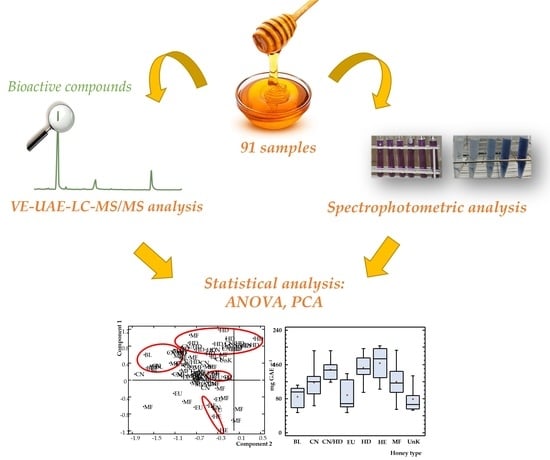
Evaluating the Presence and Contents of Phytochemicals in Honey Samples: Phenolic Compounds as Indicators to Identify Their Botanical Origin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents and Materials
2.2. Honey Samples
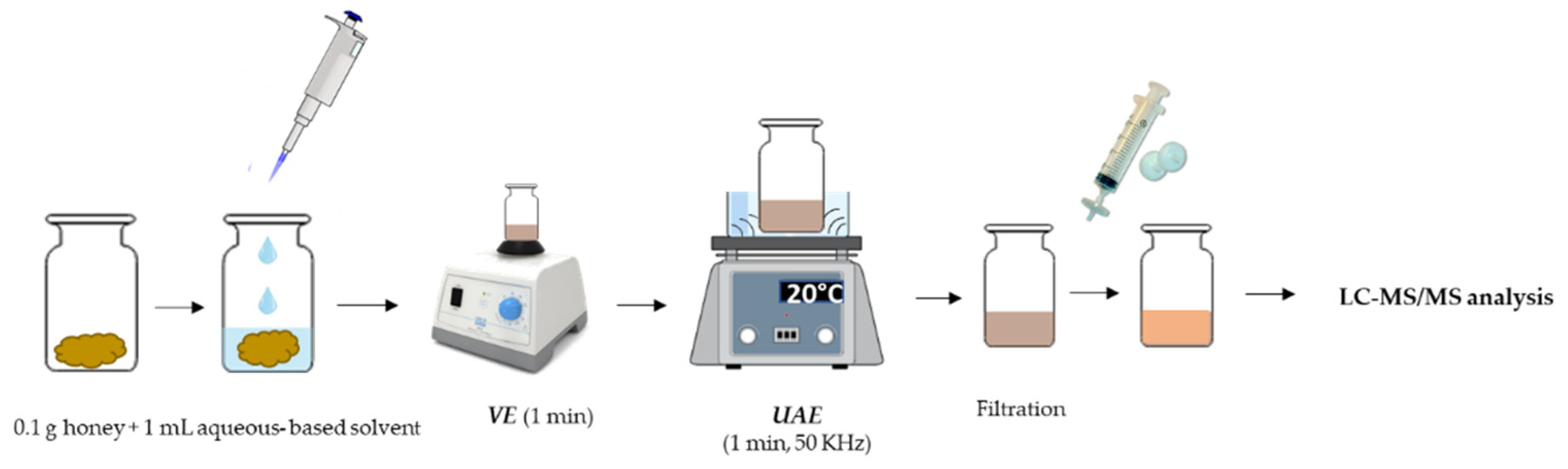
2.3. VE-UAE Procedure
2.4. Determination of TPC
2.5. Determination of AA
2.6. LC-MS/MS Analysis
2.7. Statistical Analysis
3. Results
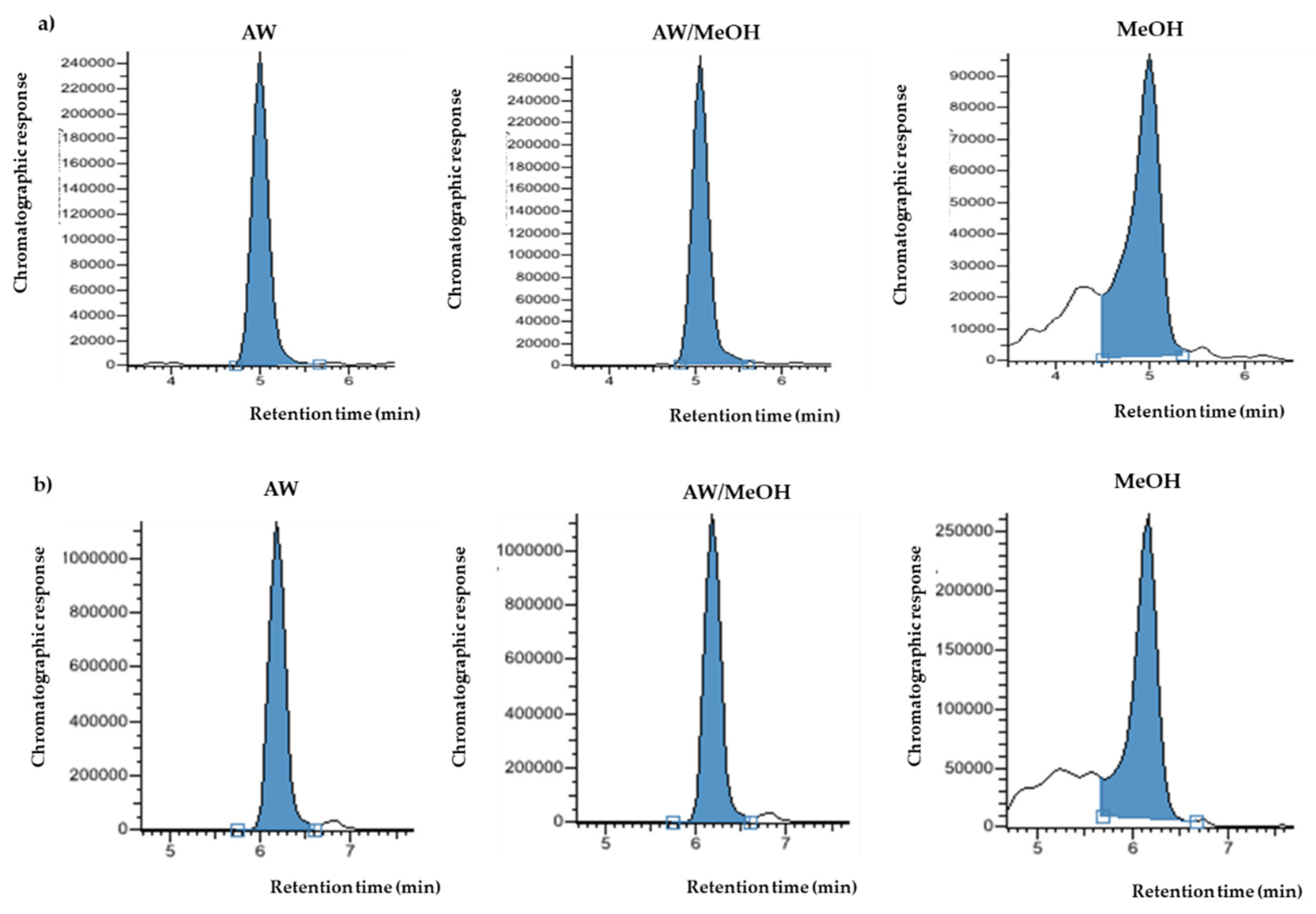
3.1. Selection of the Solvent
3.2. VE-UAE Optimization
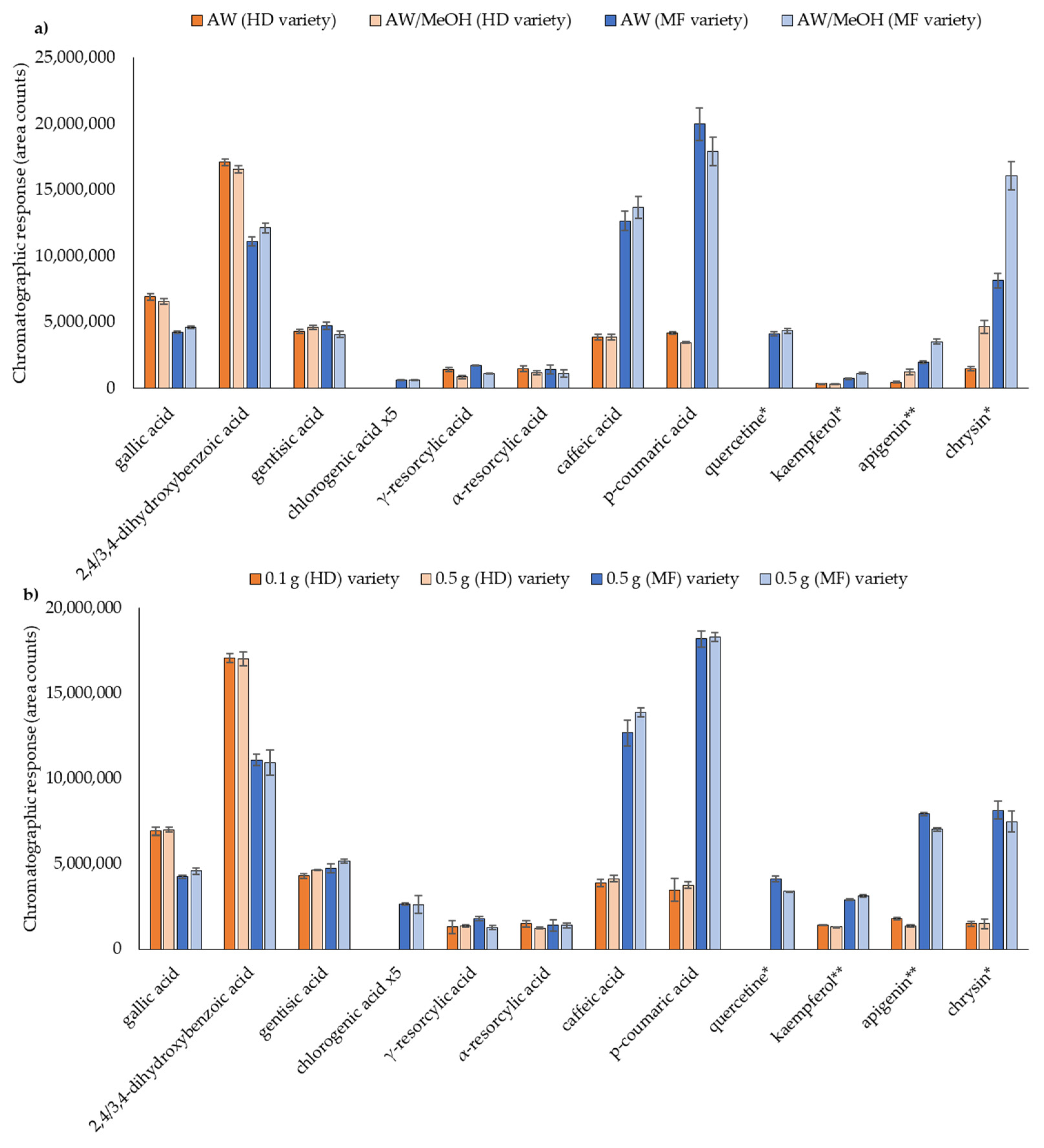
3.2.1. Extraction Solvent
3.2.2. Sample Amount
