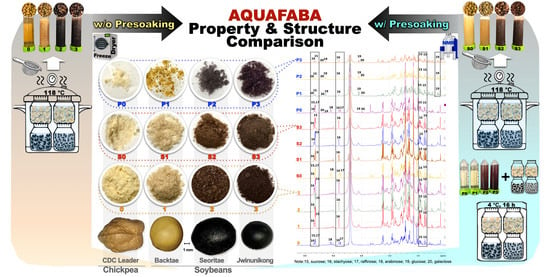
Aquafaba from Korean Soybean II: Physicochemical Properties and Composition Characterized by NMR Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. AQ Preparation from Seed
2.2.1. AQ Produced without Presoaking
2.2.2. AQ Produced with Presoaking
2.3. Seed Physical Properties
2.4. Seed Hydration Kinetics
2.5. Color
2.6. NMR Spectroscopy
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physical Characteristics of Dried Seed
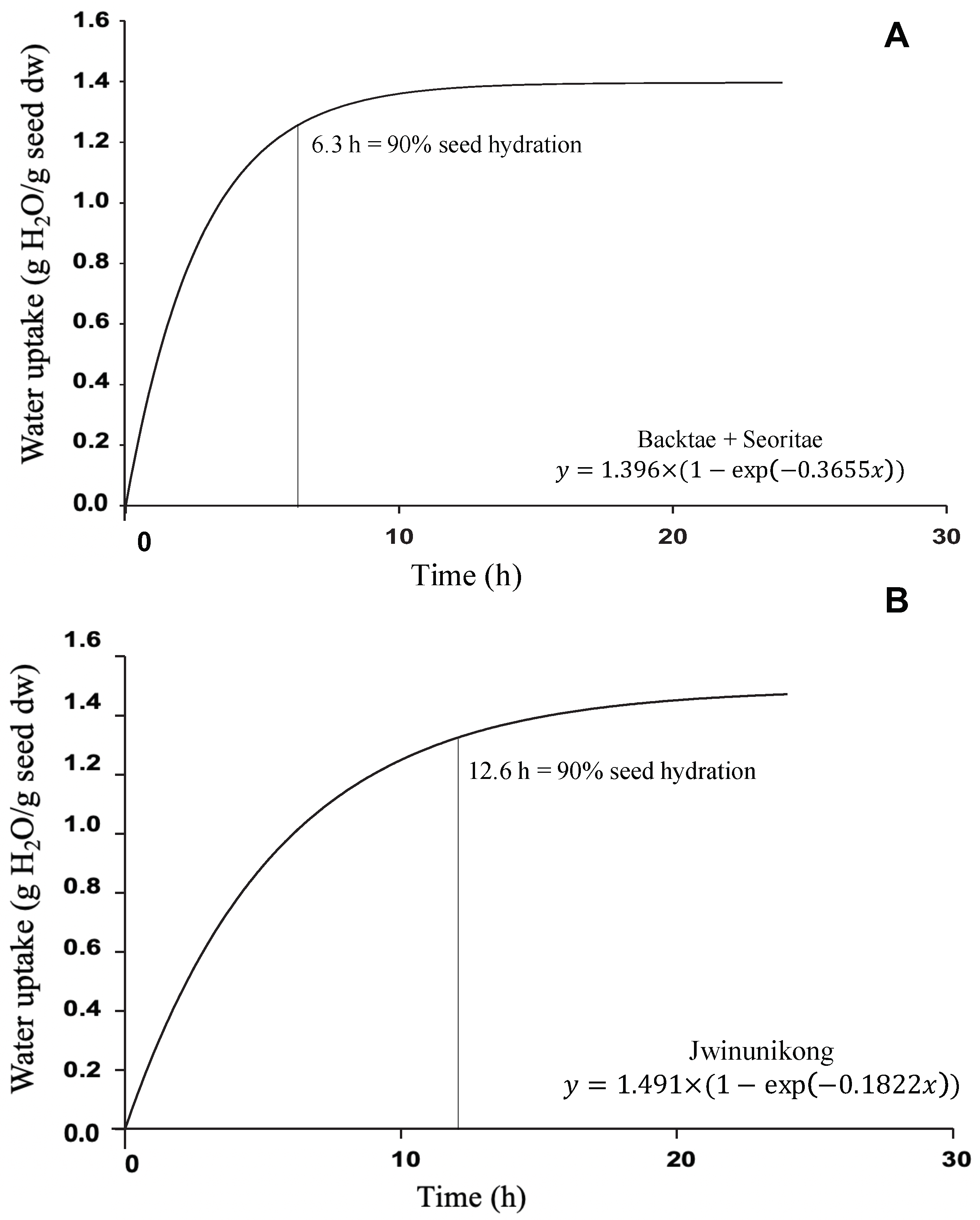
3.2. Seed Hydration Kinetics
3.3. Color Analysis
3.4. Correlation Analysis
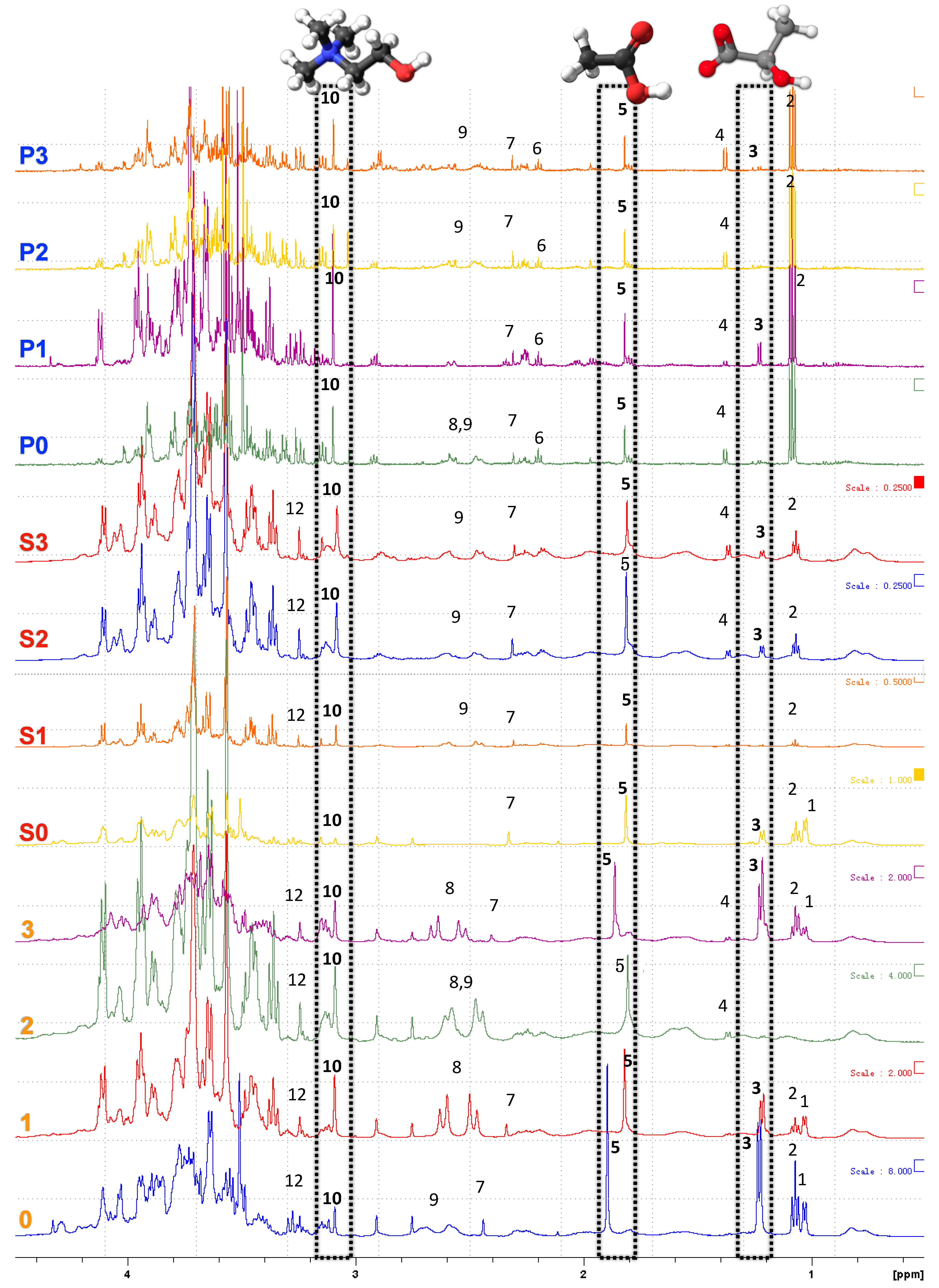
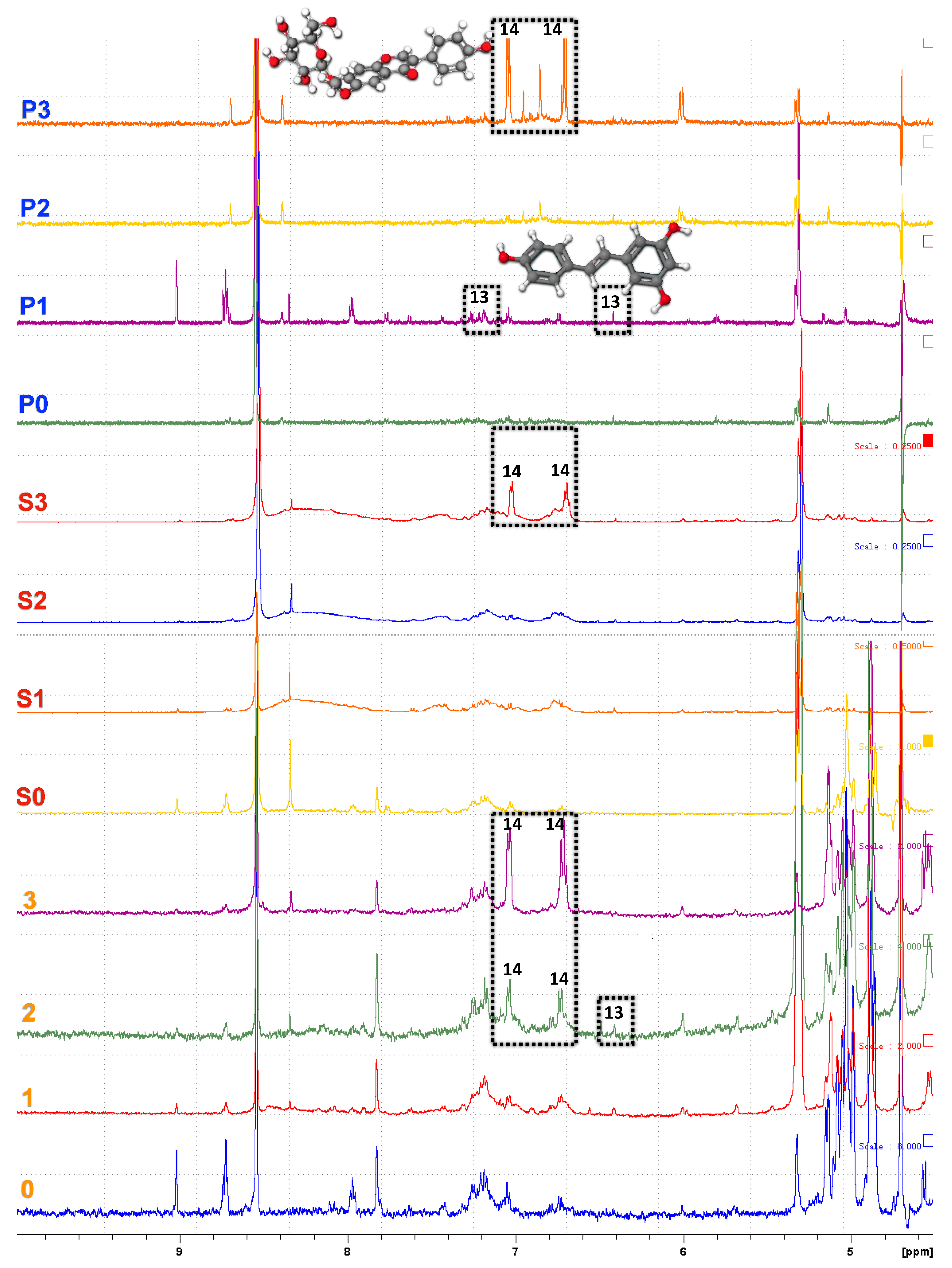
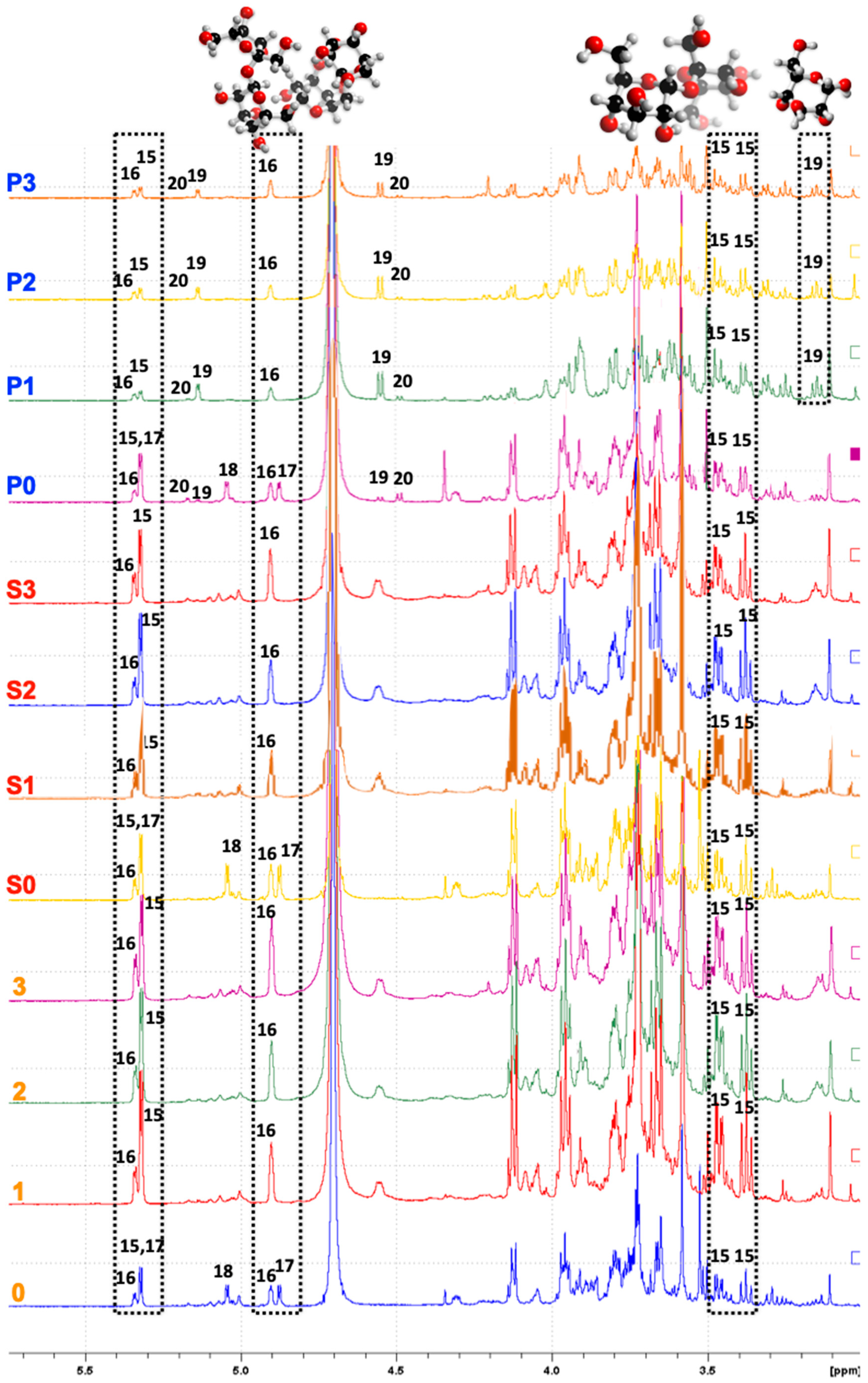
3.5. NMR Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghoush, M.A.; Samhouri, M.; Al-Holy, M.; Herald, T. Formulation and fuzzy modeling of emulsion stability and viscosity of a gum–protein emulsifier in a model mayonnaise system. J. Food Eng. 2008, 84, 348–357. [Google Scholar] [CrossRef]
- Raymundo, A.; Franco, J.M.; Empis, J.; Sousa, I. Optimization of the composition of low-fat oil-in-water emulsions stabilized by white lupin protein. J. Am. Oil Chem. Soc. 2002, 79, 783–790. [Google Scholar] [CrossRef]
- Isanga, J.; Zhang, G.N. Soybean bioactive components and their implications to health-a review. Food Rev. Int. 2008, 24, 252–276. [Google Scholar] [CrossRef]
- Uesugi, T.; Fukui, Y.; Yamori, Y. Beneficial effects of soybean isoflavone supplementation on bone metabolism and serum lipids in postmenopausal Japanese women: A four-week study. J. Am. Coll. Nutr. 2002, 21, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Y.; Park, H.M.; Hwang, T.Y.; Kim, S.L.; Kim, M.J.; Lee, S.K.; Seo, M.J.; Kim, K.J.; Kwon, Y.; Lee, S.C.; et al. A correlation between tocopherol content and antioxidant activity in seeds and germinating seeds of soybean cultivars. J. Sci. Food Agric. 2015, 95, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.J.; Kim, C.S.; Choi, M.S.; Park, T.; Sung, M.K.; Yun, J.W.; Yoo, H.; Mine, Y.; Yu, R. The soy peptide PheLeu-Val reduces TNF-α-induced inflammatory response and insulin resistance in adipocytes. J. Med. Food 2016, 19, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, R.; Reaney, M.J.T. Aquafaba, from food waste to a value-added product. In Food Wastes and by-Products: Nutraceutical & Health Potential; Campos-Vega, R., Oomah, B.D., Vergara-Castañeda, H.A., Eds.; John Wiley & Sons Ltd.: New York, NY, USA, 2020; Chapter 4; pp. 93–126. [Google Scholar]
