Polyphenols: From Theory to Practice
Abstract
:1. Introduction
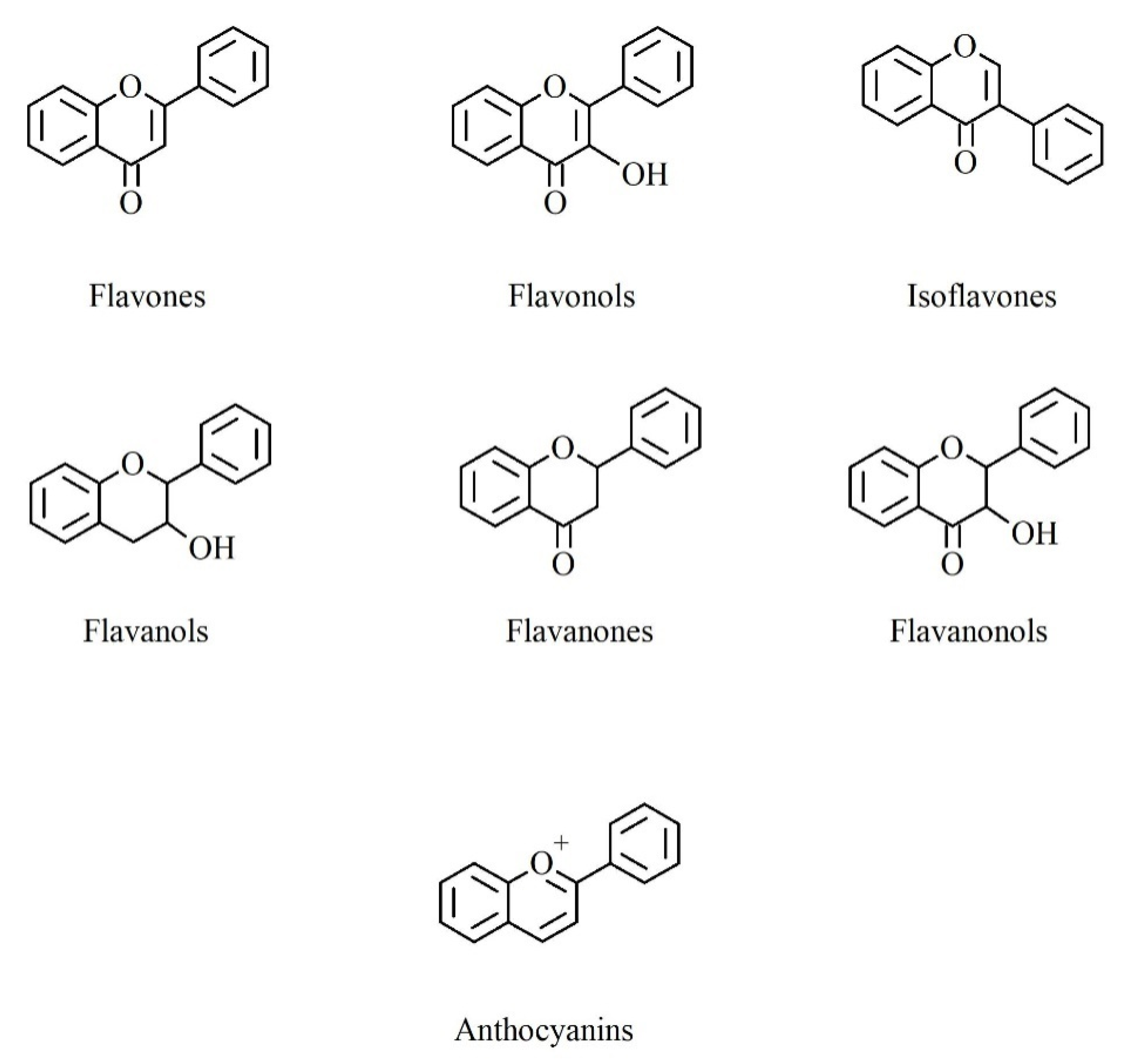
2. Polyphenols: Chemical Structure and Biosynthesis
3. Polyphenols: Not Only Conventional Antioxidants
4. The Problem of Bioavailability of Polyphenols
5. Bioavailability of Polyphenols: What In Vitro Tests Do Not Tell Us
6. From the Bench to Pre-Clinical and Clinical Studies on Polyphenols: Practical Instructions for Use
6.1. Single Polyphenols or Phytocomplex: The Importance of the Sample under Investigation
6.2. Pharmacokinetic Aspects
6.3. Study of the Mechanism of Action
7. Conclusions
Polyphenols: A Lesson from Pharmacokinetics to Transfer Theory to Practice
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, C.; Chakraborty, S. Study of dietary polyphenols from natural herbal sources for providing protection against human degenerative disorders. Biocatal. Agric. Biotechnol. 2021, 33, 101956. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutrim, C.S.; Cortez, M.A.S. A review on polyphenols: Classification, beneficial effects and their application in dairy products. Int. J. Dairy Technol. 2018, 71, 564–578. [Google Scholar] [CrossRef]
- Debelo, H.; Li, M.; Ferruzzi, M.G. Processing influences on food polyphenol profiles and biological activity. Curr. Opin. Food Sci. 2020, 32, 90–102. [Google Scholar] [CrossRef]
- Maganha, L.C.; Rosim, R.E.; Corassin, C.H.; Cruz, A.G.; Faria, J.A.F.; Oliveira, C.A.F. Viability of probiotic bacteria in fermented skim milk produced with different levels of milk powder and sugar. Int. J. Dairy Technol. 2013, 67, 89–94. [Google Scholar] [CrossRef]
- Ribeiro, A.; Caleja, C.; Barros, L.; Santos-Buelga, C.; Barreiro, M.F.; Ferreira, I.C.F.R. Rosemary extracts in functional foods: Extraction, chemical characterization and incorporation of free and microencapsulated forms in cottage cheese. Food Funct. 2016, 7, 2185–2196. [Google Scholar] [CrossRef] [Green Version]
- Balthazar, C.F.; Silva, H.; Cavalcanti, R.; Esmerino, E.; Cappato, L.; Abud, Y.; Moraes, J.; Andrade, M.; Freitas, M.; Sant’Anna, C.; et al. Prebiotics addition in sheep milk ice cream: A rheological, microstructural and sensory study. J. Funct. Foods 2017, 35, 564–573. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef] [Green Version]
- Neveu, V.; Pérez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cao, G.; Prior, R.L. Total Antioxidant Capacity of Fruits. J. Agric. Food Chem. 1996, 44, 701–705. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free. Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Cherrak, S.A.; Mokhtari-Soulimane, N.; Berroukeche, F.; Bensenane, B.; Cherbonnel, A.; Merzouk, H.; Elhabiri, M. In Vitro Antioxidant versus Metal Ion Chelating Properties of Flavonoids: A Structure-Activity Investigation. PLoS ONE 2016, 11, e0165575. [Google Scholar] [CrossRef]
- Pietta, P.-G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Wu, L.-M.; Yang, L.; Liu, Z.-L. Evidence for α-tocopherol regeneration reaction of green tea polyphenols in SDS micelles. Free. Radic. Biol. Med. 2005, 38, 78–84. [Google Scholar] [CrossRef]
