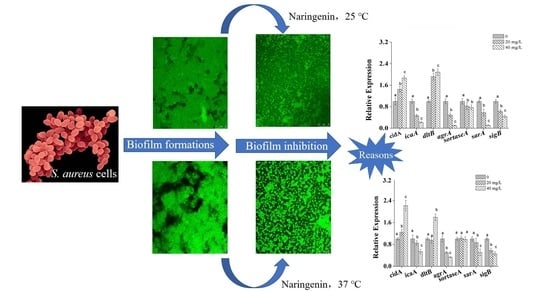
Inhibition of Biofilm Formation of Foodborne Staphylococcus aureus by the Citrus Flavonoid Naringenin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Biofilm Formation
2.2. Cell Surface Hydrophobicity of S. aureus
2.3. Quantification EPS Production of S. aureus
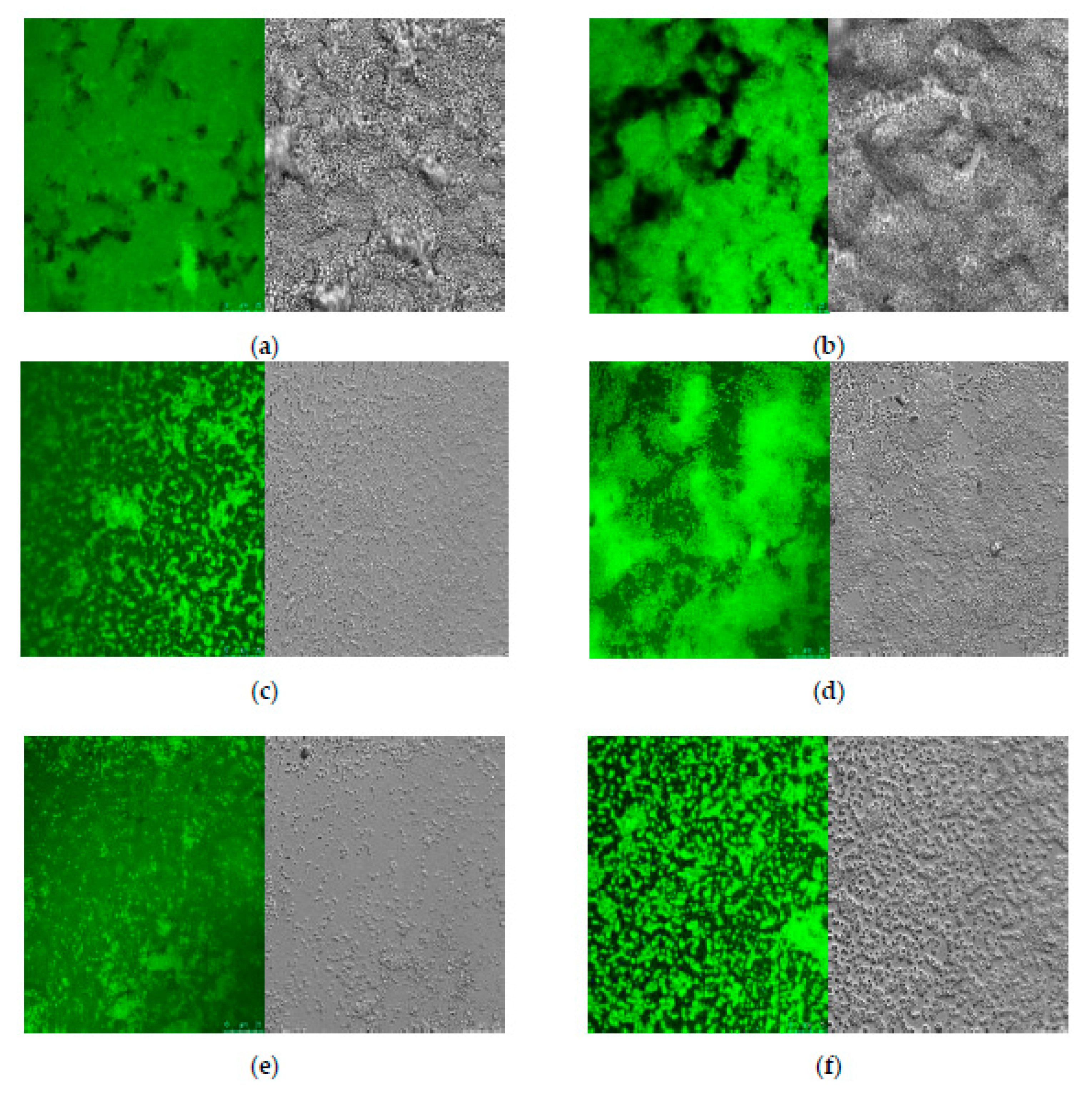
2.4. CLSM and SEM of S. aureus Biofilms
2.5. RNA Extraction and Real-Time Quantitative PCR (RT-qPCR) Analysis
2.6. Statistical Analysis
| Gene | Primer |
|---|---|
| cidA | Forward 5′-AGCGTAATTTCGGAAGCAACATCCA-3′ |
| Reverse 5′-CCCTTAGCCGGCAGTATTGTTGGTC-3′ | |
| icaA | Forward 5′-CTG GCG CAG TCA ATA CTA TTT CGG GTG TCT-3′ |
| Reverse 5′-GAC CTC CCA ATG TTT CTG GAA CCA ACA TCC-3′ | |
| dltB | Forward 5′-GTGGACATCAGATTCACTTCC-3′ |
| Reverse 5′-ATAGAACCATCACGAATTTCC-3′ | |
| agrA | Forward 5′-TGATAATCCTTATGAGGTGCTT-3′ |
| Reverse 5′-CACTGTGACTCGTAACGAAAA-3′ | |
| sortaseA | Forward 5′-AAACCACATATCGATAATTATC-3′ |
| Reverse 5′-TTATTTGACTTCTGTAGCTACAA-3′ | |
| sarA | Forward 5′-CAAACAACCACAAGTTGTTAAAGC-3′ |
| Reverse 5′-TGTTTGCTTCAGTGATTCGTTT-3′ | |
| sigB | Forward 5′-AAGTGATTCGTAAGGACGTCT-3′ |
| Reverse 5′-TCGATAACTATAACCAAAGCCT-3′ | |
| 16S rRNA | Forward 5′-CGGTGAATACGTTCYCGG-3′ |
| Reverse 5′-GGWTACCTTGTTACGACTT-3′ |
3. Results
3.1. Effects of Naringenin on S. aureus at Different Growth Temperatures
3.2. Changes in Cell Surface Hydrophobicity and EPS Production of S. aureus
3.3. Microscopic Observations of S. aureus Biofilm
3.4. Transcriptional Analysis of Biofilm-Related Genes in S. aureus Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Normanno, G.; La, S.G.; Dambrosio, A.; Quaglia, N.C.; Corrente, M.; Parisi, A.; Santagada, G.; Firinu, A.; Crisetti, E.; Celano, G.V. Occurrence, characterization and antimicrobial resistance of enterotoxigenic Staphylococcus aureus isolated from meat and dairy products. Int. J. Food Microbiol. 2007, 115, 290–296. [Google Scholar] [CrossRef]
- Tango, C.N.; Akkermans, S.; Hussain, M.S.; Khan, I.; Van Impe, J.; Jin, Y.-G.; Oh, D.H. Modeling the effect of pH, water activity, and ethanol concentration on biofilm formation of Staphylococcus aureus. Food Microbiol. 2018, 76, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Lauková, A.; Pogány Simonová, M.; Focková, V.; Kološta, M.; Tomáška, M.; Dvorožňáková, E. Susceptibility to Bacteriocins in Biofilm-Forming, Variable Staphylococci Isolated from Local Slovak Ewes’ Milk Lump Cheeses. Foods 2020, 9, 1335. [Google Scholar] [CrossRef]