3.3. VE-UAE-LC-MS/MS Performance
3.4. Analysis of Real Honey Samples
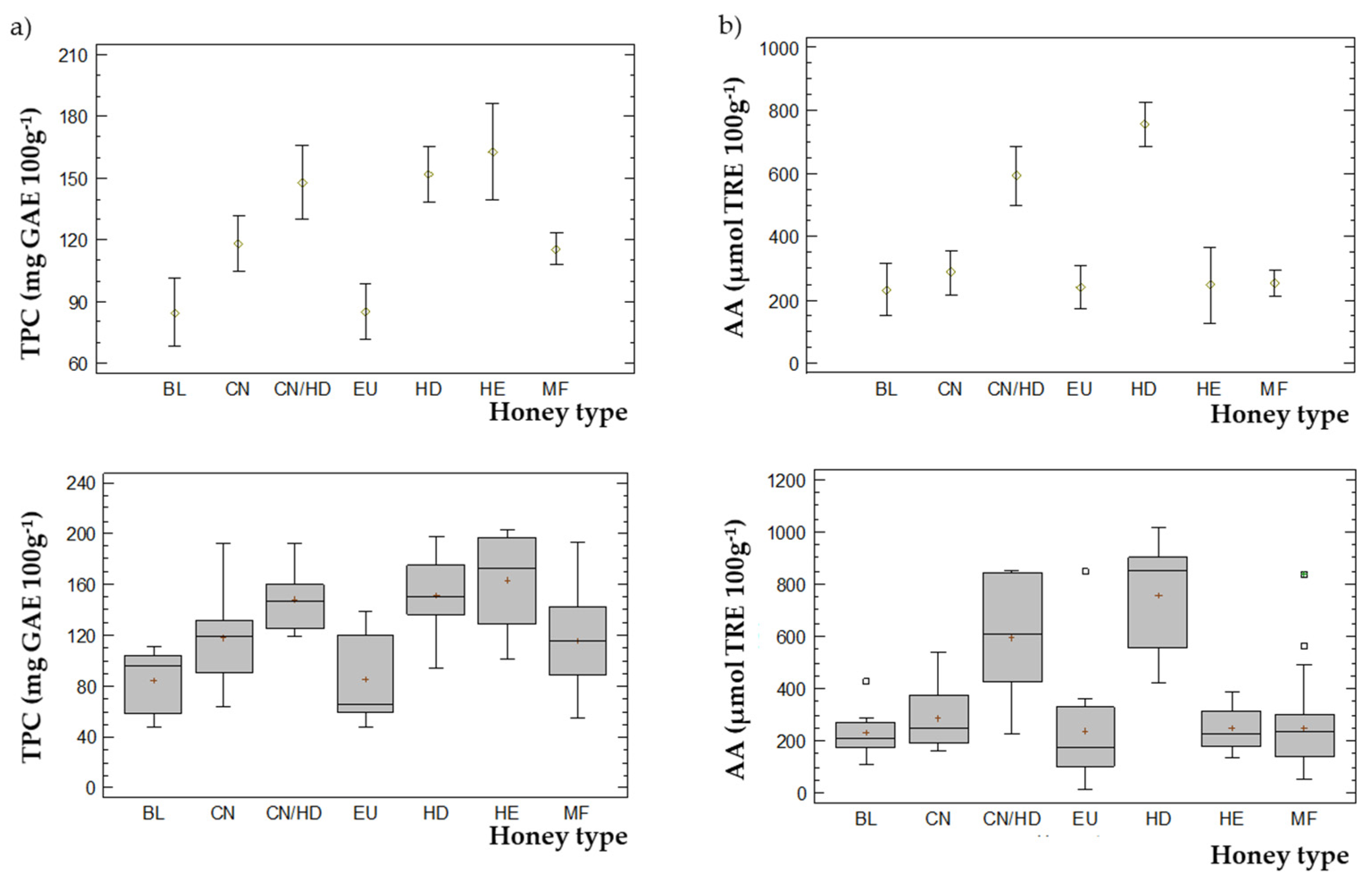
3.4.1. TPC and AA
3.4.2. Individual Phenolic Content
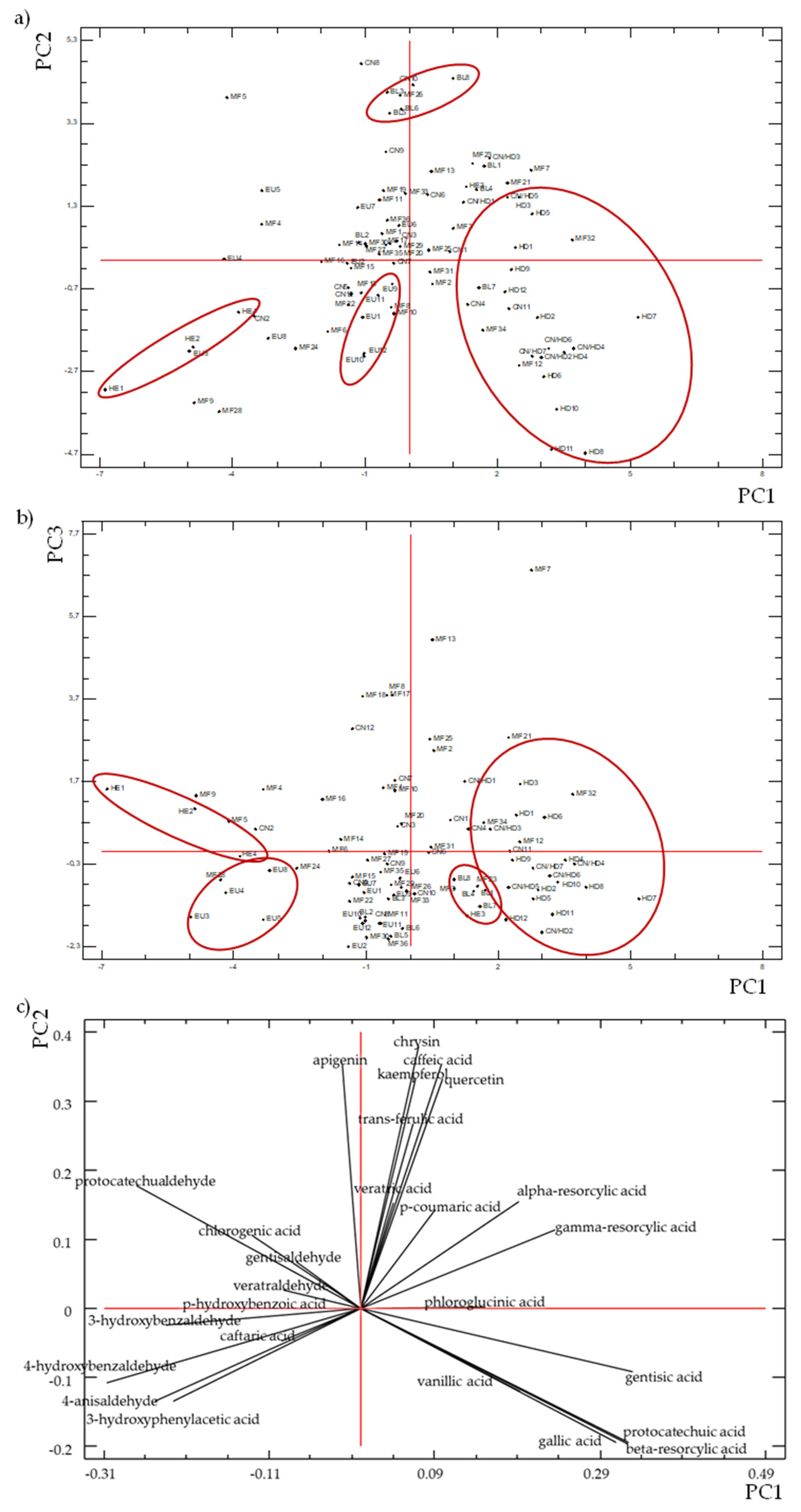
3.5. Chemometric Study
3.5.1. Analysis of Variance (ANOVA)
3.5.2. Principal Components Analysis (PCA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Lamas, L.B.; Flórez, S.M.; Toyos, P.A.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pyrzynska, K.; Biesaga, M. Analysis of phenolic acids and flavonoids in honey. TrAC Trends Anal. Chem. 2009, 28, 893–902. [Google Scholar] [CrossRef]
- Jerković, I.; Tuberso, C.I.; Gugic, M.; Bubalo, D. Composition of Sulla (Hedysarum coronarium L.) Honey Solvent Extractives Determined by GC/MS: Norisoprenoids and Other Volatile Organic Compounds. Molecules 2010, 15, 6375–6385. [Google Scholar] [CrossRef] [Green Version]
- Fakhlaei, R.; Selamat, J.; Khatib, A.; Razis, A.F.A.; Sukor, R.; Ahmad, S.; Babadi, A.A. The Toxic Impact of Honey Adulteration: A Review. Foods 2020, 9, 1538. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Codex Alimentarius Commission Standards. Ref. Nr. CL 1993/14-SH. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B12-1981%252FCXS_012e.pdf (accessed on 25 October 2021).