- Kostyuk, V.A.; Potapovich, A.I.; Suhan, T.O.; de Luca, C.; Korkina, L.G. Antioxidant and signal modulation properties of plant polyphenols in controlling vascular inflammation. Eur. J. Pharmacol. 2011, 658, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Firuzi, O.; Moosavi, F.; Hosseini, R.; Saso, L. Modulation of neurotrophic signaling pathways by polyphenols. Drug Des. Dev. Ther. 2015, 10, 23–42. [Google Scholar] [CrossRef] [Green Version]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of In Vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food Polyphenols—A Non-Systematic Review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef]
- Câmara, J.S.; Albuquerque, B.R.; Aguiar, J.; Corrêa, R.C.G.; Gonçalves, J.L.; Granato, D.; Pereira, J.A.M.; Barros, L.; Ferreira, I.C.F.R. Food Bioactive Compounds and Emerging Techniques for Their Extraction: Polyphenols as a Case Study. Foods 2020, 10, 37. [Google Scholar] [CrossRef]
- Tena, N.; Martín, J.; Asuero, A.G. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic Effect in Human Health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Aherne, S.; O’Brien, N.M. Dietary flavonols: Chemistry, food content, and metabolism. Nutrition 2002, 18, 75–81. [Google Scholar] [CrossRef]
- Hollman, P.C.; De Vries, J.H.; Van Leeuwen, S.D.; Mengelers, M.J.; Katan, M.B. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am. J. Clin. Nutr. 1995, 62, 1276–1282. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Rabaneda, F.; Jáuregui, O.; Casals, I.; Andres-Lacueva, C.; Izquierdo-Pulido, M.; Lamuela-Raventós, R.M. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of the phenolic composition of cocoa (Theobroma cacao). J. Mass Spectrom. 2003, 38, 35–42. [Google Scholar] [CrossRef]
- Tenore, G.C.; Campiglia, P.; Giannetti, D.; Novellino, E. Simulated gastrointestinal digestion, intestinal permeation and plasma protein interaction of white, green, and black tea polyphenols. Food Chem. 2015, 169, 320–326. [Google Scholar] [CrossRef]
- Flores, M.E.J. Cocoa Flavanols: Natural Agents with Attenuating Effects on Metabolic Syndrome Risk Factors. Nutrients 2019, 11, 751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Record, I.R.; Lane, J.M. Simulated intestinal digestion of green and black teas. Food Chem. 2001, 73, 481–486. [Google Scholar] [CrossRef]
- Sorrenti, V.; Ali, S.; Mancin, L.; Davinelli, S.; Paoli, A.; Scapagnini, G. Cocoa Polyphenols and Gut Microbiota Interplay: Bioavailability, Prebiotic Effect, and Impact on Human Health. Nutrients 2020, 12, 1908. [Google Scholar] [CrossRef] [PubMed]
- Sirerol, J.A.; Rodríguez, M.L.; Mena, S.; Asensi, M.A.; Estrela, J.M.; Ortega, A.L. Role of Natural Stilbenes in the Prevention of Cancer. Oxidative Med. Cell. Longev. 2016, 2016, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Machado, N.D.; Fernández, M.A.; Díaz, D.D. Recent Strategies in Resveratrol Delivery Systems. Chem. Plus. Chem. 2019, 84, 951–973. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zeng, B.; Wang, P.; Wang, L.; Wen, B.; Li, Y.; Liu, H.; Bai, S.; Jia, G. Microbiome of Total Versus Live Bacteria in the Gut of Rex Rabbits. Front. Microbiol. 2018, 9, 733. [Google Scholar] [CrossRef]
