Selenium-Enriched Soy Protein Has Antioxidant Potential via Modulation of the NRF2-HO1 Signaling Pathway
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
2.2. Preparation of Soybean Protein Isolate
2.3. Superoxide Anion Scavenging Activity
2.4. Measurement of Reducing Power
2.5. Cell Culture and Treatment
2.6. Determination of Cell Viability
2.7. Flow Cytometry Analysis of the NRF2-HO1 Signaling Pathway in Caco-2
2.8. Animals and Experimental Design
2.9. Measurement of Se Concentration in Tissues
2.10. Measurement of Superoxide Dismutase (SOD) and Glutathione Peroxidase (GPx) Activities
2.11. Statistical Analysis
3. Results
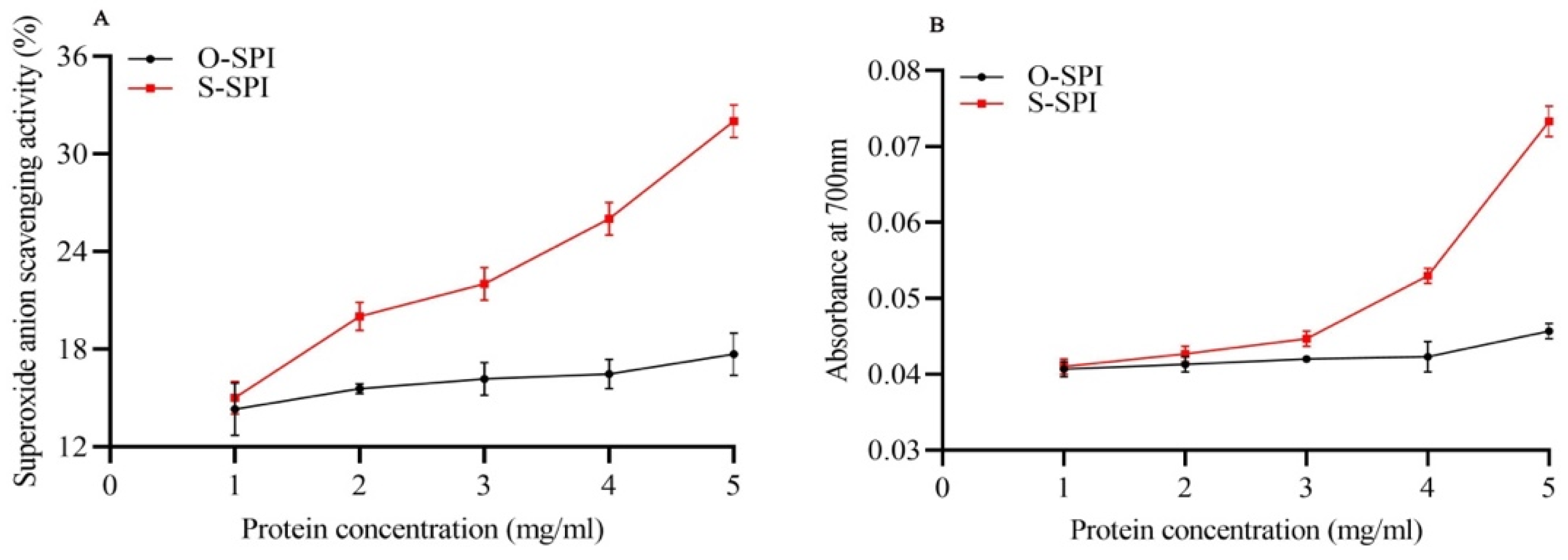
3.1. S-SPI Effectively Increases Superoxide Anion Scavenging Ability and Reducing Power of Fe3+
3.2. S-SPI Treatment Protects Caco-2 Cells from Oxidative Stress Induced by H2O2
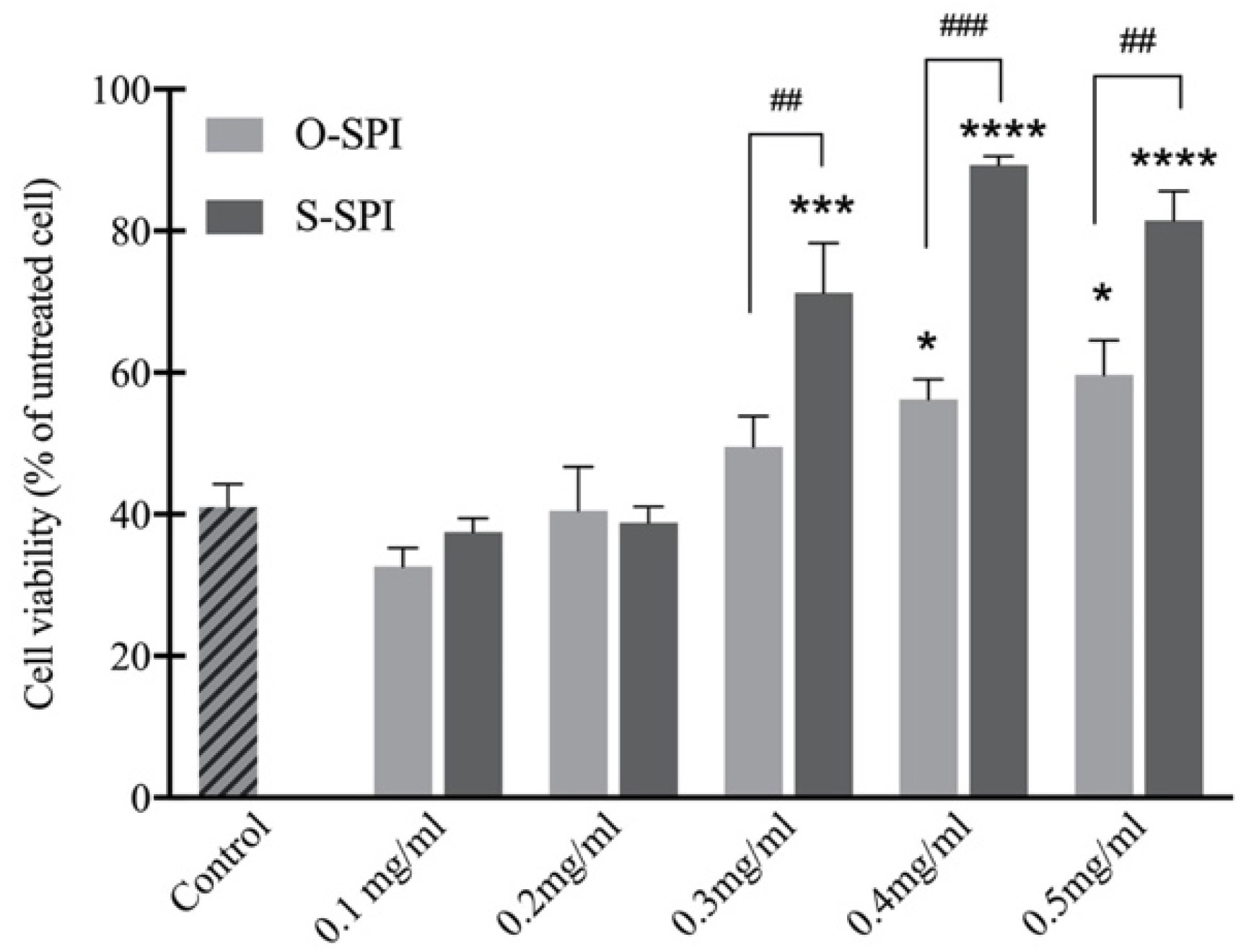
3.2.1. S-SPI Treatment Improves Cell Viability in H2O2-Exposed Caco-2 Cells
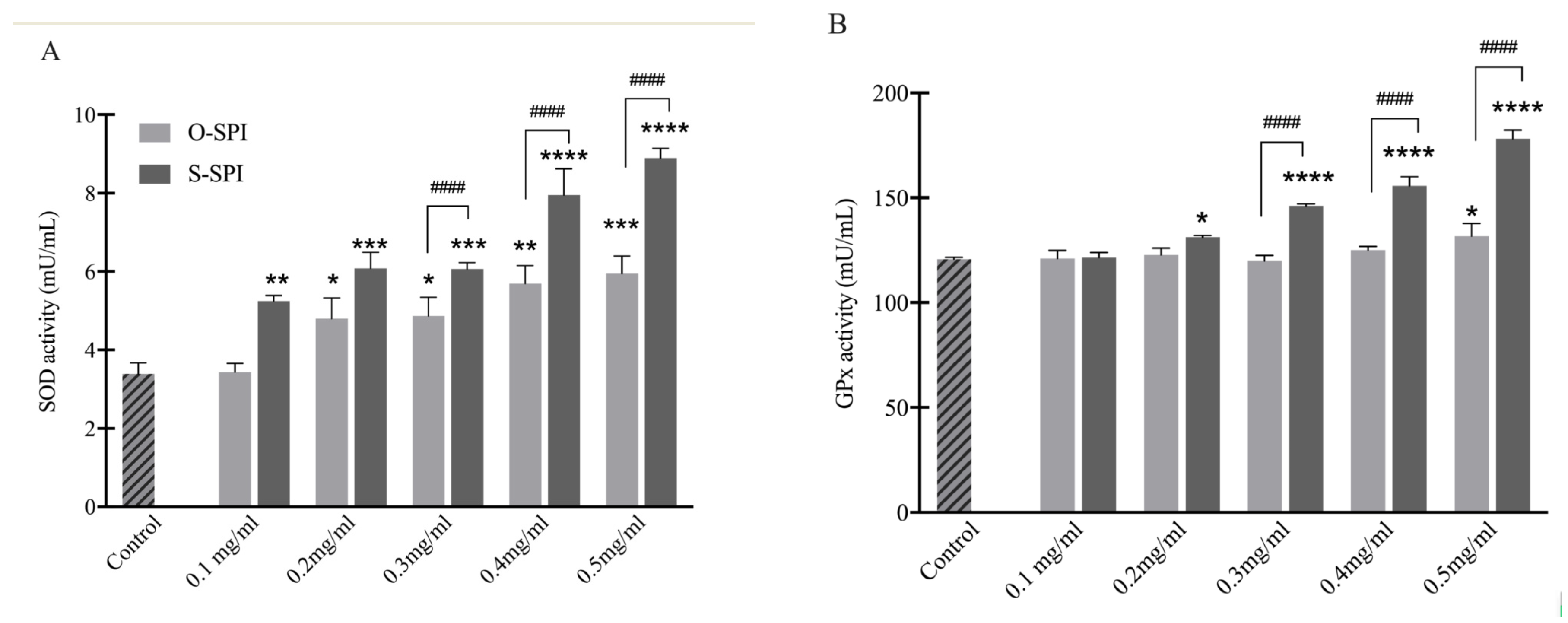
3.2.2. S-SPI Treatment Increases Activity of Key Antioxidant Enzymes SOD and GPx in Caco-2 Cells
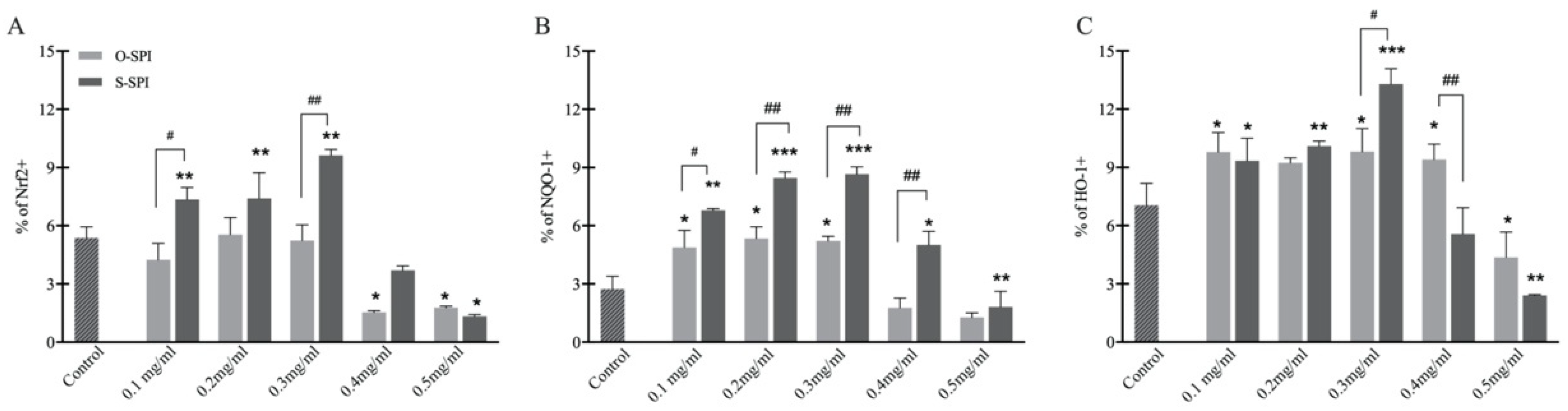
3.2.3. Suppression of Oxidative Stress in H2O2-Exposed Caco-2 Cells by S-SPI Is Related to Increased Protein Levels of the NRF2 Pathway
3.3. S-SPI Supplementation Effectively Enhances Antioxidant Activity in BALB/c Mice
3.3.1. Supplementation with S-SPI Significantly Increases Tissue Levels of BALB/c Mice
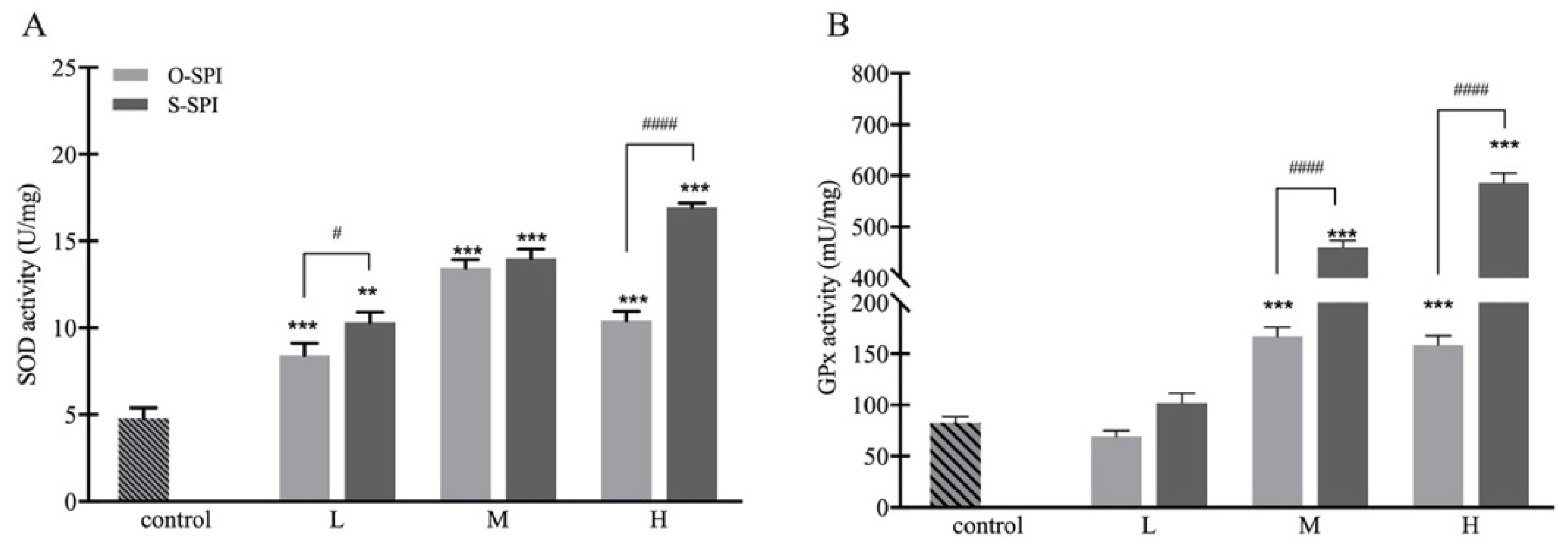
3.3.2. SPI Supplementation Increases Activity of Hepatic SOD and GPx of BALB/c Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ojeda, M.L.; Nogales, F.; Serrano, A.; Murillo, M.L.; Carreras, O. Selenoproteins and renal programming in metabolic syndrome-exposed rat offspring. Food Funct. 2020, 11, 3904–3915. [Google Scholar] [CrossRef]
