Effect of Fermentation Parameters on Natto and Its Thrombolytic Property
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Seed Preparation
2.3. Natto Preparation
2.4. One-Factor-at-a-Time Experiments
2.5. PB Design
2.6. BBD
2.7. pH Measurement and Extraction of Crude Enzyme Solution
2.8. Free Amino Nitrogen Contents (FANs)
2.9. Nattokinase (NK) Activity Assay
2.10. Sensory Properties
2.11. Thrombolytic Effect
2.12. Anticoagulant Activity
2.13. Statistical Analysis
3. Results
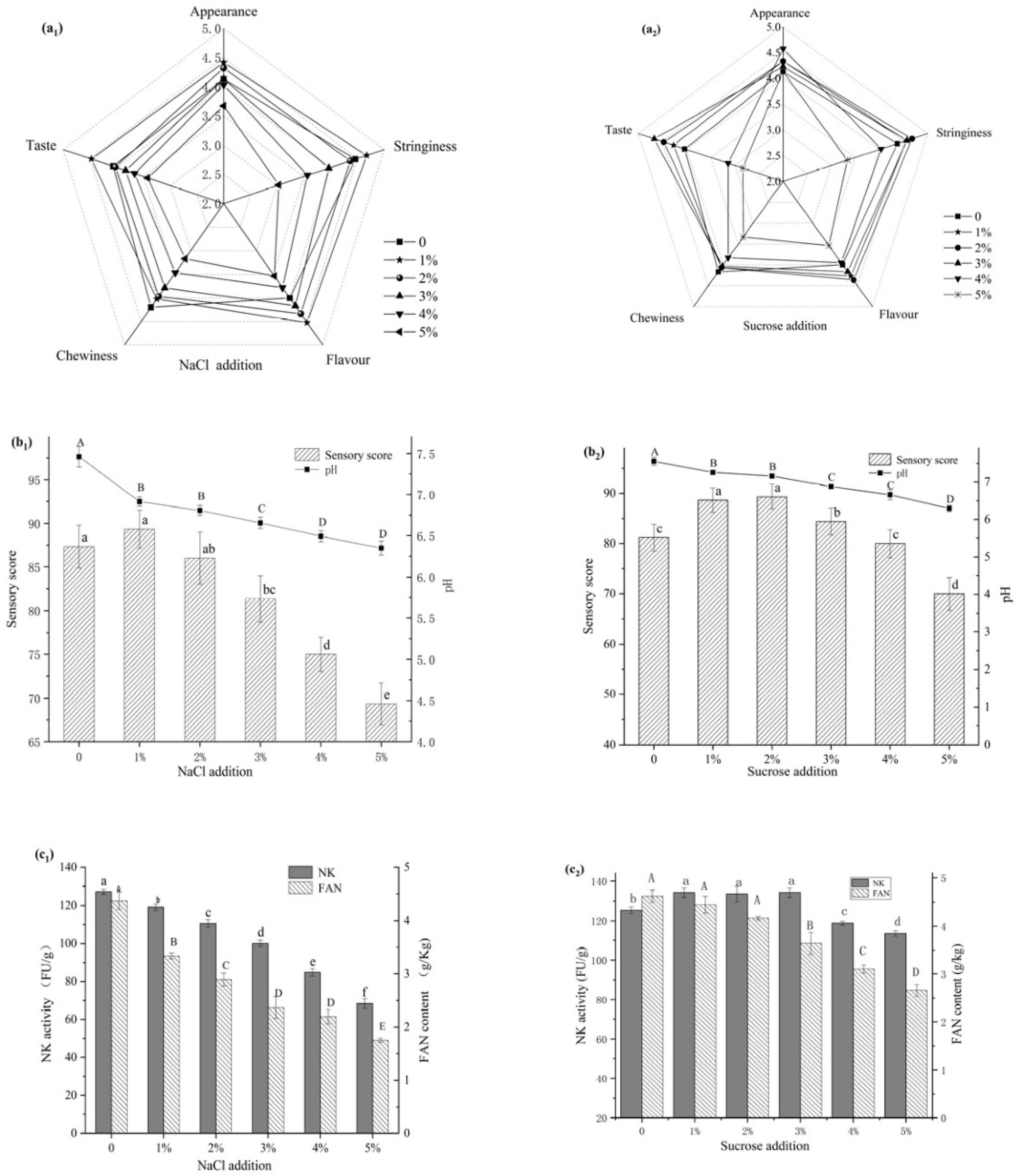
3.1. Effect of NaCl on Natto
3.2. Effect of Sucrose on Natto
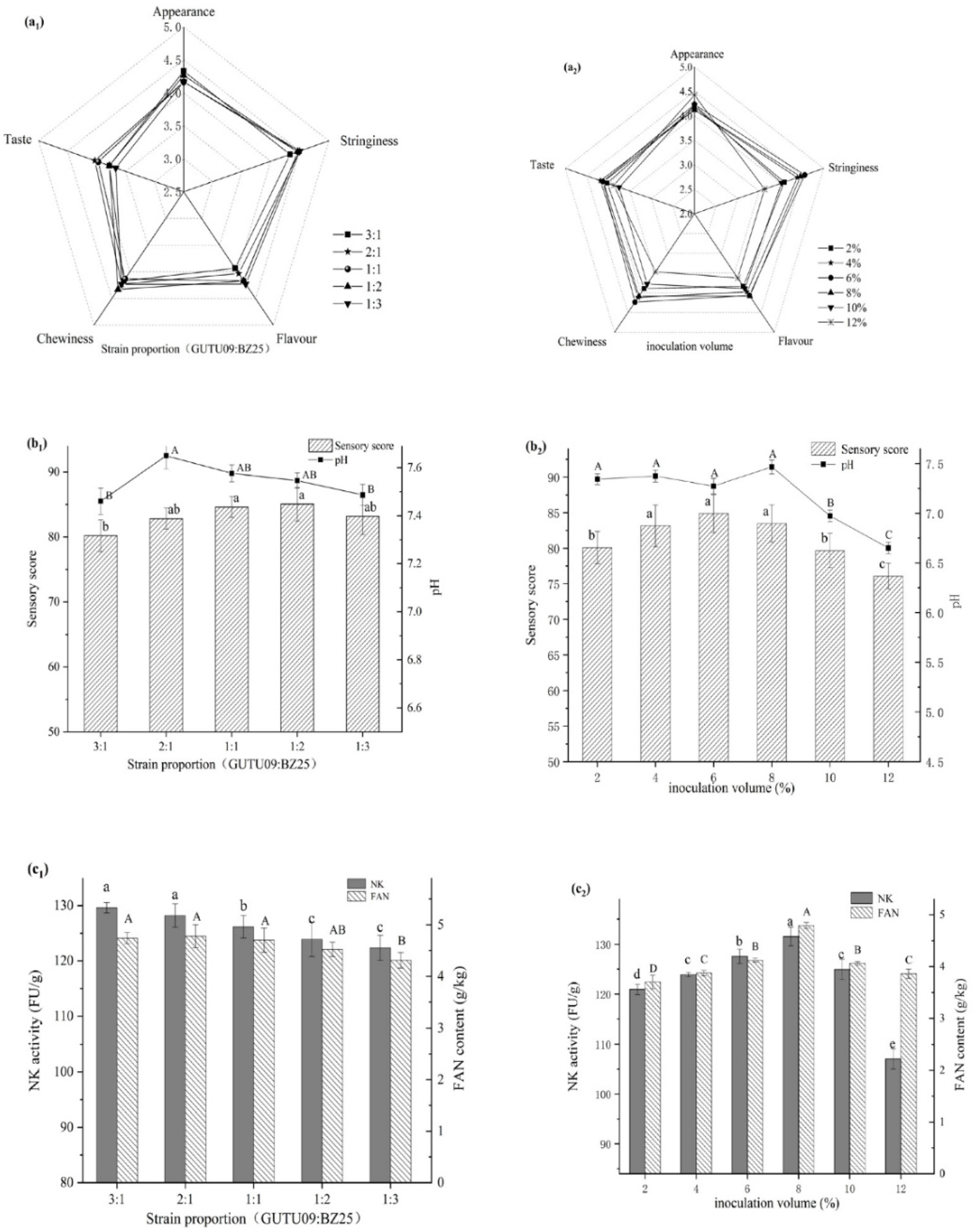
3.3. Effect of Strain Proportion on Natto
3.4. Effect of Inoculation Amount on Natto
3.5. Effect of Fermentation Temperature on Natto
3.6. Effect of Fermentation Time on Natto
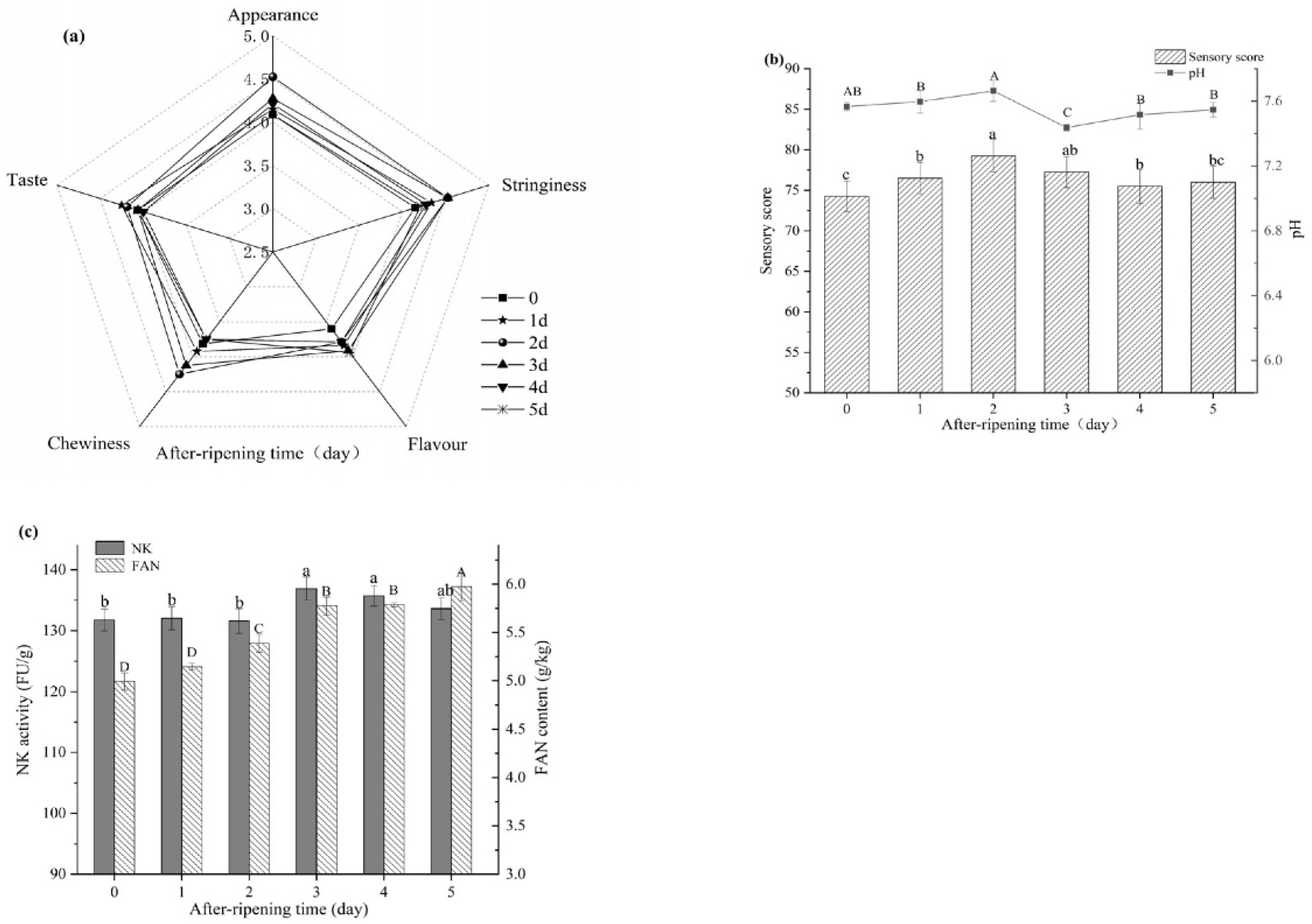
3.7. Effect of After-Ripening Time on Natto
3.8. Plackett–Burman (PB) Design
3.9. Response Surface Analysis by Box–Behnken Design (BBD)
3.10. Determination and Verification of Natto Process
3.11. Thrombolytic Activity, Fibrin Degradation Ability, and Anticoagulant Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kotb, E. The biotechnological potential of fibrinolytic enzymes in the dissolution of endogenous blood thrombi. Biotechnol. Prog. 2014, 30, 656–672. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-T.; Wang, P.-M.; Hung, Y.-F.; Chung, Y.-C. Purification and biochemical properties of a fibrinolytic enzyme from Bacillus subtilis-fermented red bean. Food Chem. 2012, 133, 1611–1617. [Google Scholar] [CrossRef]
- Ahmad, A.; Hayat, I.; Arif, S.; Masud, T.; Khalid, N.; Ahmed, A. Mechanisms involved in the therapeutic effects of soybean (Glycine Max). Int. J. Food Prop. 2014, 17, 1332–1354. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zheng, W.; Ge, C.; Cui, W.; Zhou, L.; Zhou, Z. High-level extracellular production of recombinant nattokinase in Bacillus subtilis WB800 by multiple tandem promoters. BMC Microbiol. 