- He, Y.; Meda, V.; Reaney, M.J.T.; Mustafa, R. Aquafaba, a new plant-based rheological additive for food applications. Trends Food Sci. Technol. 2021, 111, 27–42. [Google Scholar] [CrossRef]
- Bird, L.G.; Pilkington, C.L.; Saputra, A.; Serventi, L. Products of chickpea processing as texture improvers in gluten-free bread. Food Sci. Technol. Int. 2017, 23, 690–698. [Google Scholar] [CrossRef]
- He, Y.; Shim, Y.Y.; Mustafa, R.; Meda, V.; Reaney, M.J.T. Chickpea cultivar selection to produce aquafaba with superior emulsion properties. Foods 2019, 8, 685. [Google Scholar] [CrossRef] [Green Version]
- Raikos, V.; Hayes, H.; Ni, H. Aquafaba from commercially canned chickpeas as potential egg replacer for the development of vegan mayonnaise: Recipe optimisation and storage stability. Int. J. Food Sci. Technol. 2020, 55, 1935–1942. [Google Scholar] [CrossRef]
- Shim, Y.Y.; Mustafa, R.; Shen, J.; Ratanapariyanuch, K.; Reaney, M.J.T. Composition and properties of aquafaba: Water recovered from commercially canned chickpeas. J. Vis. Exp. 2018, 2018, 56305. [Google Scholar] [CrossRef]
- Buhl, T.F.; Christensen, C.H.; Hammershøj, M. Aquafaba as an egg white substitute in food foams and emulsions: Protein composition and functional behavior. Food Hydrocoll. 2019, 96, 354–364. [Google Scholar] [CrossRef]
- Alsalman, F.B.; Tulbek, M.; Nickerson, M.; Ramaswamy, H.S. Evaluation and optimization of functional and antinutritional properties of aquafaba. Legum. Sci. 2020, 2, e30. [Google Scholar] [CrossRef]
- Stantiall, S.E.; Dale, K.J.; Calizo, F.S.; Serventi, L. Application of pulses cooking water as functional ingredients: The foaming and gelling abilities. Eur. Food Res. Technol. 2018, 244, 97–104. [Google Scholar] [CrossRef]
- Damian, J.J.; Huo, S.; Serventi, L. Phytochemical content and emulsifying ability of pulses cooking water. Eur. Food Res. Technol. 2018, 244, 1647–1655. [Google Scholar] [CrossRef]
- Serventi, L.; Wang, S.; Zhu, J.; Liu, S.; Fei, F. Cooking water of yellow soybeans as emulsifier in gluten-free crackers. Eur. Food Res. Technol. 2018, 244, 2141–2148. [Google Scholar] [CrossRef]
- Shim, Y.Y.; He, Y.; Kim, J.H.; Cho, J.Y.; Meda, V.; Hong, W.S.; Shin, W.-S.; Kang, S.J.; Reaney, M.J.T. Aquafaba from Korean soybean I: A functional vegan food additive. Foods 2021, 10, 2433. [Google Scholar] [CrossRef]
- He, Y.; Purdy, S.K.; Tse, T.J.; Tar’an, B.; Meda, V.; Reaney, M.J.T.; Mustafa, R. Standardization of aquafaba production and application in vegan mayonnaise analogs. Foods 2021, 10, 1978. [Google Scholar] [CrossRef]