- Wrobel, J.K.; Power, R.; Toborek, M. Biological activity of selenium: Revisited. IUBMB Life 2016, 68, 97–105. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium intake, status, and health: A complex relationship. Hormones 2020, 19, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Mistry, H.D.; Pipkin, F.B.; Redman, C.W.; Poston, L. Selenium in reproductive health. Am. J. Obstet. Gynecol. 2012, 206, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Speckmann, B.; Steinbrenner, H. Selenium and selenoproteins in inflammatory bowel diseases and experimental colitis. Inflamm. Bowel Dis. 2014, 20, 1110–1119. [Google Scholar] [CrossRef]
- Hoffmann, P.R.; Berry, M.J. The influence of selenium on immune responses. Mol. Nutr. Food Res. 2008, 52, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Oropeza-Moe, M.; Wisloff, H.; Bernhoft, A. Selenium deficiency associated porcine and human cardiomyopathies. J. Trace Elem. Med. Biol. 2015, 31, 148–156. [Google Scholar] [CrossRef]
- Yiming, Z.; Qingqing, L.; Hang, Y.; Yahong, M.; Shu, L. Selenium deficiency causes immune damage by activating the DUSP1/NF-kappaB pathway and endoplasmic reticulum stress in chicken spleen. Food Funct. 2020, 11, 6467–6475. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.M.; Arthur, J.R. Selenium, selenoproteins and human health: A review. Public Health Nutr. 2001, 4, 593–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinceti, M.; Filippini, T.; Cilloni, S.; Crespi, C.M. The Epidemiology of Selenium and Human Cancer. Adv. Cancer Res. 2017, 136, 1–48. [Google Scholar]