2019, 19, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, X.; Luo, M.; Xie, Y.; Yang, L.; Li, H.; Xu, L.; Liu, H. Strain screening, fermentation, separation, and encapsulation for production of nattokinase functional food. Appl. Biochem. Biotech. 2012, 168, 1753–1764. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Yao, J.; Sparks, S.; Wang, K.Y. Nattokinase: An oral antithrombotic agent for the prevention of cardiovascular disease. Int. J. Mol. Sci. 2017, 18, 523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Du, Z.; Qi, S.; Zhang, X.; Wang, M.; Zhou, Y.; Lu, H.; Gu, X.; Tian, H. Food-grade expression of nattokinase in Lactobacillus delbrueckii subsp. bulgaricus and its thrombolytic activity in vitro. Biotechnol. Lett. 2020, 42, 2179–2187. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.; Li, C.; He, L.; Zeng, X.; Zhu, Q. Effects of different strains and fermentation method on nattokinase activity, biogenic amines, and sensory characteristics of natto. J. Food. Sci. Technol. 2020, 57, 4414–4423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L. The Medium Optimization and Microcapsule Preparation of Bifidobacterium with Cholesterol-Reducing Ability from Guizhou Xiang Pigs. Master’s Thesis, Guizhou University, Guiyang, China, 2015. (In Chinese). [Google Scholar]
- Gao, Z. Screening of High-Yield Nattokinase Strains and Study of Its Enzymatic Stability. Master’s Thesis, Guizhou University, Guiyang, China, 2018. (In Chinese). [Google Scholar]
- Zhang, S.; Shi, Y.; Zhang, S.; Shang, W.; Gao, X.; Wang, H. Whole soybean as probiotic lactic acid bacteria carrier food in solid-state fermentation. Food Control 2014, 41, 1–6. [Google Scholar] [CrossRef]
- Chen, T.; Wang, Y.; Yu, R. A supplement to the details of the experiment of “isolation of microorganisms decomposing cellulose”. Biol. Teach. 2019, 44, 44–45. (In Chinese) [Google Scholar]