- Pannu, N.; Bhatnagar, A. Resveratrol: From enhanced biosynthesis and bioavailability to multitargeting chronic diseases. Biomed. Pharmacother. 2019, 109, 2237–2251. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, S.J.; Yim, D.G.; Hur, S.J. Changes in the Content and Bioavailability of Onion Quercetin and Grape Resveratrol During In Vitro Human Digestion. Foods 2020, 9, 694. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Centonze, C.; Grasso, M.E.; Latronico, M.F.; Mastrangelo, P.F.; Sparascio, F.; Maffia, M. HPLC Analysis of Phenols in Negroamaro and Primitivo Red Wines from Salento. Foods 2019, 8, 45. [Google Scholar] [CrossRef] [Green Version]
- Biagi, M.; Bertelli, A.A. Wine, alcohol and pills: What future for the French paradox? Life Sci. 2015, 131, 19–22. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Tomas, M.; Ozdal, T.; Capanoglu, E. Effect of food matrix on the content and bioavailability of flavonoids. Trends Food Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Drakou, M.; Birmpa, A.; Koutelidakis, A.E.; Komaitis, M.; Panagou, E.; Kapsokefalou, M. Total antioxidant capacity, total phenolic content and iron and zinc dialyzability in selected Greek varieties of table olives, tomatoes and legumes from conventional and organic farming. Int. J. Food Sci. Nutr. 2015, 66, 197–202. [Google Scholar] [CrossRef] [PubMed]
- De Santi, C.; Pietrabissa, A.; Mosca, F.; Pacifici, G. Methylation of quercetin and fisetin, flavonoids widely distributed in edible vegetables, fruits and wine, by human liver. Int. J. Clin. Pharmacol. Ther. 2002, 40, 207–212. [Google Scholar] [CrossRef]
- De Santi, C.; Pietrabissa, A.; Mosca, F.; Rane, A.; Pacifici, G.M. Inhibition of phenol sulfotransferase (SULT1A1) by quercetin in human adult and foetal livers. Xenobiotica 2002, 32, 363–368. [Google Scholar] [CrossRef]
- Boronat, A.; Mateus, J.; Soldevila-Domenech, N.; Guerra, M.; Rodríguez-Morató, J.; Varon, C.; Muñoz, D.; Barbosa, F.; Morales, J.C.; Gaedigk, A.; et al. Cardiovascular benefits of ty-rosol and its endogenous conversion into hydroxytyrosol in humans. A randomized, controlled trial. Free Radic. Biol. Med. 2019, 143, 471–481. [Google Scholar] [CrossRef]
- Rodríguez-Morató, J.; Robledo, P.; Tanner, J.-A.; Boronat, A.; Pérez-Mañá, C.; Chen, C.-Y.O.; Tyndale, R.F.; de la Torre, R. CYP2D6 and CYP2A6 biotransform dietary tyrosol into hydroxytyrosol. Food Chem. 2017, 217, 716–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soldevila-Domenech, N.; Boronat, A.; Mateus, J.; Diaz-Pellicer, P.; Matilla, I.; Pérez-Otero, M.; Aldea-Perona, A.; De La Torre, R. Generation of the Antioxidant Hydroxytyrosol from Tyrosol Present in Beer and Red Wine in a Randomized Clinical Trial. Nutrients 2019, 11, 2241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Governa, P.; Manetti, F.; Miraldi, E.; Biagi, M. Effects of in vitro simulated digestion on the antioxidant activity of different Camellia sinensis (L.) Kuntze leaves extracts. Eur. Food Res. Technol. 2021, 1–10. [Google Scholar] [CrossRef]
- Yao, H.-T.; Li, C.-C.; Chang, C.-H. Epigallocatechin-3-Gallate Reduces Hepatic Oxidative Stress and Lowers CYP-Mediated Bioactivation and Toxicity of Acetaminophen in Rats. Nutrients 2019, 11, 1862. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.-H.; Hurh, Y.-J.; Na, H.-K.; Kim, J.H.; Chun, Y.-J.; Kim, D.H.; Kang, K.-S.; Cho, M.-H.; Surh, Y.-J. Resveratrol inhibits TCDD-induced expression of CYP1A1 and CYP1B1 and catechol estrogen-mediated oxidative DNA damage in cultured human mammary epithelial cells. Carcinogenesis 2004, 25, 2005–2013. [Google Scholar] [CrossRef]