- Yuan, C.; Li, Z.; Peng, F.; Xiao, F.; Ren, D.; Xue, H.; Chen, T.; Mushtaq, G.; Kamal, M.A. Combination of selenium-enriched green tea polysaccharides and Huo-ji polysaccharides synergistically enhances antioxidant and immune activity in mice. J. Sci. Food Agric. 2015, 95, 3211–3217. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhao, Q.; Chen, H.; Xiong, H. Distribution and effects of natural selenium in soybean proteins and its protective role in soybean β-conglycinin (7S globulins) under AAPH-induced oxidative stress. Food Chem. 2019, 272, 201–209. [Google Scholar] [CrossRef]
- Maneetong, S.; Chookhampaeng, S. Hydroponic cultivation of selenium-enriched kale (Brassica oleracea var. alboglabra L.) seedling and speciation of selenium with HPLC–ICP-MS. Microchem. J. 2013, 108, 87–91. [Google Scholar]
- Bodnar, M.; Szczyglowska, M.; Konieczka, P.; Namiesnik, J. Methods of Selenium Supplementation: Bioavailability and Determination of Selenium Compounds. Crit. Rev. Food Sci. Nutr. 2016, 56, 36–55. [Google Scholar] [CrossRef]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.Y.; Alfulaij, N.; Berry, M.J.; Seale, L.A. From Selenium Absorption to Selenoprotein Degradation. Biol. Trace Elem. Res. 2019, 192, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, H.; Sies, H. Protection against reactive oxygen species by selenoproteins. Biochim. Biophys. Acta 2009, 1790, 1478–1485. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H. Selenium as an Essential Micronutrient: Roles in Cell Cycle and Apoptosis. Molecules 2009, 14, 1263–1278. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.J.; Xie, J.H.; Nie, S.P.; Xie, M.Y. Review on cell models to evaluate the potential antioxidant activity of polysaccharides. Food Funct. 2017, 8, 915–926. [Google Scholar] [CrossRef]