- European Commission. Directive 2001/110/EC of 20 December relating honey. Off. J. EC 2001, L10, 47–52. [Google Scholar]
- Directive 2014/63/EU of the European parliament and of the council of 15 May 2014 amending council directive 2001/110/EC relating to honey. Off. J. EU 2014, L164, 1–5.
- Thrasyvoulou, A.; Tananaki, C.; Goras, G.; Karazafiris, E.; Dimou, M.; Liolios, V.; Kanelis, D.; Gounari, S. Legislation of honey criteria and standards. J. Apic. Res. 2018, 57, 88–96. [Google Scholar] [CrossRef]
- Kečkeš, S.; Gašić, U.; Velickovic, T.C.; Milojković-Opsenica, D.; Natić, M.; Tešic, Z. The determination of phenolic profiles of Serbian unifloral honeys using ultra-high-performance liquid chromatography/high resolution accurate mass spectrometry. Food Chem. 2013, 138, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Gašić, U.; Milojković-Opsenica, D.M.; Tešić, Ž.L. Polyphenols as Possible Markers of Botanical Origin of Honey. J. AOAC Int. 2017, 100, 852–861. [Google Scholar] [CrossRef] [Green Version]
- Jaganathan, S.K.; Mandal, M. Antiproliferative Effects of Honey and of Its Polyphenols: A Review. J. Biomed. Biotechnol. 2009, 2009, 830616. [Google Scholar] [CrossRef] [Green Version]
- Khalil, M.L.; Sulaiman, S.A. The potential role of honey and its polyphenols in preventing heart disease: A review. Afr. J. Tradit. Complement. Altern. Med. 2010, 7, 315–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossen, M.S.; Ali, M.Y.; Jahurul, M.H.A.; Abdel-Daim, M.M.; Gan, S.H.; Khalil, M.I. Beneficial roles of honey polyphenols against some human degenerative diseases: A review. Pharm. Reports. 2017, 69, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, F.; Tomás-Barberán, F.A.; García-Vignera, C.; Tomás-Lorente, F. Flavonoids in honey of different geographical origin. Eur. Food Res. Technol. 1993, 196, 38–44. [Google Scholar] [CrossRef]
- Pascual-Maté, A.; Osés, S.M.; Fernández-Muiño, M.A.; Sancho, M.T. Methods of analysis of honey. J. Apic. Res. 2018, 57, 38–74. [Google Scholar] [CrossRef]
- Pauliuc, D.; Dranca, F.; Oroian, M. Antioxidant Activity, Total Phenolic Content, Individual Phenolics and Physicochemical Parameters Suitability for Romanian Honey Authentication. Foods 2020, 9, 306. [Google Scholar] [CrossRef] [Green Version]
- Escuredo, O.; Rodríguez-Flores, M.S.; Rojo-Martínez, S.; Seijo, M.C. Contribution to the Chromatic Characterization of Unifloral Honeys from Galicia (NW Spain). Foods 2019, 8, 233. [Google Scholar] [CrossRef] [Green Version]
- Seijo, M.C.; Escuredo, O.; Rodríguez-Flores, M.S. Physicochemical Properties and Pollen Profile of Oak Honeydew and Evergreen Oak Honeydew Honeys from Spain: A Comparative Study. Foods 2019, 8, 126. [Google Scholar] [CrossRef] [Green Version]
- Escuredo, O.; Rodríguez-Flores, M.S.; Meno, L.; Seijo, M.C. Prediction of Physicochemical Properties in Honeys with Portable Near-Infrared (microNIR) Spectroscopy Combined with Multivariate Data Processing. Foods 2021, 10, 317. [Google Scholar] [CrossRef]
- Biesaga, M.; Pyrzyńska, K. Stability of bioactive polyphenols from honey during different extraction methods. Food Chem. 2013, 136, 46–54. [Google Scholar] [CrossRef]
- Cuevas-Glory, L.F.; Pino, J.A.; Santiago, L.S.; Sauri-Duch, E. A review of volatile analytical methods for determining the botanical origin of honey. Food Chem. 2007, 103, 1032–1043. [Google Scholar] [CrossRef]
- Vazquez, L.; Celeiro, M.; Sergazina, M.; Dagnac, T.; Llompart, M. Optimization of a miniaturized solid-phase microextraction method followed by gas chromatography mass spectrometry for the determination of twenty four volatile and semivolatile compounds in honey from Galicia (NW Spain) and foreign countries. Sustain. Chem. Pharm. 2021, 21, 100451. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vit. 1965, 16, 144–158. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Celeiro, M.; Lamas, J.P.; Arcas, R.; Lores, M. Antioxidants Profiling of By-Products from Eucalyptus Greenboards Manufacture. Antioxidants 2019, 8, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biesaga, M.; Pyrzynska, K. Liquid chromatography/tandem mass spectrometry studies of the phenolic compounds in honey. J. Chromatogr. A 2009, 1216, 6620–6626. [Google Scholar] [CrossRef]
