0.5 min isocratic with 23% B, 0.5–7.5 min from 23–30% B, 7.5–8.0 min from 30–99% B, 8.0–10.5 min isocratic with 99% B, 10.5–11 min from 99–23% B, and 11.0–16.0 min isocratic with initial conditions. For both methods, the column oven was set to 40 °C and the injection volume was 10 μL. The DAD recorded data from 200 to 600 nm with a sampling rate of 6.25 Hz, and a trace at 275 nm was used to monitor the analytes. The identity of each analyte was confirmed by the retention time and the UV spectrum. The system was controlled by the software LC solution 1.24 (Shimadzu Europa GmbH, Duisburg, Germany). The analysis method to quantify daidzein, DH-daidzein, (R, S)-equol and O-DMA as well as genistein, DH-genistein, and 6′-OH-O-DMA were validated and the parameters accuracy, precision, recovery, the limit of detection (LOD), limit of quantitation (LOQ), and linearity were determined. The results of the validation are given in Supplemental Data S13. For daidzein and genistein metabolization, S. equolifaciens DSM 24851T [17] was used as a positive control. In addition, unknown metabolites of genistein were identified by accurate LC-MS (QToF) analysis: Samples were measured using a Triple TOF 5600 mass spectrometer (AB Sciex) linked to a 1290 Infinity LC system (Agilent). The LC-DAD-MS system was controlled by the software Analyst TF (version 1.8, AB Sciex, Darmstadt, Germany). Chromatography was performed as described above. Samples were measured both in the negative and in the positive mode. The DuoSpray source operated in electrospray ionization (ESI) mode using the following source parameters: Curtain gas 45 psi, ion spray voltage −4500 V and +5500 V, respectively, ion source gas-170 psi, ion source gas-260 psi, and ion source gas-2 temperature 650 °C. The declustering potential was adjusted to −100 V and +100 V, respectively. The MS full scans were recorded from m/z 100 to 1000 with an accumulation time of 100 ms and a collision energy voltage of −10 V and +10 V, respectively. The MS/MS spectra (product ion) were recorded from m/z 50 to 1000 in the high sensitivity mode with an accumulation time of 40 ms, a collision energy voltage of −35 V, and +35 V and a collision energy spread of 15 V. Nitrogen was used as collision gas. Data were analyzed using the software PeakView 2.2.0 and FormulaFinder 2.2.0 (AB Sciex, Darmstadt, Germany).
3. Results and Discussion
In silico approach to identify the equol gene cluster. The annotated draft genome sequence (NZ_QIBZ00000000.1) of the equol and 5-OH-equol-producing strain S. isoflavoniconvertens DSM 22006T which was sequenced by our group previously [28] was used as a reference for the in silico approach. A comparison of the respective section on contig 17 (NZ_QIBZ01000017) of our annotated draft genome of S. isoflavoniconvertens DSM 22006T and the sequence of the equol gene cluster (JQ358709) generated by Sanger sequencing [23] showed an identity of 100% of the nucleotide sequences.
The aim of this in silico study was to identify strains of the Eggerthellaceae and Coriobacteriaceae which harbor genes involved in daidzein and genistein metabolism. As a comparison of amino acids sequences rather than nucleotide sequences has tremendous advantages [31], the sequences of proteins of S. isoflavoniconvertens DSM 22006T involved in daidzein metabolism, namely DZNR (AFV15453), DHDR (AFV15451), THDR (AFV15450), and putative DDRC (AFV15447), were used for BLASTp homology search. This search showed proteins with high similarity values in the following well-known equol-producing strains: A. equolifaciens subsp. equolifaciens DSM 19450T [14], A. equolifaciens subsp. celatus DSM 18785T [13,32], A. mucosicola DSM 19490T [9], S. equolifaciens DSM 24851T [17], Lactococcus garvieae 20–92 [33], Slackia sp. AUH-JLC159 [18], Slackia sp. NATTS [19], Eggerthella sp. YY7918 [34]. In addition to S. isoflavoniconvertens DSM 22006T, the occurrence of the equol gene cluster was already described for A. equolifaciens subsp. equolifaciens DSM 19450T [35], Lactococcus garvieae 20–92 [33], Slackia sp. AUH-JLC159 [18], Slackia sp. NATTS [19], Eggerthella sp. YY7918 [34] and discussed in [6]. Furthermore, this BLASTp search showed protein sequences with high similarity to DZNR, DHDR, THDR, and putative DDRC in the type strains ‘Hugonella massiliensis’ DSM 101782T [36] and Senegalimassilia faecalis KGMB04844T [37]. This suggests that these strains might be capable of metabolizing daidzein and genistein. All identified protein sequences were compared by performing a multiple alignment followed by cluster analysis using the unweighted pair group method with arithmetic mean (UPGMA) (Figure 2). These comparisons showed lower similarity values within DZNR (≥57.8%) and DDRC (≥64.3%) than within DHDR (≥89.0%) and THDR (≥80.7%). Comparable findings of the similarities of DZNR, DDRC, DHDR, and THDR were described by [23].