- Luo, S.; Jiang, X.; Jia, L.; Tan, C.; Li, M.; Yang, Q.; Du, Y.; Ding, C. In Vivo and In Vitro Antioxidant Activities of Methanol Extracts from Olive Leaves on Caenorhabditis elegans. Molecules 2019, 24, 704. [Google Scholar] [CrossRef] [Green Version]
- Vicente, S.J.; Ishimoto, E.Y.; Cruz, R.J.; Pereira, C.D.; Torres, E.A. Increase of the activity of phase II antioxidant enzymes in rats after a single dose of coffee. J. Agric. Food Chem. 2011, 59, 10887–10892. [Google Scholar] [CrossRef]
- Faraji, T.; Momeni, H.R.; Malmir, M. Protective effects of silymarin on testis histopathology, oxidative stress indicators, antioxidant defence enzymes and serum testosterone in cadmium-treated mice. Andrologia 2019, 51, e13242. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Farooq, M.U.; Zhang, G.; Tang, Z.; Zheng, T.; Su, Y.; Hussain, S.; Liang, Y.; Ye, X.; Jia, X.; et al. Dissecting the Potential of Selenoproteins Extracted from Selenium-Enriched Rice on Physiological, Biochemical and Anti-Ageing Effects In Vivo. Biol. Trace Elem. Res. 2019, 196, 119–130. [Google Scholar] [CrossRef]
- Shi, L.; Xun, W.; Yue, W.; Zhang, C.; Ren, Y.; Shi, L.; Wang, Q.; Yang, R.; Lei, F. Effect of sodium selenite, Se-yeast and nano-elemental selenium on growth performance, Se concentration and antioxidant status in growing male goats. Small Rumin. Res. 2011, 96, 49–52. [Google Scholar] [CrossRef]