- Lu, Y.; Chen, X.; Jiang, M.; Lv, X.; Rahman, N.; Dong, M.; Yan, G. Biogenic amines in Chinese soy sauce. Food Control 2009, 20, 593–597. [Google Scholar] [CrossRef]
- Deepak, V.; Kalishwaralal, K.; Ramkumarpandian, S.; Babu, S.V.; Senthilkumar, S.R.; Sangiliyandi, G. Optimization of media composition for nattokinase production by Bacillus subtilis using response surface methodology. Bioresour. Technol. 2008, 99, 8170–8174. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Jin, S.; Luo, M.; Wang, W.; Xia, X.; Zu, Y.; Li, L.; Fu, Y. Optimization of production parameters for preparation of natto-pigeon pea with immobilized Bacillus natto and sensory evaluations of the product. Innov. Food Sci. Emerg. Technol. 2015, 31, 160–169. [Google Scholar] [CrossRef]
- Yoshikawa, Y.; Chen, P.; Zhang, B.; Scaboo, A.; Orazaly, M. Evaluation of seed chemical quality traits and sensory properties of natto soybean. Food Chem. 2014, 153, 186–192. [Google Scholar] [CrossRef]
- Wei, X.; Luo, M.; Liu, H. Preparation of the antithrombotic and antimicrobial coating through layer-by-layer self-assembly of nattokinase-nanosilver complex and polyethylenimine. Colloids Surf. B 2014, 116, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Ku, T.-W.; Tsai, R.-L.; Pan, T.-M. A simple and cost-saving approach to optimize the production of subtilisin NAT by submerged cultivation of Bacillus subtilis natto. J. Agric. Food Chem. 2009, 57, 292–296. [Google Scholar] [CrossRef]
- Niu, H.; Miao, X.; Zheng, L.; Chi, Y.; Liu, J.; Gao, Y.; Wang, J.; Li, D. Changes of nutrients and bioactive substances in natto production and in cold storage. Food Res. Dev. 2021, 42, 48–54. (In Chinese) [Google Scholar] [CrossRef]