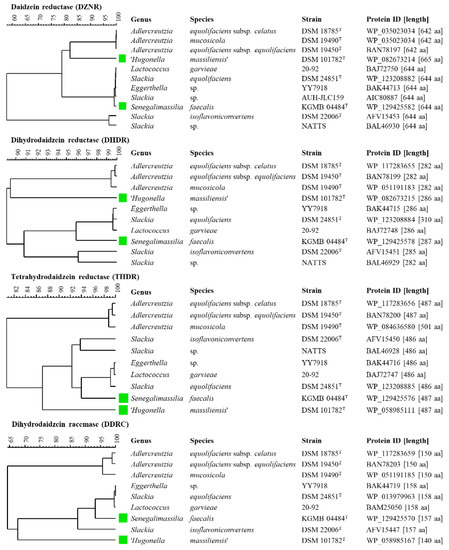
Figure 2.
Cluster analyses of amino acid sequences of daidzein reductase (DZNR), dihydrodaidzein reductase (DHDR), tetrahydrodaidzein reductase (THDR), and dihydrodaidzein racemase (DDRC) of strains of the Eggerthellaceae and Coriobacteriaceae. Clustering was performed by multiple-alignment and UPGMA (unweighted pair group method with arithmetic mean) in BioNumerics 8.0. Green labeled strains are not yet described in the literature to be associated with daidzein or genistein metabolism, to the best of our knowledge.
Alignment and comparison of the equol gene cluster. The annotated genes of the equol gene cluster of S. isoflavoniconvertens DSM 22006T and the respective homologue sequences of the above-listed strains were aligned and compared (Figure 3). Detailed information about each annotated nucleotide sequence is given in Supplemental Tables S1–S12. The genes of the equol gene cluster, the position and orientation of genes as well as the upstream and downstream genes are conserved. Noticeably, S. equolifaciens DSM 24851T and Eggerthella sp. YY7918 showed both the same insertion mutation of a NAD kinase (DMP06_RS06160 and EGYY_15680) and an interchange mutation of the genes hydG and EGYY_15670 with hydE and EGYY_15660, respectively.
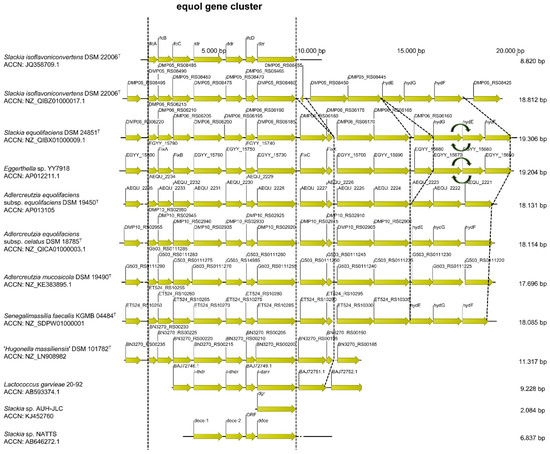
Figure 3.
Schematic representation of the gene organization within the equol gene cluster (dihydrodaidzein racemase (DDRC), tetrahydrodaidzein reductase (THDR), dihydrodaidzein reductase (DHDR), and daidzein reductase (DZNR)) as well partial downstream and upstream genes based on amino acid sequences. The respective accession numbers were obtained from the National Center for Biotechnology Information (NCBI, https://www.ncbi.nlm.nih.gov/, (accessed on 14 August 2019)) and aligned using clustalW algorithm in the CLC Sequence Viewer software (version 8, Qiagen).
The complete equol gene cluster was identified both in the already known equol-producing strains as well as in S. faecalis KGMB04844T and ‘H. massiliensis’ DSM 101782T which supported the hypothesis that these strains could be able to metabolize daidzein and genistein.
