Multiblock Analysis Applied to Fluorescence and Absorbance Spectra to Estimate Total Polyphenol Content in Extra Virgin Olive Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Fluorescence Spectral Data
2.3. Transmitance Spectral Data
2.4. Chemometrics
3. Results and Discussion
3.1. Main Features of Fluorescence Spectra
3.2. Main Features of Absorbance Spectra
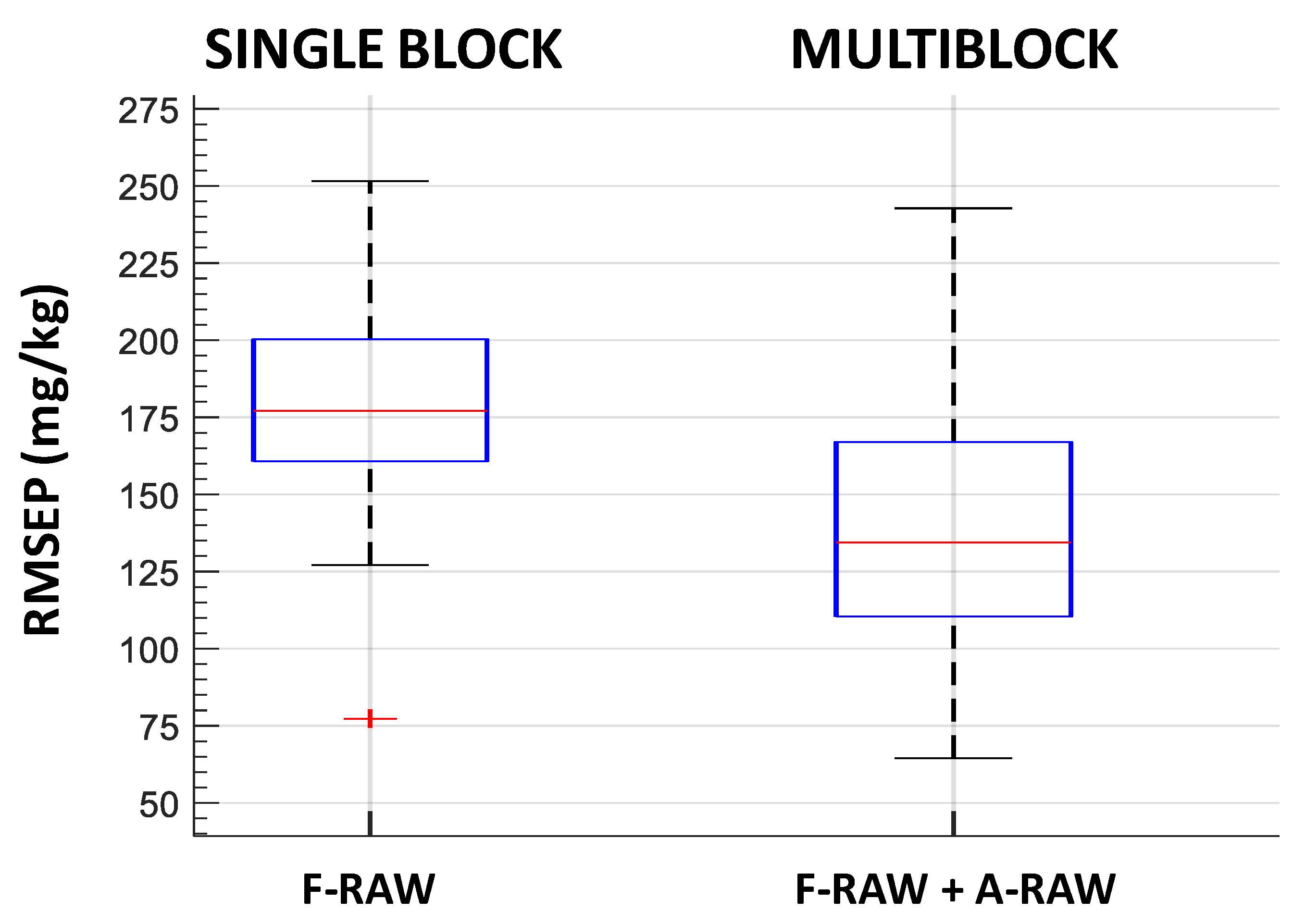
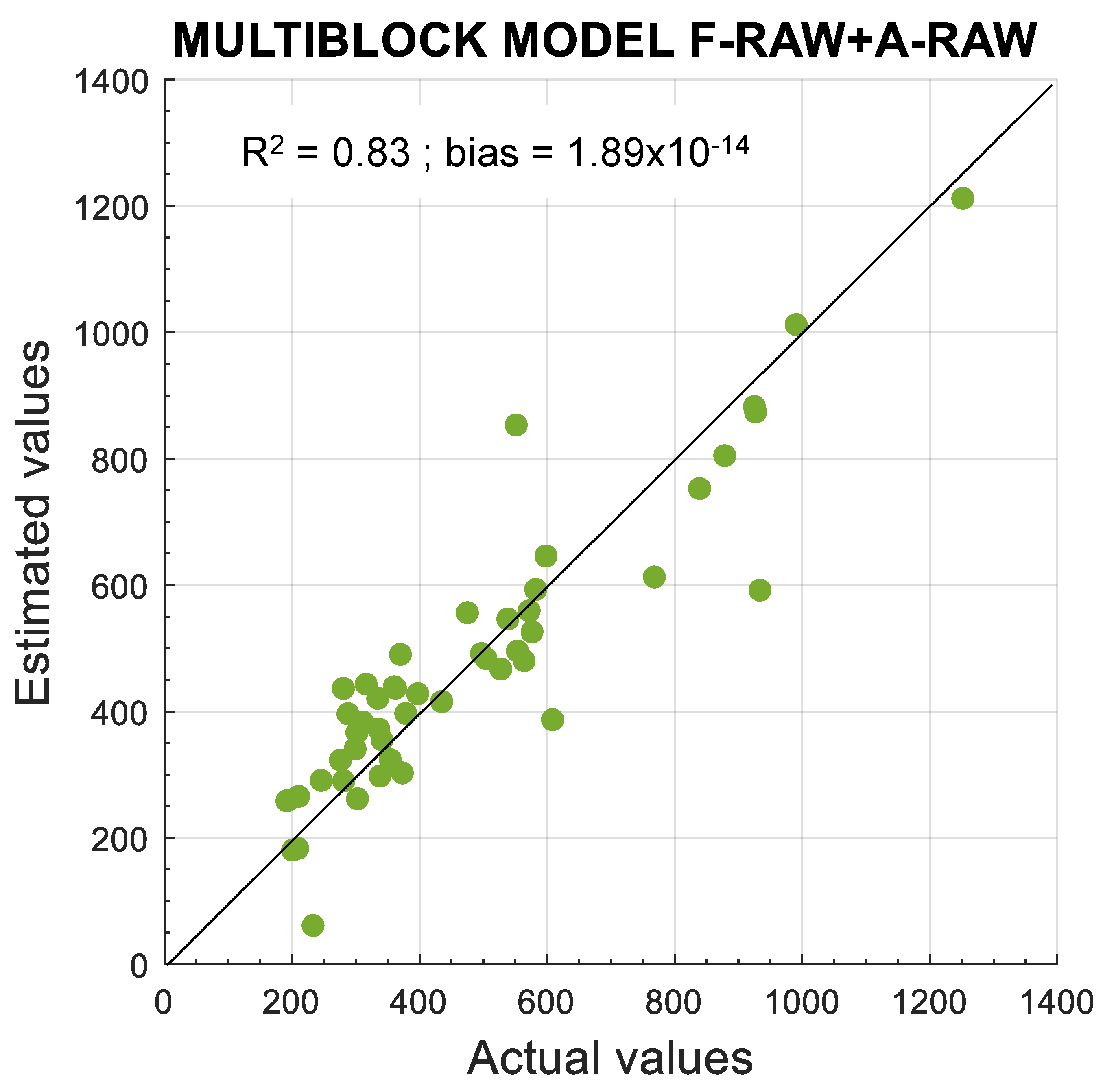
3.3. Models for Polyphenol Estimation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- European Commission. Commission Regulation (EU) No. 432/2012 of 16 May 2012 establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children’s development and health. Off. J. Eur. Union 2012, L136, 1–40. [Google Scholar]
- Gaforio, J.J.; Visioli, F.; Alarcon-de-la-Lastra, C.; Castaner, O.; Delgado-Rodriguez, M.; Fito, M.; Hernandez, A.F.; Huertas, J.R.; Martinez-Gonzalez, M.A.; Menendez, J.A.; et al. Virgin Olive Oil and Health: Summary of the III International Conference on Virgin Olive Oil and Health Consensus Report, JAEN (Spain) 2018. Nutrients 2019, 11, 2039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutierrez, F.; Arnaud, T.; Garrido, A. Contribution of polyphenols to the oxidative stability of virgin olive oil. J. Sci. Food Agric. 2001, 81, 1463–1470. [Google Scholar] [CrossRef]
- Vazquez-Roncero, A.; Janer del Valle, C.; Janer del Valle, M.L. Determination of total polyphenols in olive oils. Grasas Y Aceites 1973, 24, 350–357. [Google Scholar]