- Pascual-Maté, A.; Osés, S.M.; Fernández-Muiño, M.A.; Sancho, M.T. Analysis of Polyphenols in Honey: Extraction, Separation and Quantification Procedures. Sep. Purif. Rev. 2018, 47, 142–158. [Google Scholar] [CrossRef]
- Attanzio, A.; Tesoriere, L.; Allegra, M.; Livrea, M.A. Monofloral honeys by Sicilian black honeybee (Apis mellifera ssp. sicula) have high reducing power and antioxidant capacity. Heliyon 2016, 2, e00193. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Phenolic Compounds | CAS | Mm (g mol−1) | log Kow | Retention Time (min) | Ionization Mode 1 | MS/MS Transitions 2 |
|---|---|---|---|---|---|---|
| gallic acid | 149-91-7 | 170.1 | 0.70 | 2.61 | − | 169.02 → 125.04 (17) 169.02 → 153.1 (15) |
| phloroglucinic acid | 71989-93-0 | 188.1 | 1.28 | 4.22 | + | 168.98 → 150.99 (17) 168.98 → 83.02 (23) 168.98 → 107.02 (22) |
| β-resorcylic acid 3 | 89-86-1 | 154.1 | 1.63 | 5.00 | + | 153.00 → 109.05 (16) 153.00 → 65.09 (19) 153.00 → 67.07 (23) |
| protocatechuic acid 3 | 99-50-3 | 154.1 | 0.86 | 5.00 | + | 152.98 → 109.04 (17) 152.98 → 91.04 (28) 152.98 → 108.03 (26) |
| caftaric acid | 67879-58-7 | 312.2 | 0.21 | 4.78 | − | 310.96→ 178.97 (17) 310.96 → 148.96 (14) |
| protocatechualdehyde | 139-85-5 | 138.1 | 1.09 | 5.05 | + | 137.07 → 136.11 (21) 137.07 → 91.09 (24) 137.07 → 92.13 (25) |
| procyanidin B1 | 20315-25-7 | 578.5 | 5.07 | − | 577.03 → 407.06 (26) 577.03 → 288.93 (25) 577.03 → 424.97 (26) | |
| p-hydroxybenzoic acid | 99-96-7 | 138.1 | 1.58 | 5.35 | − | 137.00 → 93.00 (17) 137.00 → 65.00 (27) |
| gentisic acid | 490-79-9 | 117.1 | 1.74 | 5.38 | + | 152.96 → 108.00 (24) 152.96 → 81.02 (21) 152.96 → 109.01 (16) |
| catechin | 18829-70-4 | 290.3 | 0.51 | 5.50 | + | 289.00 → 245.02 (17) 289.00 → 203.11 (22) |
| 3-hydroxyphenylacetic acid | 621-37-4 | 152.2 | 0.85 | 5.70 | − | 151.00 → 65.00 (20) 151.00 → 79.00 (20) |
| procyanidin B2 | 29106-49-8 | 578.5 | 2.29 | 5.96 | − | 577.03 → 407.06 (26) 577.03 → 288.93 (25) 577.03 → 424.97 (26) |
| gentisaldehyde | 1194-98-5 | 138.1 | 1.53 | 6.11 | + | 136.99 → 108.02 (21) 136.99 → 81.08 (18) 136.99 → 109.04 (14) |
| chlorogenic acid | 327-97-9 | 354.3 | 1.01 | 6.12 | + | 353.00 → 191.07 (22) 353.00 → 85.09 (43) 353.00 → 93.07 (45) |
| 3-hydroxybenzaldehyde | 100-83-4 | 122.1 | 1.29 | 6.22 | + | 121.02 → 93.05 (20) 121.02 → 92.05 (23) 121.02 → 120.04 (19) |
| 4-hydroxybenzaldehyde | 123-08-0 | 122.1 | 1.36 | 6.25 | + | 122.97 → 95.05 (13) 122.97 → 51.10 (36) 122.97 → 77.05 (20) |
| vanillic acid | 121-34-6 | 168.2 | 1.43 | 6.29 | − | 167.00 → 108.00 (27) 167.00 → 152.00 (18) |
| γ-resorcylic acid | 303-07-1 | 154.1 | 2.20 | 6.33 | + | 153.00 → 109.05 (17) 153.00 → 65.09 (21) 153.00 → 135.02 (16) |
| α-resorcylic acid | 99-10-5 | 154.1 | 0.86 | 6.33 | + | 152.97 → 109.01 (15) 152.97 → 65.06 (16) 152.97 → 67.05 (20) |
| veratric acid | 93-07-2 | 182.2 | 1.61 | 6.45 | + | 182.96 → 137.08 (6) 182.96 → 106.99 (22) |
| caffeic acid | 331-39-5 | 180.2 | 1.15 | 6.50 | − | 178.98 → 135.03 (19) 178.98 → 134.01 (28) |
| epicatechin | 35323-91-2 | 290.3 | 0.51 | 6.56 | + | 289.00 → 245.02 (17) 289.00 → 203.11 (22) |
| epigallocatechin gallate | 989-51-5 | 458.4 | 2.56 | 6.79 | + | 457.15 → 169.05 (21) 457.15 → 125.09 (42) 457.15 → 305.09 (21) |
| gallocatechin gallate | 84650-60-2 | 458.4 | 2.56 | 7.29 | + | 457.15 → 169.05 (21) 457.15 → 125.09 (42) 457.15 → 305.09 (21) |
| procyanidin A2 | 41743-41-3 | 576.5 | 2.52 | 7.32 | − | 577.09 → 287.00 (32) 577.09 → 136.98 (62) 577.09 → 425.08 (13) |