- Kiewiet, M.B.G.; van Esch, B.; Garssen, J.; Faas, M.M.; de Vos, P. Partially hydrolyzed whey proteins prevent clinical symptoms in a cow’s milk allergy mouse model and enhance regulatory T and B cell frequencies. Mol. Nutr. Food Res. 2017, 61, 1700340. [Google Scholar] [CrossRef] [PubMed]
- Genovese, M.I.; Barbosa, A.C.; Mda, S.P.; Lajolo, F.M. Commercial soy protein ingredients as isoflavone sources for functional foods. Plant. Foods Hum. Nutr. 2007, 62, 53–58. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, Q.; Cheng, X.; Selomulya, C.; Bai, C.; Zhu, X.; Li, X.; Xiong, H. Antioxidant activities of Se-SPI produced from soybean as accumulation and biotransformation reactor of natural selenium. Food Chem. 2014, 146, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Li, C.P.; He, Z.; Wang, X.; Yang, L.; Yin, C.; Zhang, N.; Lin, J.; Zhao, H. Selenization of ovalbumin by dry-heating in the presence of selenite: Effect on protein structure and antioxidant activity. Food Chem. 2014, 148, 209–217. [Google Scholar] [CrossRef]
- García-Nebot, M.J.; Cilla, A.; Alegría, A.; Barberá, R. Caseinophosphopeptides exert partial and site-specific cytoprotection against H2O2-induced oxidative stress in Caco-2 cells. Food Chem. 2011, 129, 1495–1503. [Google Scholar] [CrossRef]
- Chen, J.; Feng, L.; Qi, C.; Li, Y.; Wang, D. Direct sampling introduction and atomic fluorescence spectrometry for determination of selenium content in mice liver and urine. Chin. J. Endem. 2018, 37, 149–151. [Google Scholar]
- Wang, Y.; Wang, X. Binding, stability, and antioxidant activity of quercetin with soy protein isolate particles. Food Chem. 2015, 188, 24–29. [Google Scholar] [CrossRef]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef]
