Aquafaba from Korean Soybean I: A Functional Vegan Food Additive
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
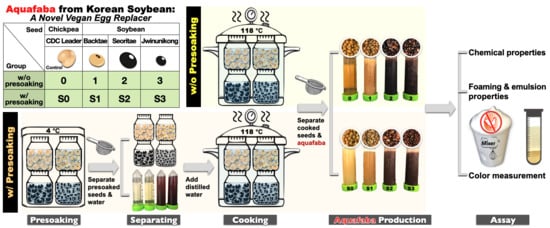
2.2. Fresh AQ Preparation
2.2.1. AQ Produced without Presoaking
2.2.2. AQ Produced with Presoaking
2.3. Chemical Properties
2.4. AQ Foaming Properties
2.5. AQ Emulsion Properties
2.5.1. Oil Emulsion Preparation
2.5.2. Emulsion Capacity
2.5.3. Emulsion Stability
2.6. Color
2.7. Statistical Analysis
3. Results and Discussion
3.1. Chemical Characteristics of Dried Seeds
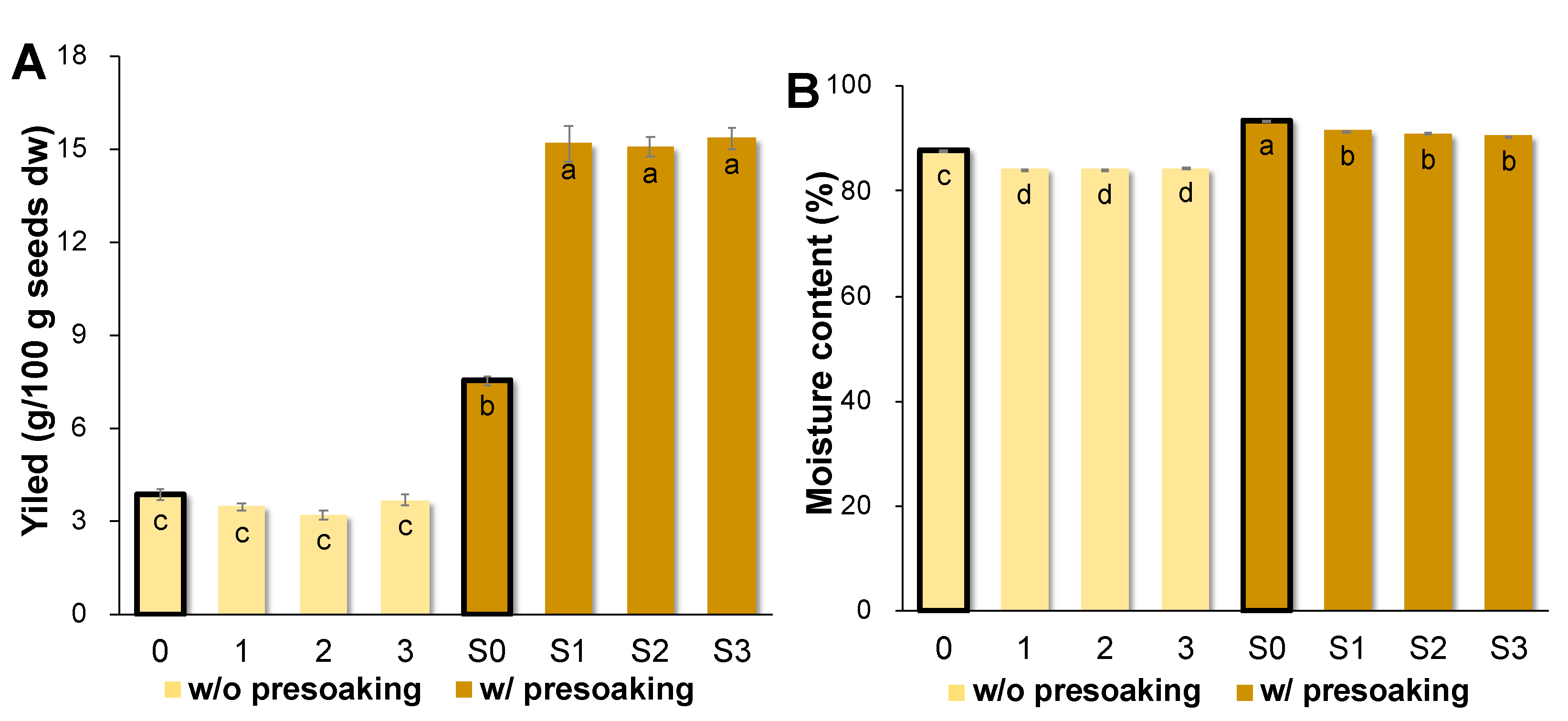
3.2. AQ Produced from Different Seed and Conditions
3.3. Color Analysis
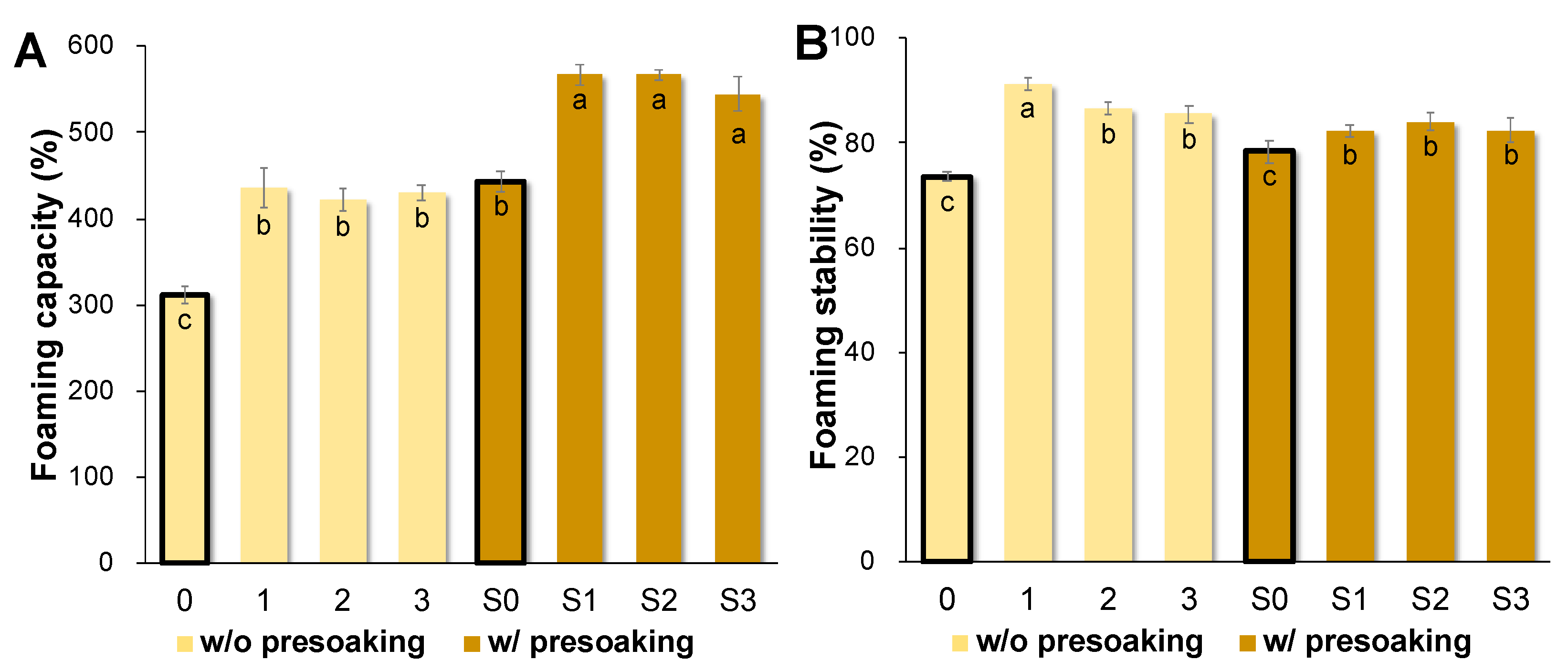
3.4. AQ Foaming Properties
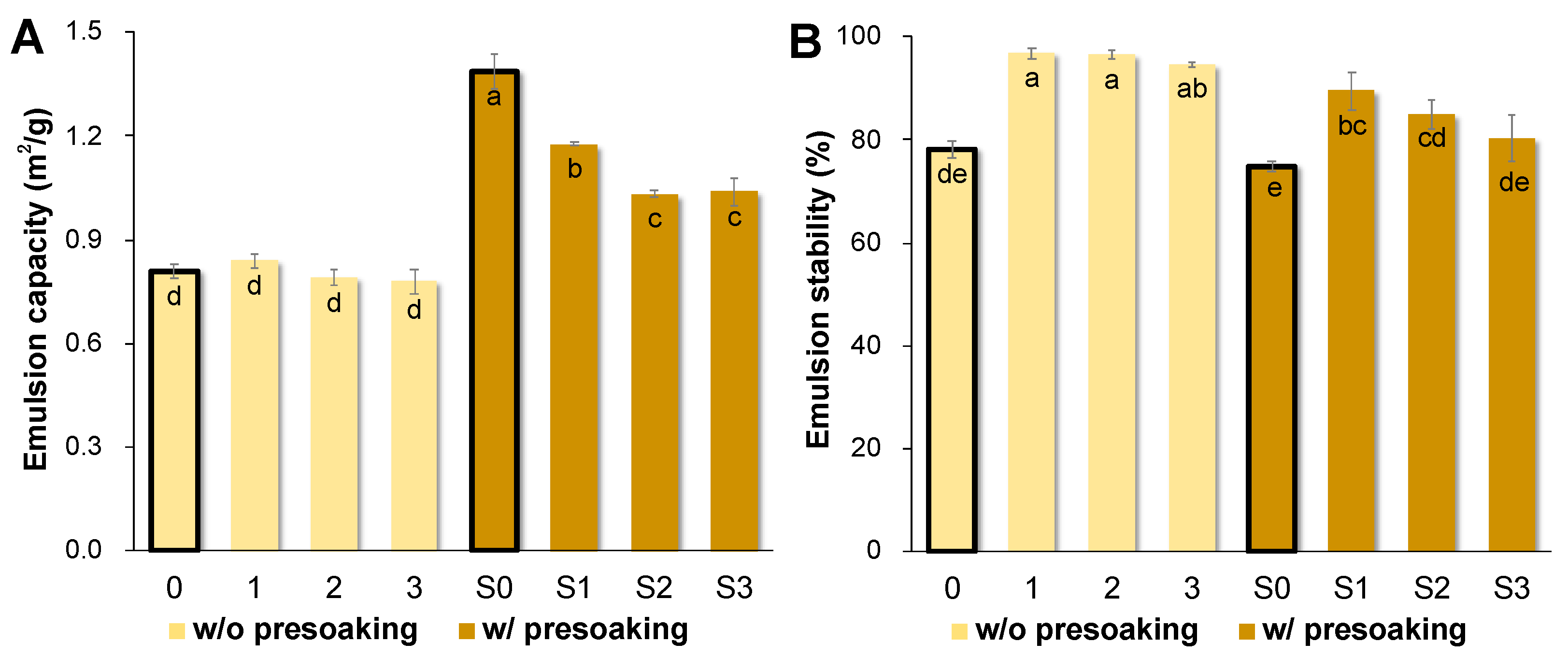
3.5. AQ Emulsion Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruby, M.B. Vegetarianism. A blossoming field of study. Appetite 2012, 58, 141–150. [Google Scholar] [CrossRef]
- Sanchez-Satate, R.; Sabaté, J. Consumer attitudes towards environmental concerns of meat consumption: A systematic review. Int. J. Environ. Res. Public Health 2019, 16, 1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Global Data. Ingredient Insights: Opportunities in Meat Substitutes; GlobalData UK Ltd.: London, UK, 2019; pp. 1–30. [Google Scholar]
- Cision PR Newswire. Egg Replacement Ingredient Market: Global Industry Analysis and Opportunity Assessment, 2016–2026. Available online: https://www.prnewswire.com/news-releases/egg-replacement-ingredient-market-global-industry-analysis-and-opportunity-assessment-2016-2026-300370861.html (accessed on 22 September 2021).