| umbelliferone | 93-35-6 | 162.1 | 1.58 | 7.80 | + | 162.99 → 107.04 (22) 162.99 → 77.05 (34) 162.99 → 91.05 (20) |
| p-coumaric acid | 501-98-4 | 164.2 | 1.79 | 7.89 | + | 163.02 → 119.07 (18) 163.02→ 93.07 (37) 163.02 → 117.05 (38) |
| catechin gallate | 130405-40-2 | 442.3 | 2.62 | 8.01 | + | 441.13 → 289.13 (20) 441.13 → 125.08 (42) 441.13 → 169.05 (24) |
| trans-ferulic acid | 537-98-4 | 194.2 | 1.51 | 8.33 | − | 192.80 → 177.90 (12) 192.80 → 133.90 (16) |
| veratraldehyde | 120-14-9 | 166.2 | 1.22 | 8.92 | + | 167.01 → 139.05 (13) 167.01 → 108.05 (21) 167.01 → 124.03 (18) |
| 4-anisaldehyde | 123-11-5 | 136.1 | 1.76 | 10.03 | + | 136.97 → 109.05 (12) 136.97 → 77.05 (23) 136.97 → 94.04 (18) |
| miquelianin | 22688-79-5 | 478.4 | 0.20 | 10.32 | + | 479.09 → 461.50 (14) 479.09 → 302.96 (18) |
| rutin | 153-18-4 | 610.5 | 0.15 | 10.35 | − | 609.18 → 270.92 (96) 609.18 → 178.87 (44) 609.18 → 300.01 (37) |
| isoquercitrin | 482-35-9 | 463.4 | 0.76 | 10.43 | + | 465.07 → 256.90 (41) 465.07 → 302.97 (14) |
| myricetin | 529-44-2 | 318.2 | 1.42 | 11.43 | + | 319.00 → 153.02 (31) 319.00 → 217.06 (31) 319.00 → 245.06 (27) |
| 3,4,5-trimethoxycinnamic acid | 90-50-6 | 238.2 | 1.58 | 11.59 | + | 239.03 → 221.04 (11) 239.03 → 162.99 (27) 239.03 → 190.01 (19) |
| 3,5-dimethoxybenzaldehyde | 7311-34-4 | 166.2 | 1.87 | 11.81 | + | 167.15 → 124.03 (17) 167.15 → 77.05 (26) |
| quercetin | 117-39-5 | 302.2 | 1.48 | 12.10 | + | 303.09 → 229.10 (28) 303.09 → 153.04 (33) |
| kaempferol | 520-18-3 | 286.2 | 1.96 | 12.57 | − | 285.07 → 184.91 (30) 285.07 → 239.12 (35) |
| apigenin | 520-36-5 | 270.2 | 3.02 | 12.63 | − | 269.09 → 117.12 (37) 269.09 → 149.12 (26) 269.09 → 151.06 (26) |
| chrysin | 480-40-0 | 254.2 | 3.52 | 13.24 | + | 253.13 → 143.18 (30) 253.13 → 63.20 (34) 253.13 → 145.16 (31) |
| Phenolic Compounds | Linearity | Precision (RSD, %) | Recovery (%) | LOD (ng g−1) | |
|---|---|---|---|---|---|
| R2 | Intra-Day (n = 4) | Inter-Day (n = 5) | |||
| gallic acid | 0.9985 | 5.3 | 3.5 | 58.3 ± 9.0 | 39 |
| phloroglucinic acid | 0.9996 | 10 | 9.1 | 84.3 ± 4.7 | 137 |
| β-resorcylic acid 1 | 0.9954 | 11 | 7.2 | 78 ± 11 | 54 |
| protocatechuic acid 1 | 0.9937 | 3.4 | 4.5 | ||
| caftaric acid | 0.9973 | 2.0 | 9.9 | 101 ± 5 | 22 |
| protocatechualdehyde | 0.9906 | 8.6 | 14 | 75.3 ± 5.2 | 16 |
| procyanidine B1 | 0.9993 | 18 | 16 | 78 ± 11 | 17 |
| gentisicacid | 0.9996 | 14 | 16 | 90.5 ± 7.3 | 20 |
| catechin | 0.9917 | 6.5 | 15 | 61 ± 12 | 46 |
| procyanidine B2 | 0.9926 | 3.9 | 18 | 59.0 ± 5.1 | 18 |
| gentisaldehyde | 0.9993 | 5.2 | 15 | 69.2 ± 1.0 | 91 |
| chlorogenic acid | 0.9992 | 4.3 | 2.6 | 59.2 ± 2.7 | 7.1 |
| 3-hydroxybenzaldehyde | 0.9986 | 8.2 | 20 | 71.7 ± 4.4 | 17 |
| 4-hydroxybenzaldehyde | 0.9978 | 13 | 15 | 113 ± 2 | 35 |
| γ-resorcylic acid | 0.9949 | 1.2 | 4.7 | 141 ± 2 | 14 |
| α-resorcylic acid | 0.9903 | 7.7 | 5.3 | 133 ± 7 | 12 |
| veratric acid | 0.9988 | 16 | 14 | 80.5 ± 4.6 | 40 |
| caffeic acid | 0.9962 | 4.3 | 5.6 | 76.1 ± 0.8 | 8.8 |
| epicatechin | 0.9946 | 11 | 9.7 | 54.7 ± 0.6 | 6.9 |
| epigallocatechin gallate | 0.9940 | 18 | 16 | 76.1 ± 8.0 | 121 |
| gallocatechin gallate | 0.9990 | 17 | 3.9 | 45.1 ± 8.3 | 79 |
| procyanidine A2 | 0.9975 | 1.6 | 6.4 | 83.8 ± 4.2 | 12 |
| umbelliferone | 0.9928 | 8.9 | 12 | 106 ± 1 | 8.0 |
| p-coumaric acid | 0.9980 | 2.6 | 6.8 | 66.6 ± 1.7 | 7.9 |
| catechin gallate | 0.9988 | 7.9 | 11 | 71.7 ± 0.4 | 16 |
| veratraldehyde | 0.9999 | 4.2 | 4.1 | 77.3 ± 1.0 | 10 |
| 4-anisaldehyde | 0.9953 | 5.2 | 9.1 | 88.9 ± 6.2 | 30 |
| miquelianin | 0.9928 | 13 | 6.1 | 115 | 192 |
| rutin | 0.9928 | 8.3 | 13 | 95.3 ± 6.7 | 29 |
| isoquercitrin | 0.9915 | 15 | 2.1 | - | 163 |
| myricetin | 0.9982 | 10 | 18 | 103 ± 19 | 216 |
| 3,4,5-trimethoxycinnamic acid | 0.9944 | 12 | 12 | 86.9 ± 5.3 | 21 |