- Avola, G.; Patanè, C. Variation among physical, chemical, and technological properties in three Sicilian cultivars of Chickpea (Cicer arietinum L.). Int. J. Food Sci. Technol. 2010, 45, 2565–2572. [Google Scholar] [CrossRef]
- Mohsenin, N.N. Physical Properties of Plant. and Animal Materials; Gordon and Breach Science Publishers: New York, NY, USA, 1970. [Google Scholar]
- Borges, C.W.C.; de Jorge, L.M.M.; Jorge, R.M.M. Modeling and thermodynamic properties of soybean cultivar BRS257 hydration. J. Food Process Eng. 2019, 42, e12970. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Hua, Y.; Chen, Y.; Kong, X.; Zhang, C. Effects of water absorption of soybean seed on the quality of soymilk and the release of flavor compounds. RSC Adv. 2019, 9, 2906. [Google Scholar] [CrossRef] [Green Version]
- Chopra, R.; Prasad, D.N. Standardization of soaking conditions for soybean seeds/cotyledons for improved quality of soymilk. Indian J. Anim. Sci. 1994, 64, 405–410. [Google Scholar]
- Pan, Z.; Tangratanavalee, W. Characteristics of soybean as affected by soaking conditions. LWT-Food Sci. Technol. 2003, 36, 143–151. [Google Scholar] [CrossRef]
- Dutta, A.; Mukherjee, R.; Sarkar, T.; Pinar, Z.; Chakraborty, R. A linear driving force (LDF) approximation of moisture uptake kinetics in soybean. Int. J. Agric. Food Sci. Technol. 2014, 5, 203–210. [Google Scholar]
- Leopold, A.C. Volumetric components of seed imbibition. Plant Physiol. 1983, 73, 677–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbo, S.; Molina, C.; Jungmann, R.; Grusak, M.A.; Berkovitch, Z.; Reifen, R.; Kahl, G.; Winter, P.; Reifen, R. Quantitative trait loci governing carotenoid concentration and weight in seeds of chickpea (Cicer arietinum L.). Theor. Appl. Genet. 2005, 111, 185–195. [Google Scholar] [CrossRef]
- Snyder, H.E.; Kwon, T.W. Soybean Utilization; Van Nostrand Reinhold Company Inc.: New York, NY, USA, 1987. [Google Scholar]
- Bayram, M.; Kaya, A.; Oner, M.D. Changes in properties of soaking water during production of soy-bulgur. J. Food Eng. 2004, 61, 221–230. [Google Scholar] [CrossRef]
- Mukherjee, R.; Chakraborty, R.; Dutta, A. Soaking of soybean meal: Evaluation of physicochemical properties and kinetic studies. J. Food Meas. Charact. 2019, 13, 390–403. [Google Scholar] [CrossRef]