- Joshi, P.K.; Parthasarathy Rao, P. Global and regional pulse economies current trends and outlook. In IFPRI Discussion Paper 01544; International Food Policy Research Institute: Washington, DC, USA, 2016; p. 149. [Google Scholar]
- Mateos-Aparicio, I.; Redondo Cuenca, A.; Villanueva-Suárez, M.J.; Zapata-Revilla, M.A. Soybean, a promising health source. Nutr. Hosp. 2008, 23, 305–312. [Google Scholar] [PubMed]
- He, Y.; Meda, V.; Reaney, M.J.T.; Mustafa, R. Aquafaba, a new plant-based rheological additive for food applications. Trends Food Sci. Technol. 2021, 111, 27–42. [Google Scholar] [CrossRef]
- Steinfeld, H.; Gerber, P.; Wassenaar, T.D.; Castel, V.; Rosales, M.; Rosales, M.; de Haan, C. Livestock’s Long Shadow: Environmental Issues and Options; Food & Agriculture Org.: Rome, Italy, 2006; ISBN 92-5-105571-8. [Google Scholar]
- Bouwman, L.; Goldewijk, K.K.; Van Der Hoek, K.W.; Beusen, A.H.; Van Vuuren, D.P.; Willems, J.; Rufino, M.C.; Stehfest, E. Exploring global changes in nitrogen and phosphorus cycles in agriculture induced by livestock production over the 1900–2050 period. Proc. Natl. Acad. Sci. USA 2013, 110, 20882–20887. [Google Scholar] [CrossRef] [Green Version]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [Green Version]
- Thornton, P.K. Livestock production: Recent trends, future prospects. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2853–2867. [Google Scholar] [CrossRef] [Green Version]
- Dauvergne, P. The Shadows of Consumption: Consequences for the Global Environment; MIT Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Shim, Y.Y.; Mustafa, R.; Shen, J.; Ratanapariyanuch, R.; Reaney, M.J.T. Composition and properties of aquafaba: Water recovered from commercially canned chickpeas. J. Vis. Exp. 2018, 132, e56305. [Google Scholar] [CrossRef]
- Stantiall, S.E.; Dale, K.J.; Calizo, F.S.; Serventi, L. Application of pulses cooking water as functional ingredients: The foaming and gelling abilities. Eur. Food Res Technol. 2018, 244, 97–104. [Google Scholar] [CrossRef]
- Patil, G.; Vuong, T.D.; Kale, S.; Valliyodan, B.; Deshmukh, R.; Zhu, C.; Wu, X.; Bai, Y.; Yungbluth, D.; Lu, F.; et al. Dissecting genomic hotspots underlying seed protein, oil, and sucrose content in an interspecific mapping population of soybean using high-density linkage mapping. Plant Biotechnol. J. 2018, 16, 1939–1953. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Purdy, S.K.; Tse, T.J.; Tar’an, B.; Meda, V.; Reaney, M.J.T.; Mustafa, R. Standardization of aquafaba production and application in vegan mayonnaise analogs. Foods 2021, 10, 1978. [Google Scholar] [CrossRef] [PubMed]
- Serventi, L.; Wang, S.; Zhu, J.; Liu, S.; Fei, F. Cooking water of yellow soybeans as emulsifier in gluten-free crackers. Eur. Food Res. Technol. 2018, 244, 2141–2148. [Google Scholar] [CrossRef]
- ASABE Standards. Moisture Measurement-Unground Grain and Seeds; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 1988. [Google Scholar]