It is not yet clear if the ability of daidzein and genistein metabolization is family, species, or strain dependent [6]. Interestingly, three type strains of the genus Adlercreutzia, i.e., A. equolifaciens subsp. equolifaciens DSM 19450T, A. equolifaciens subsp. celatus DSM 18785T, and A. mucosicola DSM 19490T harbored the equol gene cluster, whereas the other three type strains of this genus, i.e., A. caecimuris DSM 21839T, A. muris DSM 29508T and A. rubneri ResAG-91T did not. Our results are in good agreement with the study of [35], which described the presence of the complete equol operon in the genomes of A. equolifaciens subsp. equolifaciens DSM 19450T and A. equolifaciens subsp. celatus DSM 18785T, and the absence of this operon in the genome of strain ResAG-91T. The incapacity of strain A. caecimuris DSM 21839T to metabolize daidzein was already described by [38]. In addition, A. rubneri ResAG-91T did neither metabolize daidzein nor genistein which was investigated within this study (see below). [35] described that strain IPLA37004, which belongs to the well-known equol-producing species A. equolifaciens, did not produce equol and suggested that this was caused by a deletion in the equol operon. However, results of digital DNA-DNA hybridization using TYGS [39] showed that the similarity of the genome sequence of strain IPLA37004 (GCA_009874275.1) to related type strains of the genus Adlercreutzia is below the 70% threshold level for species delineation (results not shown) and therefore, the non-equol-producing strain IPLA37004 represents a potentially new species of Adlercreutzia.
PCR method to detect dzr, ddr, and tdr genes. To test the presence of nucleotide sequences coding for dzr, ddr, and tdr via PCR, primers designed by [29] were used for a total of 29 Eggerthellaceae and Coriobacteriaceae strains. The results are presented in Figure 4. The amplicon sizes of 203 bp (dzr), 205 bp (ddr), and 112 bp (tdr) as proposed by [29] were confirmed in our study. As already described by [29], the primer for dzr amplification did not amplify with the DNA of S. equolifaciens DSM 24851T as a template. Therefore, an amplicon of dzr could only be detected in the positive control S. isoflavoniconvertens DSM 22006T. The primers for amplification of ddr (the gene coding for DHDR) led to products of the expected amplicon size in all previously described equol-producing strains: A. equolifaciens subsp. equolifaciens DSM 19450T, A. equolifaciens subsp. celatus DSM 18785T, A. mucosicola DSM 19490T, S. isoflavoniconvertens DSM 22006T, and S. equolifaciens DSM 24851T. In addition, a ddr amplicon of the expected size was also detected in the strains ‘H. massiliensis’ DSM 101782T and S. faecalis KGMB04844T. Concerning the presence of the genes coding for THDR, putative tdr amplicons were detected in A. equolifaciens subsp. equolifaciens DSM 19450T, A. equolifaciens subsp. celatus DSM 18785T, A. mucosicola DSM 19490T, S. isoflavoniconvertens DSM 22006T, S. equolifaciens DSM 24851T, and S. faecalis KGMB 04844T.

Figure 4.
Phylogenetic tree based on 16S rRNA gene sequences of a total of 29 strains belonging to the Eggerthellaceae and Coriobacteriaceae. Clustering was performed based on multiple alignment and unweighted pair group method with arithmetic mean (UPGMA). Results of a PCR with primers amplifying for dzr, ddr and tdr [29] are shown next to the cluster.
Investigations of the metabolism of daidzein and genistein. The same 29 Eggerthellaceae and Coriobacteriaceae strains were screened for their ability to metabolize daidzein and genistein. For quantification of daidzein, genistein, and their derived metabolites, validated extraction- and LC-DAD-methods were used.
The concentrations at the time points 0, 24, 48, and 72 h of daidzein, genistein, and their derived metabolites in fermentation samples inoculated with strains that metabolized at least either daidzein or genistein are shown in Table 1 and Table 2, respectively. In the control samples without bacteria but supplemented with isoflavones, the initial concentration of 78.4 µM remained stable during the entire course of the fermentation both for daidzein and genistein, and no metabolites were observed. As expected, the metabolization of daidzein to DH-daidzein was conducted by A. equolifaciens subsp. celatus DSM 18785T, A. equolifaciens subsp. equolifaciens DSM 19450T, A. mucosicola DSM 19490T, S. equolifaciens DSM 24851T, and S. isoflavoniconvertens DSM 22006T. In addition, ‘H. massiliensis’ DSM 101782T and S. faecalis KGMB 04484T proved DH-daidzein production. DH-daidzein was detected after a period of 24 h in all fermentation samples inoculated with the above-listed strains. Furthermore, all strains that metabolized daidzein to DH-daidzein also metabolized DH-daidzein to equol within 24 h except S. faecalis KGMB 04484T, for which no equol production was observed at no time points. It was unexpected that no equol-production for S. faecalis KGMB 04484T was observed since this strain possesses the equol gene cluster. The presence of DH-daidzein in the fermentations inoculated with S. faecalis KGMB 04484T was also very low. Therefore, it cannot be ruled out that S. faecalis KGMB 04484T is capable to produce equol under different incubation conditions.

Table 1.
Concentrations [µM] of daidzein and its metabolites measured by LC-DAD in fermentation samples inoculated with pure cultures of Eggerthellaceae and Coriobacteriaceae strains. The initial concentration of daidzein was 78.4 µM. Results are the mean values ± standard deviations of triplicates and for their calculation, values between limit of detection (LOD) and limit of quantitation (LOQ) were set as LOD, and values <LOD were set as zero. DH-Daidzein, Dihydrodaidzein; O-DMA, O-Desmethylangolensin; SD, standard deviation; -, not detected or values below LOD.