- Eum, H.L.; Park, Y.; Yi, T.G.; Lee, J.W.; Ha, K.S.; Choi, I.Y.; Park, N.I. Effect of germination environment on the biochemical compounds and anti-inflammatory properties of soybean cultivars. PLoS ONE 2020, 15, e0232159. [Google Scholar] [CrossRef] [PubMed]
- Lyimo, M.; Mugula, J.; Elias, T. Nutritive composition of broth from selected bean varieties cooked for various periods. J. Sci. Food Agric. 1992, 58, 535–539. [Google Scholar] [CrossRef]
- Jeong, I.-H.; Oh, M.-S.; Jeon, J.-S.; Kim, H.-T.; Hong, S.-R.; Park, K.-H.; Yoon, M.-H. A Comparative Study on Anthocyanin and Polyphenol Contents in Colored Agricultural Products. J. Food Hyg. Saf. 2017, 32, 371–380. [Google Scholar] [CrossRef]
- Güçlü-Üstündağ, Ö.; Mazza, G. Saponins: Properties, Applications and Processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258. [Google Scholar] [CrossRef] [PubMed]
- Vega, C.; Grover, M.K. Physicochemical properties of acidified skim milk gels containing cocoa flavanols. J. Agric. Food Chem. 2011, 59, 6740–6747. [Google Scholar] [CrossRef]
- Wu, W.; Clifford, M.; Howell, N.K. The effect of instant green tea on the foaming and rheological properties of egg albumen proteins. J. Sci. Food Agric. 2007, 87, 1810–1819. [Google Scholar] [CrossRef]
- Boyaci Gunduz, C.P.; Gaglio, R.; Franciosi, E.; Settanni, L.; Erten, H. Molecular analysis of the dominant lactic acid bacteria of chickpea liquid starters and doughs and propagation of chickpea sourdoughs with selected Weissella Confusa. Food Microbiol. 2020, 91, 103490. [Google Scholar] [CrossRef]
- Bae, E.A.; Moon, G.S. A study on the antioxidative activities of Korean soybeans. J. Korean Soc. Food Sci. Nutr. 1997, 26, 203–208. [Google Scholar]
- Shin, J.; Joo, N. Component changes in antioxidant activity and isoflavones (β-glucoside & aglycone) contents of small black bean according to different cooking methods. Korean J. Food Cook. Sci. 2016, 32, 197–203. [Google Scholar]
- King, R.E.; Bomser, J.A.; Min, D.B. Bioactivity of resveratrol. Comp. Rev. Food Sci. Food Saf. 2006, 5, 65–70. [Google Scholar] [CrossRef]
- Filip, V.; Plockova, M.; Smidrkal, J.; Spickova, Z.; Melzoch, K.; Schmidt, S. Resveratrol and its antioxidant and antimicrobial effectiveness. Food Chem. 2003, 83, 585–593. [Google Scholar] [CrossRef]
- Attia, R.S.; Shehata, A.M.E.T.; Aman, M.E.; Hamza, M.A. Effect of cooking and decortication on the physical properties, the chemical composition, and the nutritive value of chickpea (Cicer arietinum L.). Food Chem. 1994, 50, 125–131. [Google Scholar] [CrossRef]