- Vasudevan, P.; Nair, M.; Annamalai, T.; Venkitanarayanan, K.S. Phenotypic and genotypic characterization of bovine mastitis isolates of Staphylococcus aureus for biofilm formation. Vet. Microbiol. 2003, 92, 179–185. [Google Scholar] [CrossRef]
- Kroning, I.S.; Iglesias, M.A.; Sehn, C.P.; Gandra, T.K.V.; Mata, M.M.; da Silva, W.P. Staphylococcus aureus isolated from handmade sweets: Biofilm formation, enterotoxigenicity and antimicrobial resistance. Food Microbiol. 2016, 58, 105–111. [Google Scholar] [CrossRef]
- Kruk, M.; Trząskowska, M. Analysis of Biofilm Formation on the Surface of Organic Mung Bean Seeds, Sprouts and in the Germination Environment. Foods 2021, 10, 542. [Google Scholar] [CrossRef] [PubMed]
- Ciccio, P.D.; Vergara, A.; Festino, A.R.; Paludi, D.; Zanardi, E.; Ghidini, S.; Ianieri, A. Biofilm formation by Staphylococcus aureus on food contact surfaces: Relationship with temperature and cell surface hydrophobicity. Food Control 2015, 50, 930–936. [Google Scholar] [CrossRef]
- Abdallah, M.; Chataigne, G.; Ferreira-Theret, P.; Benoliel, C.; Drider, D.; Dhulster, P.; Chihib, N.E. Effect of growth temperature, surface type and incubation time on the resistance of Staphylococcus aureus biofilms to disinfectants. Appl. Microbiol. Biotechnol. 2014, 98, 2597–2607. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Bennett, R.; Bisignano, G.; Trombetta, D.; Saija, A.; Faulds, C.; Gasson, M.; Narbad, A. Antimicrobial activity of flavonoids extracted from bergamot (Citrus bergamia Risso) peel, a byproduct of the essential oil industry. J. Appl. Microbiol. 2007, 103, 2056–2064. [Google Scholar] [CrossRef]
- Wen, Q.-H.; Wang, L.-H.; Zeng, X.-A.; Niu, D.-B.; Wang, M.-S. Hydroxyl-related differences for three dietary flavonoids as inhibitors of human purine nucleoside phosphorylase. Int. J. Biol. Macromol. 2018, 118, 588–598. [Google Scholar] [CrossRef]
- Erlund, I.; Meririnne, E.; Alfthan, G.; Aro, A. Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J. Nutr. 2001, 131, 235–241. [Google Scholar] [CrossRef]
- Hashimoto, T.; Ide, T. Activity and mRNA Levels of Enzymes Involved in Hepatic Fatty Acid Synthesis in Rats Fed Naringenin. J. Agric. Food Chem. 2015, 63, 9536–9542. [Google Scholar] [CrossRef]
- Kanaze, F.I.; Kokkalou, E.; Georgarakis, M.; Niopas, I. A validated solid-phase extraction HPLC method for the simultaneous determination of the citrus flavanone aglycones hesperetin and naringenin in urine. J. Pharm. Biomed. Anal. 2004, 36, 175–181. [Google Scholar] [CrossRef]