- Piver, B.; Fer, M.; Vitrac, X.; Merillon, J.-M.; Dreano, Y.; Berthou, F.; Lucas, D. Involvement of cytochrome P450 1A2 in the biotransformation of trans-resveratrol in human liver microsomes. Biochem. Pharmacol. 2004, 68, 773–782. [Google Scholar] [CrossRef]
- Orellana, M.; Varela, N.; Guajardo, V.; Araya, J.; Rodrigo, R. Modulation of rat liver cytochrome P450 activity by prolonged red wine consumption. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 131, 161–166. [Google Scholar] [CrossRef]
- Seki, H.; Akiyoshi, T.; Imaoka, A.; Ohtani, H. Inhibitory kinetics of fruit components on CYP2C19 activity. Drug Metab. Pharmacokinet. 2019, 34, 181–186. [Google Scholar] [CrossRef]
- Chang, T.K.; Chen, J.; Lee, W.B. Differential inhibition and inactivation of human CYP1 enzymes by trans-resveratrol: Evidence for mechanism-based inactivation of CYP1A2. J. Pharmacol. Exp. Ther. 2001, 299, 874–882. [Google Scholar] [PubMed]
- Offman, E.; Freeman, D.J.; Dresser, G.K.; Muñoz, C.; Bend, J.R.; Bailey, D.G. Red wine–cisapride interaction: Comparison with grapefruit juice. Clin. Pharmacol. Ther. 2001, 70, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Littlewood, J.; Glover, V.; Sandler, M. Red wine contains a potent inhibitor of phenolsulphotransferase. Br. J. Clin. Pharmacol. 1985, 19, 275–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maier-Salamon, A.; Böhmdorfer, M.; Thalhammer, T.; Szekeres, T.; Jaeger, W. Hepatic Glucuronidation of Resveratrol: Interspecies Comparison of Enzyme Kinetic Profiles in Human, Mouse, Rat, and Dog. Drug Metab. Pharmacokinet. 2011, 26, 364–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miksits, M.; Maier-Salamon, A.; Vo, T.P.N.; Sulyok, M.; Schuhmacher, R.; Szekeres, T.; Jäger, W. Glucuronidation of piceatannol by human liver microsomes: Major role of UGT1A1, UGT1A8 and UGT1A10. J. Pharm. Pharmacol. 2010, 62, 47–54. [Google Scholar] [CrossRef]
- Lucci, P.; Bertoz, V.; Pacetti, D.; Moret, S.; Conte, L. Effect of the Refining Process on Total Hydroxytyrosol, Tyrosol, and Tocopherol Contents of Olive Oil. Foods 2020, 9, 292. [Google Scholar] [CrossRef] [Green Version]
- Beauchamp, G.K.; Keast, R.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.-H.; Smith, A.B.; Breslin, P.A.S. Ibuprofen-like activity in extra-virgin olive oil. Nat. Cell Biol. 2005, 437, 45–46. [Google Scholar] [CrossRef]
- López-Yerena, A.; Vallverdú-Queralt, A.; Mols, R.; Augustijns, P.; Lamuela-Raventós, R.M.; Escribano-Ferrer, E. Absorption and Intestinal Metabolic Profile of Oleocanthal in Rats. Pharmaceutics 2020, 12, 134. [Google Scholar] [CrossRef] [Green Version]
- Cuyàs, E.; Verdura, S.; Lozano-Sánchez, J.; Viciano, I.; Llorach-Pares, L.; Nonell-Canals, A.; Bosch-Barrera, J.; Brunet, J.; Segura-Carretero, A.; Sanchez-Martinez, M.; et al. The extra virgin olive oil phenolic oleacein is a dual substrate-inhibitor of catechol-O-methyltransferase. Food Chem. Toxicol. 2019, 128, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Kampschulte, N.; Alasmer, A.; Empl, M.T.; Krohn, M.; Steinberg, P.; Schebb, N.H. Dietary Polyphenols Inhibit the Cytochrome P450 Monooxygenase Branch of the Arachidonic Acid Cascade with Remarkable Structure-Dependent Selectivity and Potency. J. Agric. Food Chem. 2020, 68, 9235–9244. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [Green Version]