- AOAC. Official Methods of Analysis of Association of Official Analytical Chemists International, 20th ed.; Horwitz, W., Latimer, G.W., Eds.; AOAC International: Gaithersburg, MD, USA, 2016; methods 990.03, 920.39, 942.05, and 962.09. [Google Scholar]
- Martinez, M.; Stone, A.K.; Yovchev, A.G.; Peter, R.; Vandenberg, A.; Nickerson, M.T. Effect of genotype and environment on the surface characteristics and functionality of air-classified faba bean protein concentrates. Eur. Food Res. Technol. 2016, 242, 903–1911. [Google Scholar] [CrossRef]
- Mustafa, R.; He, Y.; Shim, Y.Y.; Reaney, M.J.T. Aquafaba, wastewater from chickpea canning, functions as an egg replacer in sponge cake. Int. J. Food Sci. Tech. 2018, 53, 2247–2255. [Google Scholar] [CrossRef]
- He, Y.; Shim, Y.Y.; Mustafa, R.; Meda, V.; Reaney, M.J.T. Chickpea cultivar selection to produce aquafaba with superior emulsion properties. Foods 2019, 8, 685. [Google Scholar] [CrossRef] [Green Version]
- Nikzade, V.; Tehrani, M.M.; Saadatmand-Tarzjan, M. Optimization of low-cholesterol-low-fat mayonnaise formulation: Effect of using soy milk and some stabilizer by a mixture design approach. Food Hydrocoll. 2012, 28, 344–352. [Google Scholar] [CrossRef]
- Mustafa, R.; Reaney, M.J.T. Chapter 4. Aquafaba, from food waste to a value-added product. In Food Wastes and by-Products: Nutraceutical & Health Potential; Campos-Vega, R., Oomah, B.D., Vergara-Castañeda, H.A., Eds.; John Wiley & Sons Ltd.: New York, NY, USA, 2020; pp. 93–126. [Google Scholar]
- Kuchlan, M.; Dadlani, M.; Samuel, D. Seed coat properties and longevity of soybean seeds. J. New Seeds 2010, 11, 239–249. [Google Scholar] [CrossRef]
- Sharma, S.; Kaur, M.; Goyal, R.; Gill, B.S. Physical characteristics and nutritional composition of some new soybean (Glycine max (L.) Merrill) genotypes. J. Food Sci. Technol. 2014, 51, 551–557. [Google Scholar] [CrossRef] [Green Version]
- Kuźniar, P.; Szpunar-Krok, E.; Findura, P.; Buczek, J.; Bobrecka-Jamro, D. Physical and chemical properties of soybean seeds determine their susceptibility to mechanical damage. Zemdirbyste-Agriculture 2016, 103, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Pastor, A.; Compson, Z.G.; Dijkstra, P.; Riera, J.L.; Martí, E.; Sabater, F.; Hungate, B.A.; Marks, J.C. Stream carbon and nitrogen supplements during leaf litter decomposition: Contrasting patterns for two foundation species. Oecologia 2014, 176, 1111–1121. [Google Scholar] [CrossRef]
- Bird, L.G.; Pilkington, C.L.; Saputra, A.; Serventi, L. Products of chickpea processing as texture improvers in gluten-free bread. Food Sci. Technol. Int. 2017, 23, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Damian, J.J.; Huo, S.; Serventi, L. Phytochemical content and emulsifying ability of pulses cooking water. Eur. Food Res. Technol. 2018, 244, 1647–1655. [Google Scholar] [CrossRef]
- Lyimo, M.; Mugula, J.; Elias, T. Nutritive composition of broth from selected bean varieties cooked for various periods. J. Sci. Food Agric. 1992, 58, 535–539. [Google Scholar] [CrossRef]