- Denny, S.; West, P.W.J.; Mathew, T.C. Antagonistic interactions between the flavonoids hesperetin and naringenin and β-lactam antibiotics against Staphylococcus aureus. Br. J. Biomed. Sci. 2008, 65, 145–147. [Google Scholar] [CrossRef]
- Lee, K.-A.; Moon, S.H.; Kim, K.-T.; Mendonca, A.F.; Paik, H.-D. Antimicrobial effects of various flavonoids on Escherichia coli O157: H7 cell growth and lipopolysaccharide production. Food Sci. Biotechnol. 2010, 19, 257–261. [Google Scholar] [CrossRef]
- Vikram, A.; Jayaprakasha, G.K.; Jesudhasan, P.; Pillai, S.; Patil, B. Suppression of bacterial cell–cell signalling, biofilm formation and type III secretion system by citrus flavonoids. J. Appl. Microbiol. 2010, 109, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Yang, H.; Liu, S.; Song, F.; Guo, J.; Huang, C. Influence of naringenin on the biofilm formation of Streptococcus mutans. J. Dent. 2018, 76, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Wang, M.S.; Zeng, X.A.; Xu, X.M.; Brennan, C.S. Membrane and genomic DNA dual-targeting of citrus flavonoid naringenin against Staphylococcus aureus. Integr. Biol. 2017, 9, 820–829. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Lee, K.; Kim, S.C.; Lee, J. Temperature-dependent control of Staphylococcus aureus biofilms and virulence by thermoresponsive oligo (N-vinylcaprolactam). Biotechnol. Bioeng. 2015, 112, 716–724. [Google Scholar] [CrossRef]
- Hsu, L.C.; Fang, J.; Borcatasciuc, D.A.; Worobo, R.W.; Moraru, C.I. Effect of Micro- and Nanoscale Topography on the Adhesion of Bacterial Cells to Solid Surfaces. Appl. Environ. Microbiol. 2013, 79, 2703–2712. [Google Scholar] [CrossRef] [Green Version]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Wang, L.H.; Zeng, X.A.; Wang, M.S.; Brennan, C.S.; Gong, D. Modification of membrane properties and fatty acids biosynthesis-related genes in Escherichia coli and Staphylococcus aureus: Implications for the antibacterial mechanism of naringenin. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2018, 1860, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Minami, T.; Sasaki, H.; Sobue, S.; Hamada, S.; Ooshima, T. Inhibitory effects of oolong tea extract on caries–inducing properties of Mutans streptococci. Caries Res. 1999, 33, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, A.; Kimizuka, R.; Kato, T.; Okuda, K. Inhibitory effects of cranberry juice on attachment of oral streptococci and biofilm formation. Oral Microbiol. Immunol. 2004, 19, 150–154. [Google Scholar] [CrossRef]