- COI. Determination of Biophenols in Olive Oils by HPLC; COI/T.20/Doc. No 29; COI: Madrid, Spain, 2017. [Google Scholar]
- Squeo, G.; Caponio, F.; Paradiso, V.M.; Summo, C.; Pasqualone, A.; Khmelinskii, I.; Sikorska, E. Evaluation of total phenolic content in virgin olive oil using fluorescence excitation-emission spectroscopy coupled with chemometrics. J. Sci. Food Agric. 2019, 99, 2513–2520. [Google Scholar] [CrossRef] [PubMed]
- Birlouez-Aragon, I.; Buschmann, C.; Hashimoto, A.; Kameoka, T.; Kumke, M.U.; Lakhal, L.; Langsdorf, G.; Lichtenthaler, H.K.; Löhmannsröben, H.-G.; Rizkallaf, J.; et al. Fluorescence, 1st ed.; Zude, M., Ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Sikorska, E.; Khmelinskii, I.; Sikorski, M. Analysis of Olive Oils by Fluorescence Spectroscopy: Methods and Applications; IntechOpen: London, UK, 2012; pp. 63–88. [Google Scholar]
- Lleo, L.; Hernandez-Sanchez, N.; Ammari, F.; Roger, J.M. 3D front-face fluorescence spectroscopy for characterization of extra virgin olive oil and olive oil according to the spectral pattern. CIGR J. 2016, 18, 190–199. [Google Scholar]
- Hernandez-Sanchez, N.; Lleo, L.; Ammari, F.; Cuadrado, T.R.; Roger, J.M. Fast Fluorescence Spectroscopy Methodology to Monitor the Evolution of Extra Virgin Olive Oils Under Illumination. Food Bioprocess Technol. 2017, 10, 949–961. [Google Scholar] [CrossRef]
- Gallardo-Guerrero, L.; Gandul-Rojas, B.; Roca, M.; Minguez-Mosquera, I. Effect of storage on the original pigment profile of Spanish virgin olive oil. J. Am. Oil Chem. Soc. 2005, 82, 33–39. [Google Scholar] [CrossRef]