- Frias, J.; Vidal-Valverde, C.; Sotomayor, C.; Diaz-Pollan, C.; Urbano, G. Influence of processing on available carbohydrate content and antinutritional factors of chickpeas. Eur. Food Res. Technol. 2000, 210, 340–345. [Google Scholar] [CrossRef]
- El-Adawy, T.A. Nutritional composition and antinutritional factors of chickpeas (Cicer arietinum L.) undergoing different cooking methods and germination. Plant Foods Hum. Nutr. 2002, 57, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Mokni Ghribi, A.; Sila, A.; Maklouf Gafsi, I.; Blecker, C.; Danthine, S.; Attia, H.; Bougatef, A.; Besbes, S. Structural, functional, and ACE inhibitory properties of water-soluble polysaccharides from chickpea flours. Int. J. Biol. Macromol. 2015, 75, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Iyer, V.; Kadam, S.S.; Salunkhe, D.K. Cooking. In Handbook of World Food Legumes: Nutritional Chemistry, Processing Technology and Utilization; Salunkhe, D.K., Kadam, S.S., Eds.; CRC Press Inc.: Boca Raton, FL, USA, 1989; Volume 3, pp. 141–163. [Google Scholar]
- Chau, C.F.; Cheung, P.C.K.; Wong, Y.S. Effects of cooking on content of amino acids and antinutrients in three Chinese indigenous legume seeds. J. Sci. Food Agric. 1997, 75, 447–452. [Google Scholar] [CrossRef]
- Pedrosa, M.M.; Cuadrado, C.; Burbano, C.; Allaf, K.; Haddad, J.; Gelencsér, E.; Takács, K.; Guillamón, E.; Muzquiz, M. Effect of instant controlled pressure drop on the oligosaccharides, inositol phosphates, trypsin inhibitors and lectins contents of different legumes. Food Chem. 2012, 131, 862–868. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Singh, N.; Kaur, A. Saponins in pulses and their health promoting activities: A review. Food Chem. 2017, 233, 540–549. [Google Scholar] [CrossRef]
- Chigwedere, C.M.; Njoroge, A.M.; Van Loey, A.M.; Hendrickx, M.E. Understanding the relations among the storage, soaking, and cooking behavior of pulses: A scientific basis for innovations in sustainable foods for the future. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1135–1165. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Mu, K.; Arntfield, S.D.; Nickerson, M.T. Changes in levels of enzyme inhibitors during soaking and cooking for pulses available in Canada. J. Food Sci. Technol. 2017, 54, 1014–1022. [Google Scholar] [CrossRef] [Green Version]
- Patterson, C.A.; Curran, J.; Der, T. Effect of Processing on Antinutrient Compounds in Pulses. Cereal Chem. 2017, 94, 2–10. [Google Scholar] [CrossRef]