Table 2.
Concentrations [µM] of genistein and its metabolites measured by LC-DAD in fermentation samples inoculated with pure cultures of Eggerthellaceae and Coriobacteriaceae strains. The initial concentration of genistein was 78.4 µM. Results are the mean values ± standard deviations of triplicates, except results labeled with § which are in duplicate. For their calculation, values between limit of detection (LOD) and limit of quantitation (LOQ) were set as LOD, and values <LOD were set as zero. DH-Genistein, Dihydrogenistein; 6′OH-O-DMA, 6′-Hydroxy-O-desmethylangolensin; 5-OH-Equol, 5-Hydroxy-equol; SD, standard deviation; -, not detected or values below LOD.
As already described in the literature [8,13,14,15,17], the daidzein metabolizing capacity of strains A. equolifaciens subsp. celatus DSM 18785T, A. equolifaciens subsp. equolifaciens DSM 19450T, A. mucosicola DSM 19490T, S. equolifaciens DSM 24851T, and S. isoflavoniconvertens DSM 22006T was confirmed within this study. Moreover, the incapacity of strains S. faecicanis DSM 17537T, E. lenta DSM 2243T, P. hongkongensis DSM 16106T, E. sinensis DSM 16107T, S. exigua DSM 15923T, and S. heliotrinireducens DSM 20476T to metabolize daidzein to equol as already reported by [14] was confirmed by this study. The same strains, i.e., A. equolifaciens subsp. celatus DSM 18785T, A. equolifaciens subsp. equolifaciens DSM 19450T, A. mucosicola DSM 19490T, S. faecalis KGMB 04484T, S. equolifaciens DSM 24851T, and S. isoflavoniconvertens DSM 22006T as well as ‘H. massiliensis’ DSM 101782T that showed the ability to metabolize daidzein to DH-daidzein also metabolized genistein to DH-genistein. Noteworthy, we observed a U-shaped course of concentrations of daidzein as well as of genistein over time in fermentation samples inoculated with A. equolifaciens subsp. celatus DSM 18785T (Table 1 and Table 2). There were no hints for artifacts, however, these results cannot be explained and need further investigations.
Under the conditions used in this study, S. exigua DSM 15923T was the only strain that metabolized daidzein to O-DMA which was already reported by [8,14]. However, it has to be noted that in samples of ‘H. massiliensis’ DSM 101782T small peaks around the retention time of O-DMA were detected from 24 h onwards. However, the formation of O-DMA could not be shown with certainty. Even if the detected peaks represent O-DMA, the produced amounts were around the LOD and thus less than 0.1% of the daidzein added at the beginning of the incubations (data not shown). Further analyses using more sensitive methods should be conducted to elucidate the presence of O-DMA in fermentation samples of ‘H. massiliensis’ DSM 101782T. In addition, our study showed that S. exigua DSM 15923T is also capable to metabolize genistein to 6′-OH-O-DMA which was not found by [8]. It has to be noted that under the conditions used in this study, no other investigated strain produced 6′-OH-O-DMA in detectable amounts. Interestingly, neither DH-daidzein nor DH-genistein was detected in fermentation samples inoculated with S. exigua DSM 15923T. This leads to the assumption that O-DMA and 6′-OH-O-DMA is formed without the production of the intermediate compounds DH-daidzein and DH-genistein, respectively. This result is in line as no gene sequences homolog to DZNR could be found in the publicly available genomes of S. exigua. A recent study using lactic acid bacterial and bifidobacterial strains showed also that genistein was metabolized to 6′-OH-O-DMA without detecting DH-genistein, although daidzein was transformed to O-DMA and TH-daidzein alongside the production of DH-daidzein [40].
Interestingly, three strains metabolized genistein to an unknown metabolite which was present after 24 h of sampling and thereafter: A. equolifaciens subsp. celatus DSM 18785T, A. mucosicola DSM 19490T and ‘H. massiliensis’ DSM 101782T converted genistein into DH-genistein and an unknown metabolite at a retention time of 3.3 min. In addition, fermentation samples inoculated with A. equolifaciens subsp. equolifaciens DSM 19450T showed the same unknown metabolite from 48 h on. The UV spectra of this unknown metabolite exhibited a maximum absorption at 275 nm and showed similarities with the UV spectrum of the daidzein metabolite equol with a maximum absorption at 280 nm (data not shown). Thus, this metabolite is very likely to be 5-OH-equol. Calibration curves of the reference compound equol were used to estimate the amount of this metabolite. The production of 5-OH-equol was highest in strain ‘H. massiliensis’ DSM 101782T, followed by A. mucosicola DSM 19490T. The formation of 5-OH-equol in strains A. equolifaciens subsp. equolifaciens DSM 19450T and A. equolifaciens subsp. celatus DSM 18785T were comparable but notably lower.