- Gross, J. Pigments in Vegetables: Chlorophylls and Carotenoids; Springer: New York, NY, USA, 1991. [Google Scholar]
- Wang, H.J.; Murphy, P.A. Isoflavone content in commercial soybean foods. J. Agric. Food Chem. 1994, 42, 666–1673. [Google Scholar] [CrossRef]
- Toda, T.; Sakamoto, A.; Takayanagi, T.; Yokotsuka, K. Changes in isoflavone compositions of soybean foods during cooking process. Food Sci. Technol. Res. 2000, 6, 314–319. [Google Scholar]
- Matsuura, M.; Obata, A. β-Glucosidases from soybeans hydrolyze daidzin and genistin. J. Food Sci. 1993, 58, 144–147. [Google Scholar] [CrossRef]
- Aslan, M.; Ertas, N. Possibility of using “chickpea aquafaba” as egg replacer in traditional cake formulation. Harran Tarım ve Gıda Bilimleri Dergisi 2020, 24, 1–8. [Google Scholar] [CrossRef]
- Meurer, M.C.; de Souza, D.; Ferreira Marczak, L.D. Effects of ultrasound on technological properties of chickpea cooking water (aquafaba). J. Food Eng. 2020, 265, 109688. [Google Scholar] [CrossRef]
- Lafarga, T.; Villar’o, S.; Bobo, G.; Aguil’o-Aguayo, I. Optimisation of the pH and boiling conditions needed to obtain improved foaming and emulsifying properties of chickpea aquafaba using a response surface methodology. Int. J. Gastron. Food Sci. 2019, 18, 100177. [Google Scholar] [CrossRef]
- Aluko, R.E.; Mofolasayo, O.A.; Watts, B.M. Emulsifying and foaming properties of commercial yellow pea (Pisum sativum L.) seed flours. J. Agric. Food Chem. 2009, 57, 9793–9800. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Quintela, A.; Macarulla, M.T.; Del Barrio, A.S.; Martínez, J.A. Composition and functional properties of protein isolates obtained from commercial legumes grown in northern Spain. Plant Foods Hum. Nutr. 1997, 51, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Gowen, A. Development of Innovative, Quick-Cook Legume Products: An Investigation of the Soaking, Cooking and Dehydration Characteristics of Chickpeas (Cicer arietinum L.) and Soybeans (Glycine max L. Merr.). Ph.D. Thesis, Technological University Dublin, Dublin, Ireland, May 2006. [Google Scholar]
- Mun, S.; Kim, Y.L.; Kang, C.G.; Park, K.H.; Shim, J.Y.; Kim, Y.R. Development of reduced-fat mayonnaise using 4αGTase-modified rice starch and xanthan gum. Int. J. Biol. Macromol. 2009, 44, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Langton, M.; Jordansson, E.; Altskär, A.; Sørensen, C.; Hermansson, A.M. Microstructure and image analysis of mayonnaises. Food Hydrocoll. 1999, 13, 113–125. [Google Scholar] [CrossRef]
- Raikos, V.; Hayes, H.; Ni, H. Aquafaba from commercially canned chickpeas as potential egg replacer for the development of vegan mayonnaise: Recipe optimisation and storage stability. Int. J. Food Sci. Technol. 2020, 55, 1935–1942. [Google Scholar] [CrossRef]