- Awhangbo, L.; Bendoula, R.; Roger, J.M.; Beline, F. Multi-block SO-PLS approach based on infrared spectroscopy for anaerobic digestion process monitoring. Chemom. Intell. Lab. Syst. 2020, 196, 103905. [Google Scholar] [CrossRef]
- Naes, T.; Tomic, O.; Mevik, B.H.; Martens, H. Path modelling by sequential PLS regression. J. Chemom. 2011, 25, 28–40. [Google Scholar] [CrossRef]
- Filzmoser, P.; Liebmann, B.; Varmuza, K. Repeated double cross validation. J. Chemom. 2009, 23, 160–171. [Google Scholar] [CrossRef]
- Mínguez Mosquera, M.I.; Rejano Navarro, L.; Gandul Rojas, B.; Sánchez Gómez, A.H.; Garrido Fernández, J. Color-pigment correlation in virgin olive oil. J. Am. Oil Chem. Soc. 1991, 68, 332–336. [Google Scholar] [CrossRef]
- Zeaiter, M.; Roger, J.M.; Bellon-Maurel, V. Robustness of models developed by multivariate calibration. Part II: The influence of pre-processing methods. Trac Trends Anal. Chem. 2005, 24, 437–445. [Google Scholar] [CrossRef]
- Ali, H.; Iqbal, M.A.; Atta, B.M.; Ullah, R.; Khan, M.B. Phenolic Profile and Thermal Stability of Monovarietal Extra Virgin Olive Oils Based on Synchronous Fluorescence Spectroscopy. J. Fluoresc. 2020, 30, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Domenici, V.; Ancora, D.; Cifelli, M.; Serani, A.; Veracini, C.A.; Zandomeneghi, M. Extraction of Pigment Information from Near-UV Vis Absorption Spectra of Extra Virgin Olive Oils. J. Agric. Food Chem. 2014, 62, 9317–9325. [Google Scholar] [CrossRef] [PubMed]