- Chen, X.; Singh, M.; Bhargava, K.; Ramanathan, R. Yogurt fortification with chickpea (Cicer arietinum) flour: Physicochemical and sensory effects. J. Am. Oil Chem. Soc. 2018, 95, 1041–1048. [Google Scholar] [CrossRef]
- Hernandez-Hernandez, O.; Muthaiyan, A.; Moreno, F.J.; Montilla, A.; Sanz, M.L.; Ricke, S.C. Effect of prebiotic carbohydrates on the growth and tolerance of Lactobacillus. Food Microbiol. 2012, 30, 355–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zare, F.; Champagne, C.; Simpson, B.; Orsat, V.; Boye, J. Effect of the addition of pulse ingredients to milk on acid production by probiotic and yoghurt starter cultures. LWT-Food Sci. Technol. 2012, 45, 155–160. [Google Scholar] [CrossRef]
- Bakr, S.A. Nutritional and therapeutical values of chickpea water extract enriched yogurt made from cow and camel milk. Am. J. Drug Discov. Dev. 2013, 3, 47–59. [Google Scholar] [CrossRef] [Green Version]



| Species/Cultivar (Code) | Canadian Chickpea 1 | Korean Soybeans 2 | |||
|---|---|---|---|---|---|
| Group | Cicer arietinum (L.) | Glycine max (L.) Merr. | Rhynchosia nulubilis | ||
| CDC Leader | Backtae | Seoritae | Jwinunikong | ||
| Presoaking water | P0 | P1 | P2 | P3 | |
| w/o Presoaking AQ 3 | 0 | 1 | 2 | 3 | |
| w/Presoaking AQ 4 | S0 | S1 | S2 | S3 | |
| Characteristics | Unit | CDC Leader 1 | Backtae | Seoritae | Jwinunikong |
|---|---|---|---|---|---|
| Physical | |||||
| HSW | g | 42.90 ± 0.31 a | 27.97 ± 0.46 c | 32.52 ± 0.93 b | 11.44 ± 0.06 d |
| ED | mm | 8.49 ± 0.21 a | 7.29 ± 0.28 b | 8.20 ± 0.72 a | 5.68 ± 0.35 c |
| SCI | % | 3.89 ± 0.30 c | 5.65 ± 0.19 b | 7.79 ± 1.29 a | 8.21 ± 0.67 a |
| SSA | mm2/mg | 0.528 ± 0.03 b | 0.597 ± 0.046 b | 0.654 ± 0.111 b | 0.890 ± 0.106 a |
| WSA | mg/cm2 | 7.39 ± 0.38 c | 9.51 ± 0.74 b | 12.23 ± 2.29 a | 9.35 ± 1.22 b |
| Technological | |||||
| Hmax | g (H2O g/dw) | 1.036 ± 0.022 b | 1.436 ± 0.017 a | 1.344 ± 0.040 a | 1.491 ± 0.144 a |
| Hrate | g (H2O g/min) | 0.3537 ± 0.0064 b | 0.3289 ± 0.0282 b | 0.4263 ± 0.0481 a | 0.1822 ± 0.0282 c |
| R2 | NA | 0.9948 | 0.9413 | 0.9478 | 0.9528 |
| Hydration Solution | Hmax g H2O/g Seed dw | Hrate g/min | R2 |
|---|---|---|---|
| Backtae | |||
| H2O | 1.429 | 0.3626 | 0.9524 |
| NaCl | 1.422 | 0.3199 | 0.9314 |
| NaHCO3 | 1.455 | 0.3092 | 0.9486 |
| Seoritae | |||
| H2O | 1.327 | 0.3911 | 0.9350 |
| NaCl | 1.318 | 0.4824 | 0.9476 |
| NaHCO3 | 1.392 | 0.4106 | 0.9741 |
| Backtae and Seoritae | 1.396 | 0.3655 | 0.9410 |
| Jwinunikong | |||
| H2O | 1.662 | 0.1527 | 0.9709 |
| NaCl | 1.403 | 0.2070 | 0.9584 |