- Yang, S.-Y.; Kim, S.-W.; Kim, Y.; Lee, S.-H.; Jeon, H.; Lee, K.-W. Optimization of maillard reaction with ribose for enhancing anti-allergy effect of fish protein hydrolysates using response surface methodology. Food Chem. 2015, 176, 420–425. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, X.; Zhang, Y. Microbial fibrinolytic enzymes: An overview of source, production, properties, and thrombolytic activity in vivo. Appl. Microbiol. Biotechnol. 2005, 69, 126–132. [Google Scholar] [CrossRef]
- Gu, J.; Kang, N.; Li, D.; Zhou, C.; Du, L.; Fu, Q. Analysis of synergistic anticoagulant effect of acid auricularia auricula polysaccharide. Mod. Food Sci. Technol. 2017, 33, 45–52. (In Chinese) [Google Scholar] [CrossRef]
- Xu, J.; Du, M.; Yang, X.; Chen, Q.; Chen, H.; Lin, D. Thrombolytic effects in vivo of nattokinase in a carrageenan-induced rat model of thrombosis. Acta Haematol. 2014, 132, 247–253. [Google Scholar] [CrossRef]
- Sumi, H.; Hamada, H.; Tsushima, H.; Mihara, H.; Muraki, H.J.E. A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese natto; a typical and popular soybean food in the Japanese diet. Experientia 1987, 43, 1110–1111. [Google Scholar] [CrossRef]
- Yang, Y.; Niu, C.; Shan, W.; Zheng, F.; Liu, C.; Wang, J.; Li, Q. Physicochemical, flavor and microbial dynamic changes during low-salt doubanjiang (broad bean paste) fermentation. Food Chem. 2021, 351. [Google Scholar] [CrossRef]
- Devanthi, P.V.P.; Gkatzionis, K. Soy sauce fermentation: Microorganisms, aroma formation, and process modification. Food Res. Int. 2019, 120, 364–374. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Tan, M.; Ma, Y.; MacGregor, G.A. Salt reduction to prevent hypertension and cardiovascular disease JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020, 75, 632–647. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Chen, G.; Pan, S.; Zeng, J.; Liang, Z. Cost-effective fibrinolytic enzyme production by Bacillus subtilis WR350 using medium supplemented with corn steep powder and sucrose. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Cai, T.; Xu, Y.; Ren, L.; Lei, H.; Xu, H. Study of improving nattokinase activity through solid-state fermentation of cold-pressing walnut dregs by compound strains. Sci. Technol. Food Ind. 2017, 38, 112–117. (In Chinese) [Google Scholar] [CrossRef]