- Beermann, C.; Euler, M.; Herzberg, J.; Stahl, B. Anti-oxidative capacity of enzymatically released peptides from soybean protein isolate. Eur. Food Res. Technol. 2009, 229, 637–644. [Google Scholar] [CrossRef]
- Du, M.; Zhao, L.; Li, C.; Zhao, G.; Hu, X. Purification and characterization of a novel fungi Se-containing protein from Se-enriched Ganoderma Lucidum mushroom and its Se-dependent radical scavenging activity. Eur. Food Res. Technol. 2006, 224, 659–665. [Google Scholar] [CrossRef]
- Gao, Q.; Liu, Z.H.; Wu, J.L.; Geng, Y.; Zhang, Q.; Tie, M.; Gu, X.J.; Tanokura, M.; Xue, Y.L. Foliar application is an effective method for incorporating selenium into peanut leaf proteins with antioxidant activities. Food Res. Int. 2019, 126, 108617. [Google Scholar] [CrossRef]
- Lukashenko, N.P. Expanding genetic code: Amino acids 21 and 22, selenocysteine and pyrrolysine. Russ. J. Genet. 2010, 46, 899–916. [Google Scholar] [CrossRef]
- Chan, Q.; Afton, S.E.; Caruso, J.A. Selenium speciation profiles in selenite-enriched soybean (Glycine Max) by HPLC-ICPMS and ESI-ITMS. Metallomics 2010, 2, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Zhang, B.; Xu, R.; Wang, Y.; Ding, X.; Li, P. Antioxidant activity in vitro of the selenium-contained protein from the Se-enriched Bifidobacterium animalis 01. Anaerobe 2010, 16, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Cruz, N.; Qi, L.; Alvarez, X.; Berg, R.D.; Deitch, E.A. The Caco-2 cell monolayer system as an in vitro model for studying bacterial-enterocyte interactions and bacterial translocation. J. Burn Care Rehabil. 1994, 15, 207–212. [Google Scholar] [CrossRef]