| NaHCO3 | 1.422 | 0.1930 | 0.9483 |
| All data combined | 1.432 | 0.2798 | 0.8850 |
| Group | L* | a* | b* |
|---|---|---|---|
| Seed | |||
| CDC Leader | 62.98 ± 0.19 b | 8.54 ± 0.02 a | 16.77 ± 0.10 c |
| Backtae | 66.30 ± 0.19 a | 6.77 ± 0.10 b | 24.17 ± 0.12 a |
| Seoritae | 34.53 ± 0.11 e | −0.64 ± 0.08 h | −4.42 ± 0.06 kl |
| Jwinunikong | 34.76 ± 0.10 e | –0.61 ± 0.06 h | −4.89 ± 0.07 l |
| Presoaking water | |||
| P0 | 21.13 ± 0.13 i | −0.35 ± 0.11 h | −3.56 ± 0.22 ij |
| P1 | 19.13 ± 0.37 k | −0.70 ± 0.27 h | −3.91 ± 0.41 jk |
| P2 | 21.20 ± 0.44 i | 0.04 ± 0.06 gh | −3.29 ± 0.16 i |
| P3 | 19.97 ± 0.28 j | 0.16 ± 0.08 fg | −3.50 ± 0.08 ij |
| w/o Presoaking AQ | |||
| 0 | 46.35 ± 0.06 c | 2.55 ± 0.01 d | 21.47 ± 0.06 b |
| 1 | 33.08 ± 0.22 f | 5.27 ± 0.17 c | 14.43 ± 0.14 e |
| 2 | 16.10 ± 0.04 m | 2.05 ± 0.24 de | −1.75 ± 0.10 h |
| 3 | 17.08 ± 0.15 l | 2.20 ± 0.03 de | −1.26 ± 0.12 h |
| w/Presoaking AQ | |||
| S0 | 39.30 ± 0.14 d | 0.79 ± 0.02 fg | 15.55 ± 0.40 d |
| S1 | 38.99 ± 0.24 d | 1.25 ± 0.17 ef | 11.33 ± 0.06 f |
| S2 | 27.61 ± 0.40 g | 4.76 ± 0.12 c | 1.00 ± 0.18 g |
| S3 | 26.19 ± 0.54 h | 5.12 ± 0.25 c | 1.08 ± 0.07 g |
| AQ MO 1 | ES 1 | EC 1 | FC 1 | FS 1 | HSW | SCI | ED | SSA | WSA | CAR 1 | PRO 1 | Fiber 1 | Fat 1 | Ash 1 | Hmax | Hrate | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AQ YI (S1–S3) 1 | 0.983 | 0.911 | 0.953 | 0.209 | −0.472 | 0.536 | −0.996 | 0.396 | −0.815 | −0.223 | −0.926 | −0.948 | −0.578 | 0.988 | 0.645 | −0.104 | 0.357 |
| AQ YI (1–3) 1 | −0.952 | −0.698 | 0.201 | 0.748 | 0.108 | −0.828 | −0.122 | −0.907 | 0.555 | −0.981 | −0.404 | −0.345 | 0.799 | 0.184 | 0.782 | 0.991 | −0.924 |
| AQ MO (S1–S3) 1 | 0.971 | 0.881 | 0.385 | −0.302 | 0.682 | −0.961 | 0.558 | −0.908 | −0.040 | −0.841 | −0.873 | −0.718 | 0.942 | 0.493 | −0.285 | 0.522 | |
| AQ MO (1–3) 1 | −0.998 * | −0.513 | 0.103 | −0.592 | −0.989 | 0.581 | −0.953 | 0.971 | −0.591 | 0.320 | 0.380 | 0.995 | −0.528 | 0.156 | 0.820 | −0.940 | |
| ES (S1–S3) 1 | 0.743 | 0.594 | −0.066 | 0.837 | −0.868 | 0.740 | −0.982 | 0.199 | −0.687 | −0.732 | −0.863 | 0.835 | 0.271 | −0.506 | 0.711 | ||
| ES (1–3) 1 | 0.560 | −0.048 | 0.636 | 0.979 | −0.625 | 0.935 | −0.983 | 0.545 | −0.372 | 0.062 | −0.988 | 0.575 | −0.101 | −0.787 | 0.920 | ||
| EC (S1–S3) 1 | −0.097 | −0.717 | 0.255 | −0.977 | 0.099 | −0.602 | −0.508 | −0.997 * | −1.000 ** | −0.303 | 0.989 | 0.846 | 0.202 | 0.057 | |||
| EC (1–3) 1 | 0.801 | 0.996 | 0.382 | −0.997 * | 0.230 | −0.703 | −0.389 | −0.977 | −0.989 | −0.427 | 1.000 ** | 0.768 | 0.070 | 0.189 | |||