- Al-Shabib, N.A.; Husain, F.M.; Ahmad, I.; Khan, M.S.; Khan, R.A.; Khan, J.M. Rutin inhibits mono and multi-species biofilm formation by foodborne drug resistant Escherichia coli and Staphylococcus aureus. Food Control 2017, 79, 325–332. [Google Scholar] [CrossRef]
- Xiang, H.; Cao, F.; Ming, D.; Zheng, Y.; Dong, X.; Zhong, X.; Mu, D.; Li, B.; Zhong, L.; Cao, J. Aloe-emodin inhibits Staphylococcus aureus biofilms and extracellular protein production at the initial adhesion stage of biofilm development. Appl. Microbiol. Biotechnol. 2017, 101, 6671–6681. [Google Scholar] [CrossRef]
- Hoffman, L.R.; D’Argenio, D.A.; MacCoss, M.J.; Zhang, Z.; Jones, R.A.; Miller, S.I. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 2005, 436, 1171–1175. [Google Scholar] [CrossRef]
- Kaplan, J.B.; Izano, E.A.; Gopal, P.; Karwacki, M.T.; Kim, S.; Bose, J.L.; Bayles, K.W.; Horswill, A.R. Low Levels of β-Lactam Antibiotics Induce Extracellular DNA Release and Biofilm Formation in Staphylococcus aureus. Mbio 2012, 3, e00198-12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Regmi, S.C.; Kim, J.A.; Cho, M.H.; Yun, H.; Lee, C.S.; Lee, J. Apple Flavonoid Phloretin Inhibits Escherichia coli O157:H7 Biofilm Formation and Ameliorates Colon Inflammation in Rats. Infect. Immun. 2011, 79, 4819–4827. [Google Scholar] [CrossRef] [Green Version]
- Gopu, V.; Meena, C.K.; Shetty, P.H. Quercetin influences quorum sensing in food borne bacteria: In-vitro and in-silico evidence. PLoS ONE 2015, 10, e0134684. [Google Scholar] [CrossRef] [Green Version]
- Sivaranjani, M.; Gowrishankar, S.; Kamaladevi, A.; Pandian, S.K.; Balamurugan, K.; Ravi, A.V. Morin inhibits biofilm production and reduces the virulence of Listeria monocytogenes—An in vitro and in vivo approach. Int. J. Food Microbiol. 2016, 237, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Peschel, A.; Otto, M.; Jack, R.W.; Kalbacher, H.; Jung, G.; Götz, F. Inactivation of the dlt Operon in Staphylococcus aureus Confers Sensitivity to Defensins, Protegrins, and Other Antimicrobial Peptides. J. Biol. Chem. 1999, 274, 8405–8410. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Gu, S.; Shi, Y.; Cui, X.; Wen, S.; Ge, J. The effect of emodin on Staphylococcus aureus strains in planktonic form and biofilm formation in vitro. Arch. Microbiol. 2017, 199, 1267–1275. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, Y.; Yestrepsky, B.D.; Sorenson, R.J.; Chen, M.; Larsen, S.D.; Sun, H. Novel inhibitors of Staphylococcus aureus virulence gene expression and biofilm formation. PLoS ONE 2012, 7, e47255. [Google Scholar] [CrossRef] [Green Version]