- Margarido, M.A.; Turolla, F.A.; Bueno, C.R.F. The world market for soybeans: Price transmission into Brazil and effects from the timing of crop and trade. Nova Econ. 2007, 17, 241–270. [Google Scholar] [CrossRef] [Green Version]

| Species/Cultivar (Code) | Canadian Chickpea 1 | Korean Soybeans 2 | |||
|---|---|---|---|---|---|
| Cicer arietinum (L.) | Glycine max (L.) Merr. | Rhynchosia nulubilis | |||
| Group | CDC Leader | Backtae | Seoritae | Jwinunikong | |
| w/o Presoaking AQ 3 | 0 | 1 | 2 | 3 | |
| w/Presoaking AQ 4 | S0 | S1 | S2 | S3 | |
| Characteristics | Unit | CDC Leader 1 | Backtae | Seoritae | Jwinunikong |
|---|---|---|---|---|---|
| Moisture | % | 8.86 ± 0.07 c | 9.30 ± 0.02 b | 10.52 ± 0.07 a | 9.26 ± 0.11 b |
| Carbohydrate 2 | g (100 g/dw) | 65.4 ± 2.01 a | 29.14 ± 0.78 b | 29.16 ± 0.32 b | 27.88 ± 1.32 b |
| Crude protein 3 | g (100 g/dw) | 20.9 ± 0.09 c | 40.59 ± 0.28 b | 44.77 ± 0.15 a | 44.43 ± 0.22 a |
| Fat 4 | g (100 g/dw) | 6.49 ± 0.47 c | 18.40 ± 0.69 a | 14.91 ± 0.30 b | 14.51 ± 0.37 b |
| Ash 5 | g (100 g/dw) | 3.01 ± 0.08 d | 4.65 ± 0.26 a | 4.17 ± 0.14 c | 4.52 ± 0.01 b |
| Crude fiber 6 | g (100 g/dw) | 4.32 ± 0.26 b | 7.27 ± 0.47 a | 6.99 ± 0.32 a | 8.66 ± 1.43 a |
| Group | L* | a* | b* |
|---|---|---|---|
| Seed | |||
| CDC Leader | 62.98 ± 0.19 a | 8.54 ± 0.02 a | 25.77 ± 0.10 a |
| Backtae | 66.30 ± 0.19 a | 6.77 ± 0.10 b | 24.17 ± 0.12 a |
| Seoritae | 34.53 ± 0.11 b | –0.64 ± 0.08 g | –4.42 ± 0.06 d |
| Jwinunikong | 34.76 ± 0.10 b | –0.61 ± 0.06 g | –4.89 ± 0.07 d |
| w/o Presoaking AQ | |||
| 0 | 46.35 ± 0.06 b | 2.55 ± 0.01 d | 21.47 ± 0.06 a |
| 1 | 33.08 ± 0.22 b | 5.27 ± 0.17 c | 14.43 ± 0.14 b |
| 2 | 16.10 ± 0.04 d | 2.05 ± 0.24 e | –1.75 ± 0.10 c |
| 3 | 17.08 ± 0.15 d | 2.20 ± 0.03 e | –1.26 ± 0.12 c |
| w/Presoaking AQ | |||
| S0 | 39.30 ± 0.14 b | 0.79 ± 0.02 c | 15.55 ± 0.40 b |
| S1 | 38.99 ± 0.24 b | 1.25 ± 0.17 f | 11.33 ± 0.06 b |
| S2 | 27.61 ± 0.40 c | 4.76 ± 0.12 d | 1.00 ± 0.18 c |
| S3 | 26.19 ± 0.54 c | 5.12 ± 0.25 c | 1.08 ± 0.07 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shim, Y.Y.; He, Y.; Kim, J.H.; Cho, J.Y.; Meda, V.; Hong, W.S.; Shin, W.-S.; Kang, S.J.; Reaney, M.J.T. Aquafaba from Korean Soybean I: A Functional Vegan Food Additive. Foods 2021, 10, 2433. https://doi.org/10.3390/foods10102433
Shim YY, He Y, Kim JH, Cho JY, Meda V, Hong WS, Shin W-S, Kang SJ, Reaney MJT. Aquafaba from Korean Soybean I: A Functional Vegan Food Additive. Foods. 2021; 10(10):2433. https://doi.org/10.3390/foods10102433
Chicago/Turabian StyleShim, Youn Young, Yue He, Ji Hye Kim, Jae Youl Cho, Venkatesh Meda, Wan Soo Hong, Weon-Sun Shin, Sang Jin Kang, and Martin J. T. Reaney. 2021. "Aquafaba from Korean Soybean I: A Functional Vegan Food Additive" Foods 10, no. 10: 2433. https://doi.org/10.3390/foods10102433
APA StyleShim, Y. Y., He, Y., Kim, J. H., Cho, J. Y., Meda, V., Hong, W. S., Shin, W.-S., Kang, S. J., & Reaney, M. J. T. (2021). Aquafaba from Korean Soybean I: A Functional Vegan Food Additive. Foods, 10(10), 2433. https://doi.org/10.3390/foods10102433