| FC (S1–S3) 1 | 0.764 | 0.937 | −0.117 | 0.981 | −0.736 | 0.907 | 0.176 | 0.113 | −0.919 | 0.054 | −0.613 | −0.994 | 0.988 | ||||
| FC (1–3) 1 | 0.741 | −0.248 | −0.750 | −0.398 | −0.136 | −0.863 | −0.909 | −0.881 | 0.199 | 0.790 | 0.999 ** | 0.654 | −0.437 | ||||
| FS (S1–S3) 1 | 0.491 | 0.552 | 0.623 | −0.126 | 0.965 | 0.770 | 0.728 | −0.447 | −0.604 | −0.978 | −0.828 | 0.655 | |||||
| FS (1–3) 1 | 0.467 | −1.000 ** | 0.321 | −0.767 | −0.300 | −0.953 | −0.970 | −0.511 | 0.997 * | 0.704 | −0.024 | 0.281 | |||||
| HSW | −0.455 | 0.987 | −0.926 | 0.703 | −0.177 | −0.240 | −0.999 ** | 0.398 | −0.299 | −0.895 | 0.980 | ||||||
| SCI | −0.308 | 0.758 | 0.314 | 0.957 | 0.974 | 0.499 | −0.998 * | −0.714 | 0.010 | −0.072 | |||||||
| ED | −0.854 | 0.807 | −0.020 | 0.083 | −0.978 | 0.248 | −0.447 | −0.955 | 0.999 ** | ||||||||
| SSA | −0.382 | 0.536 | 0.589 | 0.944 | −0.715 | −0.084 | 0.661 | −0.832 | |||||||||
| WSA | 0.575 | 0.522 | −0.667 | −0.373 | −0.889 | −0.946 | 0.831 | ||||||||||
| CAR 1 | 0.998 * | 0.226 | −0.973 | −0.886 | −0.279 | 0.071 | |||||||||||
| PRO 1 | −0.288 | −0.986 | −0.854 | −0.217 | −0.041 | ||||||||||||
| Fiber 1 | −0.443 | 0.251 | 0.872 | −0.969 | |||||||||||||
| Fat 1 | 0.756 | 0.052 | 0.207 | ||||||||||||||
| Ash 1 | 0.693 | −0.484 | |||||||||||||||
| Hmax | −0.966 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Shim, Y.Y.; Shen, J.; Kim, J.H.; Cho, J.Y.; Hong, W.S.; Meda, V.; Reaney, M.J.T. Aquafaba from Korean Soybean II: Physicochemical Properties and Composition Characterized by NMR Analysis. Foods 2021, 10, 2589. https://doi.org/10.3390/foods10112589
He Y, Shim YY, Shen J, Kim JH, Cho JY, Hong WS, Meda V, Reaney MJT. Aquafaba from Korean Soybean II: Physicochemical Properties and Composition Characterized by NMR Analysis. Foods. 2021; 10(11):2589. https://doi.org/10.3390/foods10112589
Chicago/Turabian StyleHe, Yue, Youn Young Shim, Jianheng Shen, Ji Hye Kim, Jae Youl Cho, Wan Soo Hong, Venkatesh Meda, and Martin J. T. Reaney. 2021. "Aquafaba from Korean Soybean II: Physicochemical Properties and Composition Characterized by NMR Analysis" Foods 10, no. 11: 2589. https://doi.org/10.3390/foods10112589
APA StyleHe, Y., Shim, Y. Y., Shen, J., Kim, J. H., Cho, J. Y., Hong, W. S., Meda, V., & Reaney, M. J. T. (2021). Aquafaba from Korean Soybean II: Physicochemical Properties and Composition Characterized by NMR Analysis. Foods, 10(11), 2589. https://doi.org/10.3390/foods10112589