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; Baglivo, M.; et al. Hydroxytyrosol: A natural compound with promising pharmacological activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar] [CrossRef]
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: Estrogenic activity, biological effect and bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 15–25. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xiao, P.; Ou-Yang, D.-S.; Fan, L.; Guo, D.; Wang, Y.-N.; Han, Y.; Tu, J.-H.; Zhou, G.; Huang, Y.-F.; et al. Simultaneous action of the flavonoid quercetin on cytochrome p450 (cyp) 1a2, cyp2a6,n-acetyltransferase and xanthine oxidase activity in healthy volunteers. Clin. Exp. Pharmacol. Physiol. 2009, 36, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Khakimov, B.; Engelsen, S.B. Resveratrol in the foodomics era: 1:25,000. Ann. N. Y. Acad. Sci. 2017, 1403, 48–58. [Google Scholar] [CrossRef]
- Neha, K.; Haider, R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Conidi, C.; Loizzo, M.R.; Sicari, V.; Cassano, A. Olive Mill Wastewater Polyphenol-Enriched Fractions by Integrated Membrane Process: A Promising Source of Antioxidant, Hypolipidemic and Hypoglycaemic Compounds. Antioxidants 2020, 9, 602. [Google Scholar] [CrossRef] [PubMed]
- Biagi, M.; Noto, D.; Corsini, M.; Baini, G.; Cerretani, D.; Cappellucci, G.; Moretti, E. Antioxidant Effect of the Castanea sativa Mill. Leaf Extract on Oxidative Stress Induced upon Human Spermatozoa. Oxidative Med. Cell. Longev. 2019, 2019, 8926075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, S.-M.; Chan, E.; Zhou, S.-F. ADME Properties of Herbal Medicines in Humans: Evidence, Challenges and Strategies. Curr. Pharm. Des. 2011, 17, 357–407. [Google Scholar] [CrossRef]
- Pradhan, P.C.; Saha, S. Anthocyanin profiling of Berberis lycium Royle berry and its bioactivity evaluation for its nutraceutical potential. J. Food Sci. Technol. 2015, 53, 1205–1213. [Google Scholar] [CrossRef] [Green Version]
- Hangun-Balkir, Y.; McKenney, M.L. Determination of antioxidant activities of berries and resveratrol. Green Chem. Lett. Rev. 2012, 5, 147–153. [Google Scholar] [CrossRef]
- Abdel-Tawab, M. Considerations to Be Taken When Carrying Out Medicinal Plant Research—What We Learn from an Insight into the IC50 Values, Bioavailability and Clinical Efficacy of Exemplary Anti-Inflammatory Herbal Components. Pharmaceuticals 2021, 14, 437. [Google Scholar] [CrossRef]
- Cuomo, J.; Appendino, G.; Dern, A.S.; Schneider, E.; McKinnon, T.P.; Brown, M.J.; Togni, S.; Dixon, B.M. Comparative Absorption of a Standardized Curcuminoid Mixture and Its Lecithin Formulation. J. Nat. Prod. 2011, 74, 664–669. [Google Scholar] [CrossRef]
- Koeberle, A.; Werz, O. Multi-target approach for natural products in inflammation. Drug Discov. Today 2014, 19, 1871–1882. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, F.; Jandaghi, S.H.S.S.; Janani, L.; Sarebanhassanabadi, M.; Emamat, H.; Vafa, M. Effects of quercetin supplementation on inflammatory factors and quality of life in post-myocardial infarction patients: A double blind, placebo-controlled, randomized clinical trial. Phytother. Res. 2021, 35, 2085–2098. [Google Scholar] [CrossRef] [PubMed]