LC-MS analysis of the unknown microbial genistein metabolite. To characterize this unknown metabolite, fermentation samples of genistein inoculated with ‘H. massiliensis’ DSM 101782T at time points 0 h and 72 h were analysed by high-resolution LC-MS. The unknown metabolite peak in the 72 h fermentation sample eluted a bit earlier in the LC-MS analysis (other HPLC device with different dead volume) and exhibited in full-scan MS a mass/charge ratio (m/z) of 259.0972 and m/z 257.0829 in the positive and negative ionization mode, respectively. These m/z values concurred with the chemical formula of 5-OH-equol (C15H14O4, mass error 2.8 ppm and 3.8 ppm in positive and negative mode, respectively). The positive MS/MS spectrum of the precursor ion 259.1 of the unknown metabolite peak exhibited the following characteristic fragment ions (m/z) 165.0547, 153.0545, 139.0381, 133.0647, 121.028, and 107.048. This matched the MS/MS spectrum of 5-OH-equol described by [9]. LC-MS measurements of A. mucosicola DSM 19490T 72 h-sample supplemented with genistein revealed similar results.
Conclusively, the unknown metabolite peak in fermentation samples of genistein inoculated with A. mucosicola DSM 19490T and ‘H. massiliensis’ DSM 101782T was putatively identified as 5-OH-equol. Due to the lack of a reference standard for 5-OH-equol, the final confirmation of the identity as well as the quantitation in the samples should be conducted in further studies. The genistein fermentation samples of A. equolifaciens subsp. celatus DSM 18785T and A. equolifaciens subsp. equolifaciens DSM 19450T were not measured by LC-MS within this study. Nevertheless, the results of the LC-DAD analysis (retention time and UV spectra) led to the assumption that the unknown peak in fermentation samples of A. equolifaciens subsp. celatus DSM 18785T and of A. equolifaciens subsp. equolifaciens DSM 19450T represents also 5-OH-equol. It must be noted that the type strain of S. isoflavoniconvertens DSM 22006T was described to be capable of 5-OH-equol production in the original strain description [8], although this metabolite could not be detected in genistein fermentation samples inoculated with this strain under comparable conditions in this study, e.g., medium, gas atmosphere, temperature, concentration of isoflavone supplementation.
4. Conclusions
Amino acid sequences comprising genes coding for daidzein reductase (DZNR), dihydrodaidzein reductase (DHDR), tetrahydrodaidzein reductase (THDR) and dihydrodaidzein racemase (DDRC) were successfully used to search for similar protein sequences within draft genome sequences of Eggerthellaceae and Coriobacteriaceae strains. Homolog genes of the equol gene cluster were detected and aligned in already described equol-producing strains. Furthermore, this cluster was newly detected in strains of the Eggerthellaceae and Coriobacteriaceae that have so far not been associated with equol production. The presence of genes of the equol gene cluster was confirmed via PCR amplification. In addition, the metabolism of daidzein and genistein was investigated using pure cultures of Eggerthellaceae and Coriobacteriaceae strains. In conclusion, this study led to the first description of the human gut bacterial strains ‘Hugonella massiliensis’ DSM 101782T and Senegalimassilia faecalis KGMB 04484T as capable of metabolizing daidzein and genistein.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/foods10112741/s1, Tables S1: Annotation of the conserved open reading frames (orfs) within the equol biosynthesis gene cluster of Slackia isoflavoniconvertens DSM 22006T (GenBank: JQ358709), Tables S2: Annotation of the conserved open reading frames (orfs) within the equol biosynthesis gene cluster of Slackia isoflavoniconvertens DSM 22006T (NCBI reference sequence: NZ_QIBZ01000017.1), Tables S3: Annotation of the conserved open reading frames (orfs) within the equol biosynthesis gene cluster of Slackia equolifaciens DSM 24851T, Tables S4: Annotation of the conserved open reading frames (orfs) within the equol biosynthesis gene cluster of Eggerthella sp. YY918, Tables S5: Annotation of the conserved open reading frames (orfs) within the equol biosynthesis gene cluster of Adlercreutzia equolifaciens subsp. equolifaciens DSM 19450T, Tables S6: Annotation of the conserved open reading frames (orfs) within the equol biosynthesis gene cluster of Adlercreutzia equolifaciens subsp. celatus DSM 18785T, Tables S7: Annotation of the conserved open reading frames (orfs) within the equol biosynthesis gene cluster of Adlercreutzia mucosicola DSM 19490T, Tables S8: Annotation of the conserved open reading frames (orfs) within the equol biosynthesis gene cluster of Senegalimassilia faecalis KGMB04484T, Tables S9: Annotation of the conserved open reading frames (orfs) within the equol biosynthesis gene cluster of ‘Hugonella massiliensis’ AT8T, Tables S10: Annotation of the conserved open reading frames (orfs) within the equol biosynthesis gene cluster of Lactococcus garvieae 20-92, Tables S11: Annotation of the conserved open reading frames (orfs) within the equol biosynthesis gene cluster of Slackia sp. AUH-JLC159, Tables S12: Annotation of the conserved open reading frames (orfs) within the equol biosynthesis gene cluster of Slackia sp. NATTS, Data S13: Results of validation (accuracy, intra-day precision, recovery, LOD, LOQ and linearity) of LC-DAD analyses of daidzein, genistein and corresponding microbial metabolites in fermentation samples of pure cultures.