- Yu, H.; Ge, Y.; Wang, Y.; Lin, C.T.; Li, J.; Liu, X.; Zang, T.; Xu, J.; Liu, J.; Luo, G.; et al. A fused selenium-containing protein with both GPx and SOD activities. Biochem. Biophys. Res. Commun. 2007, 358, 873–878. [Google Scholar] [CrossRef]
- Farhat, Z.; Browne, R.W.; Bonner, M.R.; Tian, L.; Deng, F.; Swanson, M.; Mu, L. How do glutathione antioxidant enzymes and total antioxidant status respond to air pollution exposure? Environ. Int. 2018, 112, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhang, Y.; Miao, W.; Cheng, T. Reactive Oxygen Species and Nrf2: Functional and Transcriptional Regulators of Hematopoiesis. Oxidative Med. Cell. Longev. 2019, 2019, 5153268. [Google Scholar] [CrossRef] [PubMed]
- Pacini, N.; Elia, A.C.; Abete, M.C.; Dorr, A.J.; Brizio, P.; Gasco, L.; Righetti, M.; Prearo, M. Antioxidant response versus selenium accumulation in the liver and kidney of the Siberian sturgeon (Acipenser baeri). Chemosphere 2013, 93, 2405–2412. [Google Scholar] [CrossRef] [PubMed]
- Haratake, M.; Yoshida, S.; Mandai, M.; Fuchigami, T.; Nakayama, M. Elevated amyloid-beta plaque deposition in dietary selenium-deficient Tg2576 transgenic mice. Metallomics 2013, 5, 479–483. [Google Scholar] [CrossRef] [Green Version]
- Kiermayer, C.; Michalke, B.; Schmidt, J.; Brielmeier, M. Effect of selenium on thioredoxin reductase activity in Txnrd1 or Txnrd2 hemizygous mice. Biol. Chem. 2007, 388, 1091–1097. [Google Scholar] [CrossRef]
- Oster, O.; Schmiedel, G.; Prellwitz, W. The organ distribution of selenium in German adults. Biol. Trace Elem. Res. 1988, 15, 23–45. [Google Scholar] [CrossRef]
| Control mg/kg | L-S-SPI mg/kg | M-S-SPI mg/kg | H-S-SPI mg/kg | L-O-SPI mg/kg | M-O-SPI mg/kg | H-O-SPI mg/kg | |