- Javadi, F.; Ahmadzadeh, A.; Eghtesadi, S.; Aryaeian, N.; Zabihiyeganeh, M.; Foroushani, A.R.; Jazayeri, S. The Effect of Quercetin on Inflammatory Factors and Clinical Symptoms in Women with Rheumatoid Arthritis: A Double-Blind, Randomized Controlled Trial. J. Am. Coll. Nutr. 2017, 36, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Brüll, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Müller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Stehle, P.; et al. No effects of quercetin from onion skin extract on serum leptin and adiponectin concentrations in over-weight-to-obese patients with (pre-) hypertension: A randomized double-blinded, placebo-controlled crossover trial. Eur. J. Nutr. 2017, 56, 2265–2275. [Google Scholar] [CrossRef] [PubMed]
- Samsami-Kor, M.; Daryani, N.E.; Asl, P.R.; Hekmatdoost, A. Anti-Inflammatory Effects of Resveratrol in Patients with Ulcerative Colitis: A Randomized, Double-Blind, Placebo-controlled Pilot Study. Arch. Med. Res. 2015, 46, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Brenjian, S.; Moini, A.; Yamini, N.; Kashani, L.; Faridmojtahedi, M.; Bahramrezaie, M.; Khodarahmian, M.; Amidi, F. Resveratrol treatment in patients with polycystic ovary syndrome decreased pro-inflammatory and endoplasmic reticulum stress markers. Am. J. Reprod. Immunol. 2020, 83, e13186. [Google Scholar] [CrossRef]
- Khojah, H.M.; Ahmed, S.; Abdel-Rahman, M.S.; Elhakeim, E.H. Resveratrol as an effective adjuvant therapy in the management of rheumatoid arthritis: A clinical study. Clin. Rheumatol. 2018, 37, 2035–2042. [Google Scholar] [CrossRef]
- Bertelli, A.; Mannari, C.; Santi, S.; Filippi, C.; Migliori, M.; Giovannini, L. Immunomodulatory activity of shikimic acid and quercitin in comparison with oseltamivir (Tamiflu) in an “in vitro” model. J. Med. Virol. 2008, 80, 741–745. [Google Scholar] [CrossRef]
- Goc, A.; Sumera, W.; Rath, M.; Niedzwiecki, A. Phenolic compounds disrupt spike-mediated receptor-binding and entry of SARS-CoV-2 pseudo-virions. PLoS ONE 2021, 16, e0253489. [Google Scholar] [CrossRef]
- Liu, X.; Raghuvanshi, R.; Ceylan, F.D.; Bolling, B.W. Quercetin and Its Metabolites Inhibit Recombinant Human Angiotensin-Converting Enzyme 2 (ACE2) Activity. J. Agric. Food Chem. 2020, 68, 13982–13989. [Google Scholar] [CrossRef]
- Ramdani, L.H.; Bachari, K. Potential therapeutic effects of Resveratrol against SARS-CoV-2. Acta Virol. 2020, 64, 276–280. [Google Scholar] [CrossRef]
- Kandeil, A.; Mostafa, A.; Kutkat, O.; Moatasim, Y.; Al-Karmalawy, A.A.; Rashad, A.A.; Kayed, A.E.; Kayed, A.E.; El-Shesheny, R.; Kayali, G.; et al. Bioactive Polyphenolic Compounds Showing Strong Antiviral Activities against Severe Acute Respiratory Syndrome Coronavirus 2. Pathogens 2021, 10, 758. [Google Scholar] [CrossRef] [PubMed]
- Mehany, T.; Khalifa, I.; Barakat, H.; Althwab, S.A.; Alharbi, Y.M.; El-Sohaimy, S. Polyphenols as promising biologically active substances for preventing SARS-CoV-2: A review with research evidence and underlying mechanisms. Food Biosci. 2021, 40, 100891. [Google Scholar] [CrossRef]