Author Contributions
Conceptualization, S.T.S., D.A.S. and M.H.; methodology, S.T.S.; validation, S.T.S. and A.S.; formal analysis, S.T.S., D.A.S., N.D. and A.S.; investigation, N.D. and A.S.; resources, S.E.K. and M.H.; data curation, S.T.S., D.A.S. and M.H.; writing—original draft preparation, S.T.S., D.A.S., N.D. and M.H.; writing—review and editing, S.T.S., D.A.S., S.E.K. and M.H.; visualization, N.D. and M.H.; supervision, S.T.S., D.A.S., S.E.K. and M.H.; project administration, S.E.K. and M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We thank Stephanie Stricker and Lilia Wiest for excellent technical assistance in the anaerobe and molecular biological laboratories. Furthermore, we thank Bettina Schindler for the excellent technical support regarding the LC-DAD analyses. We acknowledge the KGMB (Korean Gut Microbiome Bank), especially Jung-Sook Lee, for the supply of the type strain Senegalimassilia faecalis KGMB04484T (=KCTC 15721).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Hwang, K.A.; Choi, K.C. Chapter One—Endocrine-Disrupting Chemicals with Estrogenicity Posing the Risk of Cancer Progression in Estrogen-Responsive Organs. In Advances in Molecular Toxicology; Fishbein, J.C., Heilman, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–33. [Google Scholar]
- Messina, M. Soy and Health Update: Evaluation of the Clinical and Epidemiologic Literature. Nutrients 2016, 8, 754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hüser, S.; Guth, S.; Joost, H.G.; Soukup, S.T.; Köhrle, J.; Kreienbrock, L.; Diel, P.; Lachenmeier, D.W.; Eisenbrand, G.; Vollmer, G.; et al. Effects of isoflavones on breast tissue and the thyroid hormone system in humans: A comprehensive safety evaluation. Arch. Toxicol. 2018, 92, 2703–2748. [Google Scholar] [CrossRef] [Green Version]
- Clavel, T.; Lepage, P.; Charrier, C. The Family Coriobacteriaceae, in The Prokaryotes—Actinobacteria; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Mayo, B.; Vázquez, L.; Flórez, A.B. Equol: A Bacterial Metabolite from The Daidzein Isoflavone and Its Presumed Beneficial Health Effects. Nutrients 2019, 11, 2231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthies, A.; Blaut, M.; Braune, A. Isolation of a Human Intestinal Bacterium Capable of Daidzein and Genistein Conversion. Appl. Environ. Microbiol. 2009, 75, 1740–1744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthies, A.; Clavel, T.; Gütschow, M.; Engst, W.; Haller, D.; Blaut, M.; Braune, A. Conversion of Daidzein and Genistein by an Anaerobic Bacterium Newly Isolated from the Mouse Intestine. Appl. Environ. Microbiol. 2008, 74, 4847–4852. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.-S.; Nishihata, T.; Kakiuchi, N.; Hattori, M. Biotransformation of C-Glucosylisoflavone Puerarin to Estrogenic (3S)-Equol in Co-culture of Two Human Intestinal Bacteria. Biol. Pharm. Bull. 2008, 31, 1621–1625. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.S.; Chen, W.J.; Adeolu, M.; Chai, Y. Molecular signatures for the class Coriobacteriia and its different clades; proposal for division of the class Coriobacteriia into the emended order Coriobacteriales, containing the emended family Coriobacteriaceae and Atopobiaceae fam. nov., and Eggerthellales ord. nov., containing the family Eggerthellaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2013, 63, 3379–3397. [Google Scholar] [CrossRef] [Green Version]
- Oren, A.; Garrity, G.M. Notification that new names of prokaryotes and new combinations have appeared in volume 63, part 9, of the IJSEM. Int. J. Syst. Evol. Microbiol. 2013, 63, 4371–4373. [Google Scholar] [CrossRef]
- Minamida, K.; Tanaka, M.; Abe, A.; Sone, T.; Tomita, F.; Hara, H.; Asano, K. Production of equol from daidzein by gram-positive rod-shaped bacterium isolated from rat intestine. J. Biosci. Bioeng. 2006, 102, 247–250. [Google Scholar] [CrossRef] [Green Version]