- Lee, B.; Lai, Y.; Wu, S. Antioxidation, angiotensin converting enzyme inhibition activity, nattokinase, and antihypertension of Bacillus subtilis (natto)-fermented pigeon pea. J. Food Drug Anal. 2015, 23, 750–757. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Li, J.; Zhang, M.; Yang, Z.; Liang, P.; Mou, J. Study on the technology of natto fermentation by mixed bacteria. China Brew 2019, 38, 49–53. CNKI:SUN:ZNGZ.0.2019-06-009(In Chinese) [Google Scholar]
- Jhan, J.-K.; Chang, W.-F.; Wang, P.-M.; Chou, S.-T.; Chung, Y.-C. Production of fermented red beans with multiple bioactivities using co-cultures of Bacillus subtilis and Lactobacillus delbrueckii subsp. bulgaricus. LWT-Food Sci. Technol. 2015, 63, 1281–1287. [Google Scholar] [CrossRef]
- Luo, S.; Liu, H.; Yang, S.; Yan, Q.; Wu, S.; Jiang, Z. Nutrient composition, thrombolysis and antioxidant activity of three kinds of tempeh fermented by Bacillus amyloliticus. J. Chin. Inst. Food Sci. Technol. 2021, 21, 37–44. (In Chinese) [Google Scholar] [CrossRef]
- Mozzetti, V.; Grattepanche, F.; Moine, D.; Berger, B.; Rezzonico, E.; Meile, L.; Arigoni, F.; Lacroix, C. New method for selection of hydrogen peroxide adapted bifidobacteria cells using continuous culture and immobilized cell technology. Microb. Cell Factories 2010, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosoi, T.; Ametani, A.; Kiuchi, K.; Kaminogawa, S. Improved growth and viability of lactobacilli in the presence of Bacillus subtilis (natto), catalase, or subtilisin. Can. J. Microbiol. 2000, 46, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-R.; Xiong, H.-R.; Guo, X.-H. Enhanced viability of Lactobacillus reuteri for probiotics production in mixed solid-state fermentation in the presence of Bacillus subtilis. Folia Microbiol. 