- Jang, M.; Park, Y.-I.; Cha, Y.-E.; Park, R.; Namkoong, S.; Lee, J.I.; Park, J. Tea Polyphenols EGCG and Theaflavin Inhibit the Activity of SARS-CoV-2 3CL-Protease In Vitro. Evid.-Based Complement. Altern. Med. 2020, 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Wang, J.; An, X.; Dai, M.; Jiang, Z.; Zhang, L.; Yu, S.; Huang, X. UPLC-MS/MS Method for the Determination of Hyperoside and Application to Pharmacokinetics Study in Rat After Different Administration Routes. Chromatographia 2021, 84, 249–256. [Google Scholar] [CrossRef]
- Steensma, A.; Faassen-Peters, M.A.W.; Noteborn, H.P.J.M.; Rietjens, I. Bioavailability of Genistein and Its Glycoside Genistin As Measured in the Portal Vein of Freely Moving Unanesthetized Rats. J. Agric. Food Chem. 2006, 54, 8006–8012. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhou, Y.; Li, L.; Shen, X.; Chen, G.; Wang, X.; Liang, X.; Tan, M.; Huang, Z. Computational Approaches in Preclinical Studies on Drug Discovery and Development. Front. Chem. 2020, 8, 726. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.; Sanderson, I.R.; MacDonald, T.T. Curcumin as a therapeutic agent: The evidence fromin vitro, animal and human studies. Br. J. Nutr. 2010, 103, 1545–1557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef] [PubMed]

| Polyphenol or Polyphenol Class | Oral Bioavailability | Main Cytochrome Interactions | Polyphenol-Polyphenol Interaction | Nutrients Interaction |
|---|---|---|---|---|
| Anthocyanidins | 1–2% [22] | Weak CYP450 inhibitors [60] | Not known | Lipids, carotenoids, digestible carbohydrates, hydrophilic and lipophilic vitamins, alkaloids, P-glycoprotein inhibitors improve flavonoids and curcumin bioavailability Minerals, proteins and dietary fibers decrease flavonoids bioavailability [38] |
| Curcumin | <1% [61] | CYP3A4 (inhibition) [38] | Not known | |
| Flavan-3-ols | 2–15% in green tea; 5–10% in cocoa beans [26,27] | EGCG: inhibition of the activity of CYP1A2 CYP3A4 CYP2E1 [46] | Green, black and oolong tea phenolic complex improve EGCG bioavailability [45] | |
| Hydroxytyrosol | High [62] | Plausible interaction with CYP450 [62] | In olive oil tyrosol is converted in hydroxytyrosol by CYP2A6 and CYP2D6 [42,43] | |
| Isoflavones | High [63] | Genistein: CYP450 ω-hydroxylase subfamily inhibitor [60] | Not known | |
| Quercetin | <1% (up to 17% when ingested as glycoside) [64] | CYP1A2 CYP2A6 (inhibition) [65] | Not known | |
| Resveratrol | <1% [37] | CYP3A4 CYP1B1 CYP1A1 CYP1A2 (inhibition) [31,37,51] | Red wine phenolic complex improves resveratrol bioavailability [35,37] Quercetin improves resveratrol bioavailability [40,41] |
| Polyphenol | Studied Effects | Models | Findings | Main Concerns | Possible Suggestions |
|---|---|---|---|---|---|
| Quercetin | Pro-inflammatory cytokines release inhibition Cyclooxygenase and lipoxygenase inhibition Inhibition of Src- and Syk-mediated PI3K-(p85) Inhibition of intracellular calcium influx and PKC signaling | Human umbilical cord blood-derived cultured mast cells (hCBMCs) Human normal peripheral blood mononuclear cells (PBMC) Human monocytes (THP-1) RAW 264.7 macrophages T lymphocytes Mast cells Microglial cells BV-2 | Anti-inflammatory activity only exerted at concentrations >1 μM, more often in the range 10–100 