- Maruo, T.; Sakamoto, M.; Ito, C.; Toda, T.; Benno, Y. Adlercreutzia equolifaciens gen. nov., sp. nov., an equol-producing bacterium isolated from human faeces, and emended description of the genus Eggerthella. Int. J. Syst. Evol. Microbiol. 2008, 58, 1221–1227. [Google Scholar] [CrossRef]
- Clavel, T.; Charrier, C.; Braune, A.; Wenning, M.; Blaut, M.; Haller, D. Isolation of bacteria from the ileal mucosa of TNFdeltaARE mice and description of Enterorhabdus mucosicola gen. nov., sp. nov. Int. J. Syst. Evol. Microbiol. 2009, 59, 1805–1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawada, Y.; Yokoyama, S.-I.; Yanase, E.; Niwa, T.; Suzuki, T. The production of S-equol from daidzein is associated with a cluster of three genes in Eggerthella sp. YY7918. Biosci. Microbiota Food Health 2016, 35, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.S.; Kitahara, M.; Sakamoto, M.; Hattori, M.; Benno, Y. Slackia equolifaciens sp. nov., a human intestinal bacterium capable of producing equol. Int. J. Syst. Evol. Microbiol. 2010, 60, 1721–1724. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.N.; Hao, Q.H.; Zhang, H.L.; Zhou, B.; Yu, X.M.; Wang, X.L. Reduction of soy isoflavones by use of Escherichia coli whole-cell biocatalyst expressing isoflavone reductase under aerobic conditions. Lett. Appl. Microbiol. 2016, 63, 111–116. [Google Scholar] [CrossRef]
- Tsuji, H.; Moriyama, K.; Nomoto, K.; Miyanaga, N.; Akaza, H. Isolation and characterization of the equol-producing bacterium Slackia sp. strain NATTS. Arch. Microbiol. 2010, 192, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.; Yasuda, S.; Takahashi, M.; Hayashi, T.; Miyazawa, N.; Sato, I.; Abiru, Y.; Uchiyama, S.; Hishigaki, H. Cloning and Expression of a Novel NADP(H)-Dependent Daidzein Reductase, an Enzyme Involved in the Metabolism of Daidzein, from Equol-Producing Lactococcus Strain 20–92. Appl. Environ. Microbiol. 2010, 76, 5892–5901. [Google Scholar] [CrossRef] [Green Version]
- Shimada, Y.; Takahashi, M.; Miyazawa, N.; Ohtani, T.; Abiru, Y.; Uchiyama, S.; Hishigaki, H. Identification of Two Novel Reductases Involved in Equol Biosynthesis in Lactococcus Strain 20–92. J. Mol. Microbiol. Biotechnol. 2011, 21, 160–172. [Google Scholar] [CrossRef]
- Shimada, Y.; Takahashi, M.; Miyazawa, N.; Abiru, Y.; Uchiyama, S.; Hishigaki, H. Identification of a Novel Dihydrodaidzein Racemase Essential for Biosynthesis of Equol from Daidzein in Lactococcus sp. Strain 20–92. Appl. Environ. Microbiol. 2012, 78, 4902–4907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schröder, C.; Matthies, A.; Engst, W.; Blaut, M.; Braune, A. Identification and Expression of Genes Involved in the Conversion of Daidzein and Genistein by the Equol-Forming Bacterium Slackia isoflavoniconvertens. Appl. Environ. Microbiol. 2013, 79, 3494–3502. [Google Scholar] [CrossRef] [Green Version]
- Tsuji, H.; Moriyama, K.; Nomoto, K.; Akaza, H. Identification of an Enzyme System for Daidzein-to-Equol Conversion in Slackia sp. Strain NATTS. Appl. Environ. Microbiol. 2012, 78, 1228–1236. [Google Scholar] [CrossRef] [Green Version]
- Flórez, A.B.; Vázquez, L.; Rodríguez, J.; Redruello, B.; Mayo, B. Transcriptional Regulation of the Equol Biosynthesis Gene Cluster in Adlercreutzia equolifaciens DSM19450T. Nutrients 2019, 11, 993. [Google Scholar] [CrossRef] [Green Version]