2014, 59, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ng, Y.K.; Koh, E.; Yao, L.; Chien, A.S.; Lin, H.X.; Lee, Y.K. RNA-Seq reveals transcriptomic interactions of Bacillus subtilis natto and Bifidobacterium animalis subsp. lactis in whole soybean solid-state co-fermentation. Food Microbiol. 2015, 51, 25–32. [Google Scholar] [CrossRef] [PubMed]




| Variables | Units | Levels | |
|---|---|---|---|
| 1 | −1 | ||
| A—NaCl content | % | 0 | 1 |
| B—Sucrose addition | % | 1 | 3 |
| D—Fermentation temperature | °C | 37 | 34 |
| E—Fermentation time | h | 30 | 24 |
| G—strain proportion | 2:1 | 1:1 | |
| J—inoculation volume | % | 8 | 6 |
| L—after-ripening time | d | 3 | 1 |
| C, F, H, K | 1 | −1 | |
| Variables | Units | Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| D—Fermentation temperature | °C | 31 | 34 | 37 |
| E—Fermentation time | h | 24 | 30 | 36 |
| J—Inoculation volume | % | 4 | 6 | 8 |
| Source | Nattokinase Activity | Amino Acid Nitrogen | Sensory Scores | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sum of Squares | df | Meansquare | F-Value | p | Significance | Sum of Squares | df | Meansquare | F-Value | p | Significance | Sum of Squares | df | Meansquare | F-Value | p | Significance | |
| Model | 492.89 | 7 | 70.41 | 8.92 | 0.0258 | * | 29.87 | 4 | 7.47 | 31.9 | 0.0001 | ** | 280.06 | 3 | 93.35 | 13.10 | 0.0019 | ** |
| A—NaCl content | 118.94 | 1 | 118.9 | 15.07 | 0.0178 | * | 17.74 | 1 | 17.74 | 75.77 | <0.0001 | ** | ||||||
| B—Sucrose addition | 131.87 | 1 | 131.9 | 16.71 | 0.015 | * | 5.45 | 1 | 5.45 | 23.30 | 0.0019 | ** | ||||||
| D—Fermentation temperature | 88.35 | 1 | 88.35 | 11.19 | 0.0287 | * | 3.19 | 1 | 3.19 | 13.64 | 0.0077 | ** | 55.04 | 1 | 55.04 | 7.72 | 0.024 | * |
| E—Fermentation time | 62.38 | 1 | 62.38 | 7.9 | 0.0482 | * | 3.49 | 1 | 3.49 | 14.9 | 0.0062 | ** | 101.5 | 1 | 101.5 | 14.24 | 0.0054 | ** |