- Reboredo-Rodríguez, P.; Valli, E.; Bendini, A.; Di Lecce, G.; Simal-Gándara, J.; Gallina Toschi, T. A widely used spectrophotometric assay to quantify olive oil biophenols according to the health claim (EU Reg. 432/2012). Eur. J. Lipid Sci. Technol. 2016, 118, 1593–1599. [Google Scholar] [CrossRef]


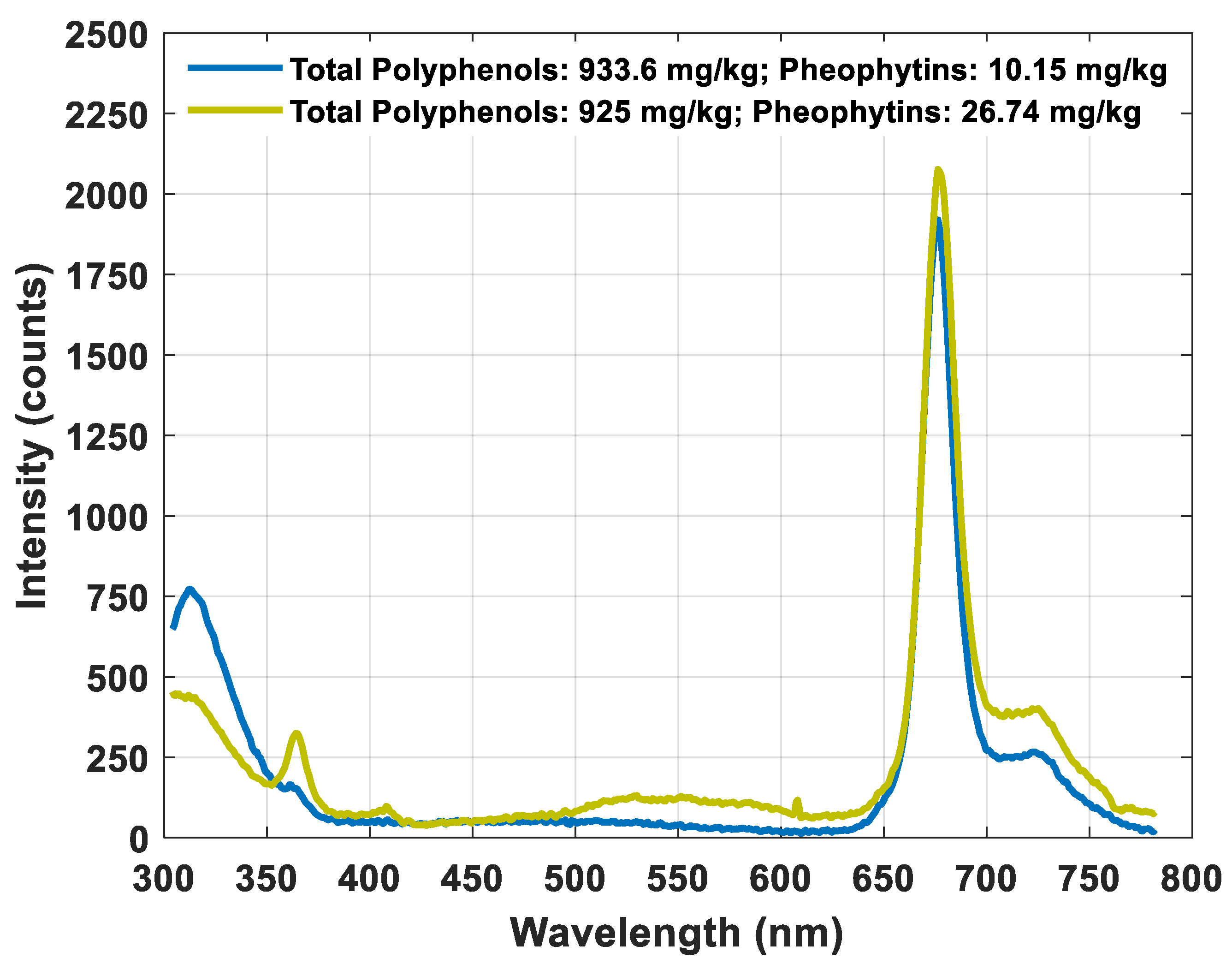


| Statistics (n = 48) | Polyphenols (mg·kg−1) | Pheophytins (mg·kg−1) | Lutein (mg·kg−1) |
|---|---|---|---|
| Mean | 478.90 | 10.22 | 10.84 |
| Median | 375.73 | 7.06 | 7.99 |
| STD | 244.66 | 8.65 | 10.11 |
| Min | 192.00 | 1.97 | 2.84 |
| Max | 1251.00 | 43.75 | 59.51 |
| Code | Preprocessing | Ref |
|---|---|---|
| RAW | None | [16] |
| SG1 | First derivative by Savitsky and Golay algorithm, width 21 wavelengths, polynomial degree 3, order 1 | |
| SG2 | Second derivative by Savitsky and Golay algorithm, width 21 wavelengths, polynomial degree 3, order 2 | |
| SNV | Standard normal variate | |
| NORM | Normalization by root mean square |
| Raw Data | ||||
| PLSR single block | RMSEP median (mg·kg−1) | RPD | LV median fluorescence | |
| F-RAW | 177.11 | 1.4 | 8 | |
| SO-PLS multiblock | RMSEP median (mg·kg−1) | RPD | LV median fluorescence | LV median absorbance |
| F-RAW + A-RAW | 134.45 | 3 | 1 | |
| Pre-treated data | ||||
| PLSR single block | RMSEP median (mg·kg−1) | RPD | LV median fluorescence | |
| F-SG1 | 174.22 | 1.4 | 3.5 | |
| F-SG2 | 219.67 | 1.1 | 3 | |
| F-SNV | 134.59 | 1.8 | 3.5 | |
| F-NORM | 145.02 | 1.7 | 8 | |
| SO-PLS multiblock | RMSEP median (mg·kg−1) | RPD | LV median fluorescence | LV median absorbance |
| F-RAW + A-SG1 | 136.29 | 1.8 | 3.5 | 2 |