| 3,5-dimethoxybenzaldehyde | 0.9985 | 15 | 13 | 76.5 ± 9.5 | 156 |
| quercetin | 0.9992 | 10 | 19 | - | 39 |
| kaempferol | 0.9906 | 5.9 | 9.7 | 61.5 ± 5.9 | 45 |
| apigenin | 0.9168 | 7.5 | 5.4 | 53.8 ± 2.8 | 7.0 |
| chrysin | 0.9948 | 10 | 6.6 | 70.3 ± 13 | 55 |
| trans-ferulic acid | 0.9972 | 7.5 | 4.2 | 105 ± 2.2 | 41 |
| vanillic acid | 0.9980 | 2.1 | 5.5 | 87.0 ± 5.7 | 24 |
| p-hydroxybenzoic acid | 0.9922 | 9.8 | 4.9 | n.c. | 7.0 |
| 3-hydroxyphenylacetic acid | 0.9977 | 7.1 | 4.4 | n.c. | 60 |
| Phenolic Compounds | EU (N = 12) | BL (N = 8) | CN (N = 12) | CN/HD (N = 7) | HD (N = 12) | HE (N = 4) | MF (N = 36) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Range | Mean | Median | N | Range | Mean | Median | N | Range | Mean | Median | N | Range | Mean | Median | N | Range | Mean | Median | N | Range | Mean | Median | N | Range | Mean | Median | |
| gallic acid | 7 | 0.13–0.47 | 0.26 | 0.22 | 8 | 0.29–2.1 | 0.97 | 0.56 | 9 | 0.18–3.3 | 0.92 | 0.46 | 7 | 1.6–6.8 | 4.3 | 5.2 | 12 | 1.6–9.8 | 5.5 | 6.4 | 2 | 0.20–1.1 | 0.65 | 0.65 | 28 | 0.15–4.5 | 0.73 | 0.30 |
| phloroglucinic acid | 0 | 2 | 0.21–0.34 | 0.28 | 0.28 | 2 | 0.13–0.48 | 0.12 | 0.12 | 4 | 0.29–0.87 | 0.47 | 0.36 | 2 | 0.3–1.4 | 0.86 | 0.86 | 1 | 0.38 | 0.38 | 0.38 | 3 | 0.18–0.32 | 0.27 | 0.32 | |||
| β-resorcylic acid/protocatechuic acid | 12 | 0.22–0.55 | 0.40 | 0.41 | 8 | 0.42–4.6 | 1.9 | 1.5 | 12 | 0.11–0.13 | 1.5 | 0.74 | 7 | 2.5–8.0 | 5.5 | 6.1 | 12 | 3.3–10 | 7.0 | 6.8 | 4 | 0.36–2.5 | 0.95 | 0.49 | 36 | 0.26–11 | 1.5 | 0.72 |
| caftaric acid | 2 | 0.06–0.10 | 0.08 | 0.08 | 1 | 0.05 | 0.05 | 0.05 | 0 | 0 | 0 | 0 | 1 | 0.08 | 0.08 | 0.08 | ||||||||||||
| protocatechualdehyde | 8 | 0.05–0.27 | 0.12 | 0.09 | 5 | 0.07–0.19 | 0.14 | 0.15 | 9 | 0.09–0.25 | 0.17 | 0.19 | 1 | 0.05 | 0.05 | 0.05 | 0 | 3 | 0.16–0.23 | 0.20 | 0.22 | 29 | 0.05–0.43 | 0.15 | 0.12 | |||
| gentisic acid | 11 | 0.07–0.44 | 0.17 | 0.11 | 8 | 0.10–1.3 | 0.69 | 0.70 | 12 | 0.16–2.0 | 0.90 | 0.80 | 7 | 1.7–3.0 | 2.4 | 2.6 | 12 | 1.6–3.8 | 2.7 | 2.7 | 4 | 0.12–0.98 | 0.36 | 0.18 | 32 | 0.06–5.2 | 1.2 | 0.87 |
| gentisaldehyde | 3 | 0.31–0.54 | 0.45 | 0.49 | 0 | 4 | 0.18–0.43 | 0.27 | 0.23 | 1 | 0.64 | 0.64 | 0.64 | 0 | 1 | 0.32 | 0.32 | 0.32 | 2 | 0.22–0.27 | 0.25 | 0.25 | ||||||
| chlorogenic acid | 5 | 0.05–0.10 | 0.09 | 0.10 | 6 | 0.05–0.09 | 0.07 | 0.07 | 10 | 0.05–0.18 | 0.08 | 0.07 | 2 | 0.06–0.15 | 0.11 | 0.11 | 4 | 0.06–0.10 | 0.08 | 0.08 | 3 | 0.05–0.12 | 0.09 | 0.11 | 25 | 0.05–0.19 | 0.10 | 0.08 |
| 3-hydroxybenzaldehyde | 4 | 0.13–0.87 | 0.59 | 0.68 | 2 | 0.10–0.22 | 0.16 | 0.16 | 1 | 0.30 | 0.30 | 0.30 | 0 | 1 | 0.10 | 0.10 | 0.10 | 2 | 0.73–1.2 | 0.97 | 0.97 | 4 | 0.12–0.97 | 0.44 | 0.33 | |||
| 4-hydroxybenzaldehyde | 4 | 1.2–1.3 | 1.3 | 1.3 | 1 | 0.28 | 0.28 | 0.28 | 4 | 0.33–1.5 | 0.71 | 0.51 | 0 | 0 | 3 | 1.2–1.8 | 1.5 | 1.7 | 9 | 0.10–2.0 | 0.82 | 0.32 | ||||||
| γ-resorcylic acid | 3 | 0.05–0.07 | 0.06 | 0.06 | 6 | 0.05–0.09 | 0.07 | 0.07 | 10 | 0.05–0.12 | 0.09 | 0.10 | 6 | 0.06–0.10 | 0.09 | 0.10 | 11 | 0.05–0.12 | 0.08 | 0.07 | 1 | 0.08 | 0.08 | 0.08 | 18 | 0.05–0.31 | 0.10 | 0.10 |
| α-resorcylic acid | 2 | 0.06–0.07 | 0.07 | 0.07 | 6 | 0.05–0.08 | 0.06 | 0.06 | 9 | 0.08–0.11 | 0.09 | 0.09 | 6 | 0.05–0.10 | 0.08 | 0.09 | 7 | 0.06–0.13 | 0.09 | 0.08 | 1 | 0.07 | 0.07 | 0.07 | 23 | 0.06–0.28 | 0.09 | 0.08 |
| veratric acid | 0 | 2 | 0.2–1.9 | 1.6 | 1.6 | 1 | 1.6 | 1.6 | 1.6 | 0 | 1 | 1.8 | 1.8 | 1.8 | 0 | 2 | 0.89–1.1 | 1.1 | 1.1 | |||||||||
| caffeic acid | 12 | 0.07–0.37 | 0.20 | 0.17 | 8 | 0.35–0.81 | 0.55 | 0.57 | 12 | 0.06–0.85 | 0.38 | 0.30 | 7 | 0.22–0.62 | 0.37 | 0.33 | 12 | 0.07–0.57 | 0.28 | 0.30 | 4 | 0.12–0.42 | 0.22 | 0.17 | 36 | 0.05–0.64 | 0.27 | 0.26 |
| p-coumaric acid | 12 | 0.48–4.2 | 1.3 | 0.85 | 8 | 0.44–4.1 | 2.2 | 2.1 | 12 | 0.5–11 | 3.3 | 2.0 | 7 | 1.4–6.7 | 3.3 | 1.7 | 12 | 0.27–3.5 | 1.7 | 1.5 | 4 | 0.05–2.6 | 0.87 | 0.41 | 36 | 0.14–12 | 4.02 | 2.25 |