- Setchell, K.D.R.; Clerici, C.; Lephart, E.D.; Cole, S.J.; Heenan, C.; Castellani, D.; Wolfe, B.E.; Nechemias-Zimmer, L.; Brown, N.M.; Lund, T.D.; et al. S-Equol, a potent ligand for estrogen receptor β, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am. J. Clin. Nutr. 2005, 81, 1072–1079. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, S.-I.; Suzuki, T. Isolation and Characterization of a Novel Equol-Producing Bacterium from Human Feces. Biosci. Biotechnol. Biochem. 2008, 72, 2660–2666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danylec, N.; Stoll, D.A.; Dötsch, A.; Huch, M. Draft Genome Sequences of Type Strains of Gordonibacter faecihominis, Paraeggerthella hongkongensis, Parvibacter caecicola, Slackia equolifaciens, Slackia faecicanis, and Slackia isoflavoniconvertens. Microbiol. Resour. Announc. 2019, 8, e01532-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vázquez, L.; Guadamuro, L.; Giganto, F.; Mayo, B.; Flórez, A.B. Development and Use of a Real-Time Quantitative PCR Method for Detecting and Quantifying Equol-Producing Bacteria in Human Faecal Samples and Slurry Cultures. Front. Microbiol. 2017, 8, 1155. [Google Scholar] [CrossRef] [PubMed]
- Bode, L.M.; Bunzel, D.; Huch, M.; Cho, G.-S.; Ruhland, D.; Bunzel, M.; Bub, A.; Franz, C.M.A.P.; Kulling, S.E. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am. J. Clin. Nutr. 2013, 97, 295–309. [Google Scholar] [CrossRef]
- Koonin, E.V.; Galperin, M.Y. Sequence—Evolution—Function: Computational Approaches in Comparative Genomics; Kluwer Academic: Boston, MA, USA, 2003. [Google Scholar]
- Minamida, K.; Ota, K.; Nishimukai, M.; Tanaka, M.; Abe, A.; Sone, T.; Tomita, F.; Hara, H.; Asano, K. Asaccharobacter celatus gen. nov., sp. nov., isolated from rat caecum. Int. J. Syst. Evol. Microbiol. 2008, 58, 1238–1240. [Google Scholar] [CrossRef]
- Uchiyama, S.; Ueno, T.; Suzuki, T. Identification of a Newly Isolated Equol-Producing Lactic Acid Bacterium from the Human Feces. J. Intest. Microbiol. 2007, 21, 217–220. [Google Scholar]
- Yokoyama, S.-I.; Oshima, K.; Nomura, I.; Hattori, M.; Suzuki, T. Complete Genomic Sequence of the Equol-Producing Bacterium Eggerthella sp. Strain YY7918, Isolated from Adult Human Intestine. J. Bacteriol. 2011, 193, 5570–5571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vázquez, L.; Flórez, A.B.; Redruello, B.; Mayo, B. Metabolism of Soy Isoflavones by Intestinal Bacteria: Genome Analysis of an Adlercreutzia equolifaciens Strain That Does Not Produce Equol. Biomolecules 2020, 10, 950. [Google Scholar] [CrossRef]
- Elsawi, Z.; Togo, A.H.; Beye, M.; Dubourg, G.; Andrieu, C.; Armsrtong, N.; Richez, M.; Di Pinto, F.; Bittar, F.; Labas, N.; et al. Hugonella massiliensis gen. nov., sp. nov., genome sequence, and description of a new strictly anaerobic bacterium isolated from the human gut. MicrobiologyOpen 2017, 6, e00458. [Google Scholar] [CrossRef] [Green Version]
- Han, K.-I.; Kim, J.-S.; Lee, K.C.; Eom, M.K.; Suh, M.K.; Kim, H.S.; Park, S.-H.; Lee, J.H.; Kang, S.W.; Park, J.-E.; et al. Lee1,4, Senegalimassilia faecalis sp. nov., an anaerobic actinobacterium isolated from human faeces, and emended description of the genus Senegalimassilia. Int. J. Syst. Evol. Microbiol. 2020, 70, 1684–1690. [Google Scholar] [CrossRef] [PubMed]
- Clavel, T.; Duck, W.; Charrier, C.; Wenning, M.; Elson, C.; Haller, D. Enterorhabdus caecimuris sp. nov., a member of the family Coriobacteriaceae isolated from a mouse model of spontaneous colitis, and emended description of the genus Enterorhabdus Clavel et al. 2009. Int. J. Syst. Evol. Microbiol. 2010, 60, 1527–1531. [Google Scholar] [CrossRef] [Green Version]
- Meier-Kolthoff, J.P.; Göker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef]
- Peirotén, Á.; Gaya, P.; Álvarez, I.; Landete, J.M. Production of O-desmethylangolensin, tetrahydrodaidzein, 6′-hydroxy-O-desmethylangolensin and 2-(4-hydroxyphenyl)-propionic acid in fermented soy beverage by lactic acid bacteria and Bifidobacterium strains. Food Chem. 2020, 318, 126521. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).