μM [73,75] | Effective concentrations are high if compared with those normally achievable in vivo [73] Quercetin is considered one of the most impacting dietary flavonoids, but it mostly occurs in food as glycoside [23] In vitro demonstrated effects could only be referred to repeated administration of high dosesof quercetin [76,77] | Quercetin should be tested in vitro at nanomolar level Quercetin should be investigated both as single compound and in matrix when its dietary role is taken into account Investigation on quercetin should consider simulated digestion in order to evaluate the role of metabolites |
| Curcumin | Upstream signaling and modulation of transduction and transcription factors Downstream level of pro-inflammaotry markers | Different human immune cell lines Human umbilical vein endothelial cells (HUVEC) Tracheal smooth muscle cells Head and neck cancer cells RAW 264.7 macrophages Oesophageal epithelial cells Microglial cells | Strong anti-inflammatory activity exerted at concentrations >10 μM [73,92] | Effective concentrations are high if compared with those normally achievable in vivoand in vitro tests hardly could explain clinical findings [73] Curcumin occurs in food and food supplements in complex with other curcuminoids [23] | Curcumin and curcuminoids should be tested in vitroat nanomolar level Curcumin should be investigated both as single compound and in matrix together with other curcuminoids |
| Resveratrol | Arachidonic acid pathways MAPKs pathways NF-κB signaling AP-1 pathways Pro-inflammatory cytokines release inhibition | A549 adenocarcinomic human alveolar basal epithelial cells Human keratinocytes Human mammary epithelial cells Human T lymphocytes THP-1 HUVEC RAW 264.7 macrophages Myeloid leukemia cells Cardiomyocytes Chondrocytes Mesangial cells Osteoblasts Pancreatic cancer cells Benign prostatic hyperplasia epithelial cell line (BPH-1) | Anti-inflammatory activityexerted at concentrations >1 μM [73,93] | Effective concentrations are high if compared with those normally achievable in vivoand in vitro tests hardly could explain clinical findings [73] In vitro effects could be not referred to dietary resveratrol contained in grape, wine or in other source, given its poor content [37,93] | Resveratrol should be tested in vitro at nanomolar level Investigation on resveratrol should consider simulated digestion in order to evaluate the role of metabolites |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertelli, A.; Biagi, M.; Corsini, M.; Baini, G.; Cappellucci, G.; Miraldi, E. Polyphenols: From Theory to Practice. Foods 2021, 10, 2595. https://doi.org/10.3390/foods10112595
Bertelli A, Biagi M, Corsini M, Baini G, Cappellucci G, Miraldi E. Polyphenols: From Theory to Practice. Foods. 2021; 10(11):2595. https://doi.org/10.3390/foods10112595
Chicago/Turabian StyleBertelli, Alberto, Marco Biagi, Maddalena Corsini, Giulia Baini, Giorgio Cappellucci, and Elisabetta Miraldi. 2021. "Polyphenols: From Theory to Practice" Foods 10, no. 11: 2595. https://doi.org/10.3390/foods10112595
APA StyleBertelli, A., Biagi, M., Corsini, M., Baini, G., Cappellucci, G., & Miraldi, E. (2021). Polyphenols: From Theory to Practice. Foods, 10(11), 2595. https://doi.org/10.3390/foods10112595