| F-RAW + A-SG2 | 145.12 | 1.7 | 3 | 2 |
| F-RAW + A-SNV | 145.23 | 1.7 | 3 | 1 |
| F-RAW + A-NORM | 146.6 | 1.7 | 3 | 2 |
| F-SNV + A-RAW | 136.87 | 1.8 | 3 | 1 |
| F-SNV + A-SG1 | 130.15 | 1.9 | 3 | 1 |
| F-SNV + A-SG2 | 134.4 | 1.8 | 3 | 1 |
| F-SNV + A-SNV | 130.31 | 1.9 | 3 | 1 |
| F-SNV + A-NORM | 130.37 | 1.9 | 3 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Sánchez, N.; Lleó, L.; Diezma, B.; Correa, E.C.; Sastre, B.; Roger, J.-M. Multiblock Analysis Applied to Fluorescence and Absorbance Spectra to Estimate Total Polyphenol Content in Extra Virgin Olive Oil. Foods 2021, 10, 2556. https://doi.org/10.3390/foods10112556
Hernández-Sánchez N, Lleó L, Diezma B, Correa EC, Sastre B, Roger J-M. Multiblock Analysis Applied to Fluorescence and Absorbance Spectra to Estimate Total Polyphenol Content in Extra Virgin Olive Oil. Foods. 2021; 10(11):2556. https://doi.org/10.3390/foods10112556
Chicago/Turabian StyleHernández-Sánchez, Natalia, Lourdes Lleó, Belén Diezma, Eva Cristina Correa, Blanca Sastre, and Jean-Michel Roger. 2021. "Multiblock Analysis Applied to Fluorescence and Absorbance Spectra to Estimate Total Polyphenol Content in Extra Virgin Olive Oil" Foods 10, no. 11: 2556. https://doi.org/10.3390/foods10112556
APA StyleHernández-Sánchez, N., Lleó, L., Diezma, B., Correa, E. C., Sastre, B., & Roger, J.-M. (2021). Multiblock Analysis Applied to Fluorescence and Absorbance Spectra to Estimate Total Polyphenol Content in Extra Virgin Olive Oil. Foods, 10(11), 2556. https://doi.org/10.3390/foods10112556