|---|---|---|---|---|---|---|---|
| Liver | 0.31 ± 0.09 | 0.61 ± 0.06 | 0.87 ± 0.05 b | 1.34 ± 0.15 a | 0.51 ± 0.03 | 0.62 ± 0.03 | 0.43 ± 0.05 |
| Kidney | 0.46 ± 0.03 | 0.54 ± 0.05 | 0.77 ± 0.03 b | 0.92 ± 0.05 a | 0.53 ± 0.03 | 0.69 ± 0.02 c | 0.66 ± 0.03 c |
| Spleen | 0.17 ± 0.04 | 0.35 ± 0.01 c | 0.40 ± 0.03 b | 0.51 ± 0.08 a | 0.29 ± 0.05 | 0.32 ± 0.04 | 0.31 ± 0.04 |
| Stomach | 0.07 ± 0.05 | 0.13 ± 0.01 | 0.15 ± 0.01 b | 0.18 ± 0.01 a | 0.12 ± 0.02 | 0.13 ± 0.02 | 0.12 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Gao, J.; Hogenkamp, A.; Knippels, L.M.J.; Garssen, J.; Bai, J.; Yang, A.; Wu, Y.; Chen, H. Selenium-Enriched Soy Protein Has Antioxidant Potential via Modulation of the NRF2-HO1 Signaling Pathway. Foods 2021, 10, 2542. https://doi.org/10.3390/foods10112542
Zhao X, Gao J, Hogenkamp A, Knippels LMJ, Garssen J, Bai J, Yang A, Wu Y, Chen H. Selenium-Enriched Soy Protein Has Antioxidant Potential via Modulation of the NRF2-HO1 Signaling Pathway. Foods. 2021; 10(11):2542. https://doi.org/10.3390/foods10112542
Chicago/Turabian StyleZhao, Xiaoli, Jinyan Gao, Astrid Hogenkamp, Leon M. J. Knippels, Johan Garssen, Jing Bai, Anshu Yang, Yong Wu, and Hongbing Chen. 2021. "Selenium-Enriched Soy Protein Has Antioxidant Potential via Modulation of the NRF2-HO1 Signaling Pathway" Foods 10, no. 11: 2542. https://doi.org/10.3390/foods10112542
APA StyleZhao, X., Gao, J., Hogenkamp, A., Knippels, L. M. J., Garssen, J., Bai, J., Yang, A., Wu, Y., & Chen, H. (2021). Selenium-Enriched Soy Protein Has Antioxidant Potential via Modulation of the NRF2-HO1 Signaling Pathway. Foods, 10(11), 2542. https://doi.org/10.3390/foods10112542