| G—Strain proportion | 28.34 | 1 | 28.34 | 3.59 | 0.131 | |||||||||||||
| J—Inoculation volume | 9.08 | 1 | 9.08 | 1.15 | 0.3438 | 123.52 | 1 | 123.52 | 17.33 | 0.0032 | ** | |||||||
| L—After-ripening time | 53.93 | 1 | 53.93 | 6.83 | 0.0592 | |||||||||||||
| Residual | 31.57 | 4 | 7.89 | 8.92 | 0.0258 | 1.64 | 7 | 0.23 | 57.02 | 8 | 7.13 | |||||||
| sum | 524.46 | 11 | 15.07 | 31.51 | 11 | 337.08 | 11 | |||||||||||
| Center Hole Area 1.98 ± 0.39 mm2 | GUTU09 Single-Strain | Double-Strain Fermentation | ||
|---|---|---|---|---|
| Dissolving Circle Diameter (mm) | Thrombolytic Area (mm2) | Dissolving Circle Diameter (mm) | Thrombolytic Area (mm2) | |
| 6 h | 5.46 ± 0.45 g | 21.53 ± 3.4 G | 4.77 ± 0.69 g | 16.16 ± 4.75 G |
| 12 h | 14.17 ± 0.31 f | 155.76 ± 6.55 F | 13.75 ± 0.51 f | 146.64 ± 10.66 F |
| 18 h | 16.13 ± 0.48 e | 202.46 ± 11.86 E | 16.12 ± 0.45 e | 202.11 ± 11.02 E |
| 24 h | 17.1 ± 0.20 d | 232.64 ± 5.08 D | 17.77 ± 0.2 c | 245.83 ± 5.18 C |
| 30 h | 18.28 ± 0.38 b | 272.8 ± 10.66 B | 19.70 ± 0.35 a | 302.73 ± 10.37 A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Lan, G.; Tian, X.; He, L.; Li, C.; Zeng, X.; Wang, X. Effect of Fermentation Parameters on Natto and Its Thrombolytic Property. Foods 2021, 10, 2547. https://doi.org/10.3390/foods10112547
Yang Y, Lan G, Tian X, He L, Li C, Zeng X, Wang X. Effect of Fermentation Parameters on Natto and Its Thrombolytic Property. Foods. 2021; 10(11):2547. https://doi.org/10.3390/foods10112547
Chicago/Turabian StyleYang, Yun, Guangqun Lan, Xueyi Tian, Laping He, Cuiqin Li, Xuefeng Zeng, and Xiao Wang. 2021. "Effect of Fermentation Parameters on Natto and Its Thrombolytic Property" Foods 10, no. 11: 2547. https://doi.org/10.3390/foods10112547
APA StyleYang, Y., Lan, G., Tian, X., He, L., Li, C., Zeng, X., & Wang, X. (2021). Effect of Fermentation Parameters on Natto and Its Thrombolytic Property. Foods, 10(11), 2547. https://doi.org/10.3390/foods10112547