- Pratten, J.; Foster, S.J.; Chan, P.F.; Wilson, M.; Nair, S.P. Staphylococcus aureus accessory regulators: Expression within biofilms and effect on adhesion. Microbes Infect. 2001, 3, 633–637. [Google Scholar] [CrossRef]
- Coelho, L.R.; Souza, R.R.; Ferreira, F.A.; Guimaraes, M.A.; Ferreira-Carvalho, B.T.; Figueiredo, A. agr RNAIII divergently regulates glucose-induced biofilm formation in clinical isolates of Staphylococcus aureus. Microbiology 2008, 154, 3480–3490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valle, J.; Toledo-Arana, A.; Berasain, C.; Ghigo, J.M.; Amorena, B.; Penadés, J.R.; Lasa, I. SarA and not σB is essential for biofilm development by Staphylococcus aureus. Mol. Microbiol. 2003, 48, 1075–1087. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Huang, P.; Chen, M.W. Curcumin reduces Streptococcus mutans biofilm formation by inhibiting sortase A activity. Arch. Oral Biol. 2013, 58, 1343–1348. [Google Scholar] [CrossRef]
- Cascioferro, S.; Totsika, M.; Schillaci, D. Sortase A: An ideal target for anti-virulence drug development. Microb. Pathog. 2014, 77, 105–112. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, Q.-H.; Wang, R.; Zhao, S.-Q.; Chen, B.-R.; Zeng, X.-A. Inhibition of Biofilm Formation of Foodborne Staphylococcus aureus by the Citrus Flavonoid Naringenin. Foods 2021, 10, 2614. https://doi.org/10.3390/foods10112614
Wen Q-H, Wang R, Zhao S-Q, Chen B-R, Zeng X-A. Inhibition of Biofilm Formation of Foodborne Staphylococcus aureus by the Citrus Flavonoid Naringenin. Foods. 2021; 10(11):2614. https://doi.org/10.3390/foods10112614
Chicago/Turabian StyleWen, Qing-Hui, Rui Wang, Si-Qi Zhao, Bo-Ru Chen, and Xin-An Zeng. 2021. "Inhibition of Biofilm Formation of Foodborne Staphylococcus aureus by the Citrus Flavonoid Naringenin" Foods 10, no. 11: 2614. https://doi.org/10.3390/foods10112614
APA StyleWen, Q.-H., Wang, R., Zhao, S.-Q., Chen, B.-R., & Zeng, X.-A. (2021). Inhibition of Biofilm Formation of Foodborne Staphylococcus aureus by the Citrus Flavonoid Naringenin. Foods, 10(11), 2614. https://doi.org/10.3390/foods10112614