| veratraldehyde | 7 | 0.05–0.12 | 0.07 | 0.07 | 1 | 0.09 | 0.09 | 0.09 | 0 | 0 | 2 | 0.06–0.07 | 0.07 | 0.07 | 2 | 0.06–0.10 | 0.08 | 0.08 | 12 | 0.05–0.45 | 0.14 | 0.06 | ||||||
| 4-anisaldehyde | 1 | 0.28 | 0.28 | 0.28 | 0 | 3 | 0.13–0.41 | 0.26 | 0.24 | 0 | 0 | 3 | 0.14–0.77 | 0.50 | 0.59 | 10 | 0.12–0.72 | 0.33 | 0.22 | |||||||||
| quercetin | 7 | 0.25–0.80 | 0.51 | 0.51 | 7 | 0.25–1.4 | 0.83 | 1.02 | 10 | 0.22–1.3 | 0.46 | 0.38 | 5 | 0.39–0.62 | 0.46 | 0.42 | 11 | 0.16–0.63 | 0.35 | 0.31 | 1 | 0.79 | 0.79 | 0.79 | 28 | 0.29–1.1 | 0.56 | 0.51 |
| kaempferol | 3 | 0.23–0.44 | 0.31 | 0.25 | 6 | 0.25–0.49 | 0.40 | 0.45 | 3 | 0.27–0.60 | 0.44 | 0.44 | 4 | 0.24–0.41 | 0.34 | 0.35 | 7 | 0.22–0.38 | 0.28 | 0.26 | 2 | 0.25–0.31 | 0.28 | 0.28 | 15 | 0.22–0.71 | 0.34 | 0.33 |
| apigenin | 10 | 0.06–0.39 | 0.15 | 0.12 | 8 | 0.13–0.31 | 0.22 | 0.23 | 12 | 0.07–0.31 | 0.15 | 0.13 | 7 | 0.09–0.21 | 0.14 | 0.10 | 11 | 0.06–0.20 | 0.13 | 0.15 | 4 | 0.08–0.19 | 0.13 | 0.13 | 32 | 0.05–0.43 | 0.15 | 0.13 |
| chrysin | 12 | 0.50–3.4 | 1.9 | 1.9 | 8 | 2.2–5.9 | 3.8 | 3.4 | 12 | 0.53–4.5 | 2.8 | 2.9 | 7 | 1.7–4.2 | 2.6 | 2.2 | 12 | 0.94–4.4 | 2.6 | 2.5 | 4 | 1.8–3.1 | 2.3 | 2.1 | 36 | 0.51–5.9 | 2.63 | 2.40 |
| trans-ferulic acid | 11 | 0.48–0.91 | 0.69 | 0.69 | 8 | 0.62–1.7 | 1.1 | 1.2 | 8 | 0.36–2.0 | 0.93 | 0.90 | 7 | 0.42–1.0 | 0.72 | 0.74 | 10 | 0.27–0.96 | 0.6 | 0.7 | 4 | 0.15–1.1 | 0.48 | 0.34 | 23 | 0.19–1.5 | 0.64 | 0.64 |
| p-hydroxybenzoic acid | 12 | 0.77–2.3 | 1.4 | 1.3 | 8 | 0.68–2.3 | 1.4 | 1.4 | 12 | 1.2–3.4 | 2.0 | 1.8 | 7 | 1.1–2.5 | 1.8 | 1.7 | 12 | 0.83–2.9 | 1.6 | 1.6 | 4 | 1.4–4.0 | 2.7 | 2.6 | 36 | 0.77–5.1 | 2.3 | 2.1 |
| 3-hydroxyphenylacetic acid | 5 | 12–123 | 66 | 61 | 3 | 3.7–63 | 32 | 29 | 3 | 2.8–12 | 7.5 | 8.0 | 2 | 1.7–9.5 | 5.6 | 5.6 | 7 | 0.41–11 | 4.2 | 2.0 | 3 | 54–242 | 140 | 125 | 10 | 1.3–138 | 24 | 9.9 |
| vanillic acid | 0 | 1 | 0.27 | 0.27 | 0.27 | 2 | 0.07–0.20 | 0.14 | 0.14 | 2 | 0.15–0.40 | 0.28 | 0.28 | 8 | 0.14–6.2 | 1.1 | 0.30 | 2 | 0.10–0.12 | 0.11 | 0.11 | 6 | 0.07–0.58 | 0.31 | 0.28 | |||
| ∑ [phenolic compounds] | 12 | 3.4–130 | 35 | 11 | 8 | 11–71 | 27 | 16 | 12 | 7.1–31 | 16 | 15 | 7 | 21–30 | 25 | 23 | 12 | 18–35 | 26 | 26 | 4 | 15–252 | 116 | 99 | 36 | 6.5–144 | 21 | 15 |
| TPC (mg GAE 100g−1) | 12 | 48–139 | 85 | 66 | 8 | 48–111 | 85 | 96 | 12 | 64–192 | 118 | 119 | 7 | 119–192 | 148 | 147 | 12 | 95–197 | 152 | 151 | 4 | 102–203 | 163 | 173 | 36 | 55–193 | 116 | 116 |
| AA (µmol TRE 100g−1) | 12 | 15–846 | 239 | 176 | 8 | 111–428 | 232 | 211 | 12 | 140–540 | 265 | 210 | 7 | 228–854 | 592.43 | 611 | 12 | 420–1017 | 756 | 852 | 4 | 138–392 | 272 | 279 | 36 | 56–837 | 252 | 236 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vazquez, L.; Armada, D.; Celeiro, M.; Dagnac, T.; Llompart, M. Evaluating the Presence and Contents of Phytochemicals in Honey Samples: Phenolic Compounds as Indicators to Identify Their Botanical Origin. Foods 2021, 10, 2616. https://doi.org/10.3390/foods10112616
Vazquez L, Armada D, Celeiro M, Dagnac T, Llompart M. Evaluating the Presence and Contents of Phytochemicals in Honey Samples: Phenolic Compounds as Indicators to Identify Their Botanical Origin. Foods. 2021; 10(11):2616. https://doi.org/10.3390/foods10112616
Chicago/Turabian StyleVazquez, Lua, Daniel Armada, Maria Celeiro, Thierry Dagnac, and Maria Llompart. 2021. "Evaluating the Presence and Contents of Phytochemicals in Honey Samples: Phenolic Compounds as Indicators to Identify Their Botanical Origin" Foods 10, no. 11: 2616. https://doi.org/10.3390/foods10112616
APA StyleVazquez, L., Armada, D., Celeiro, M., Dagnac, T., & Llompart, M. (2021). Evaluating the Presence and Contents of Phytochemicals in Honey Samples: Phenolic Compounds as Indicators to Identify Their Botanical Origin. Foods, 10(11), 2616. https://doi.org/10.3390/foods10112616