The Effect of Trans Fatty Acids on Human Health: Regulation and Consumption Patterns
Abstract
:1. Introduction
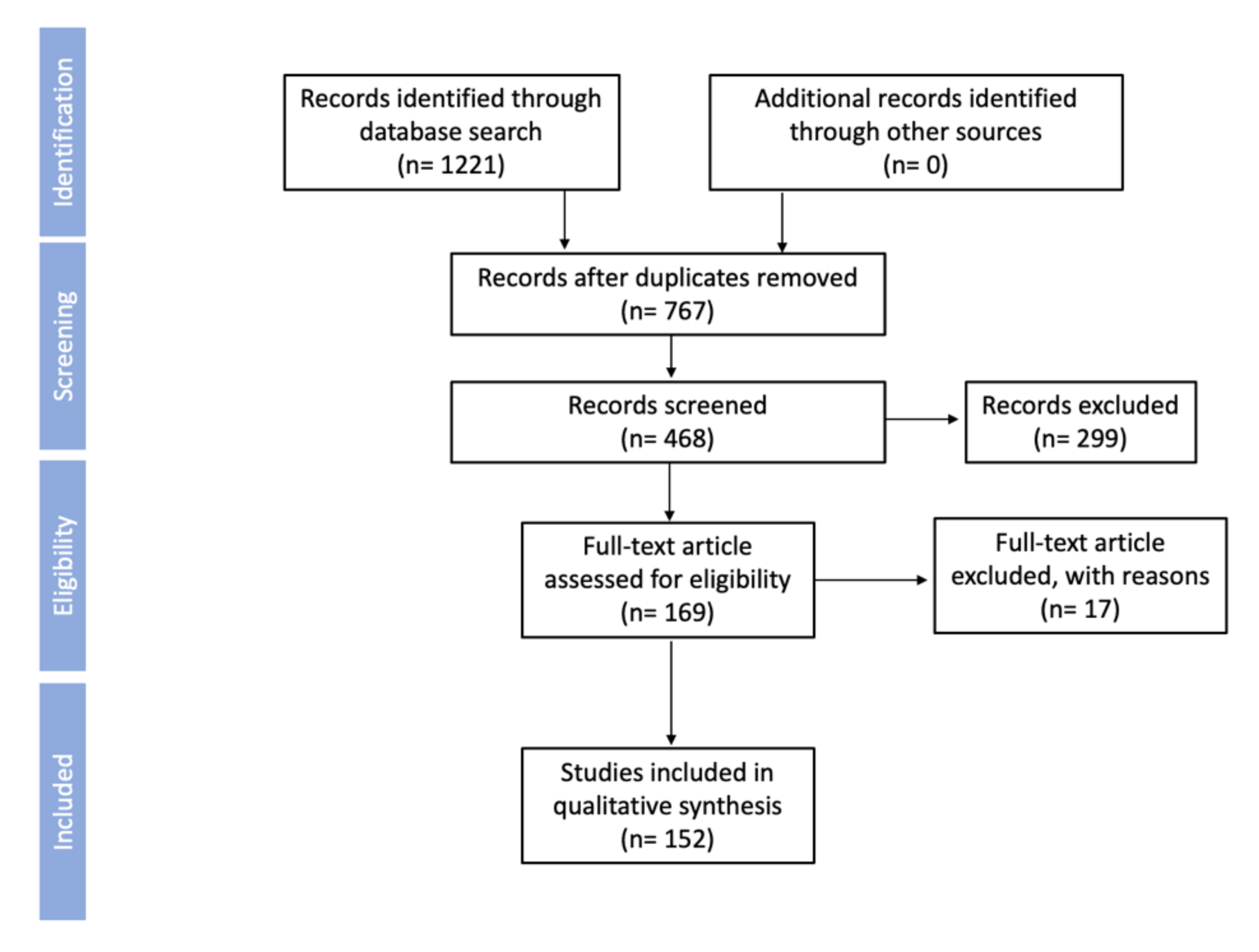
2. Objectives and Methods
3. Health Effects of TFAs
3.1. Obesity
3.2. Insulin Resistance
3.3. Cardiovascular Disease (CVD)
3.4. Cancer
3.5. Inflammation
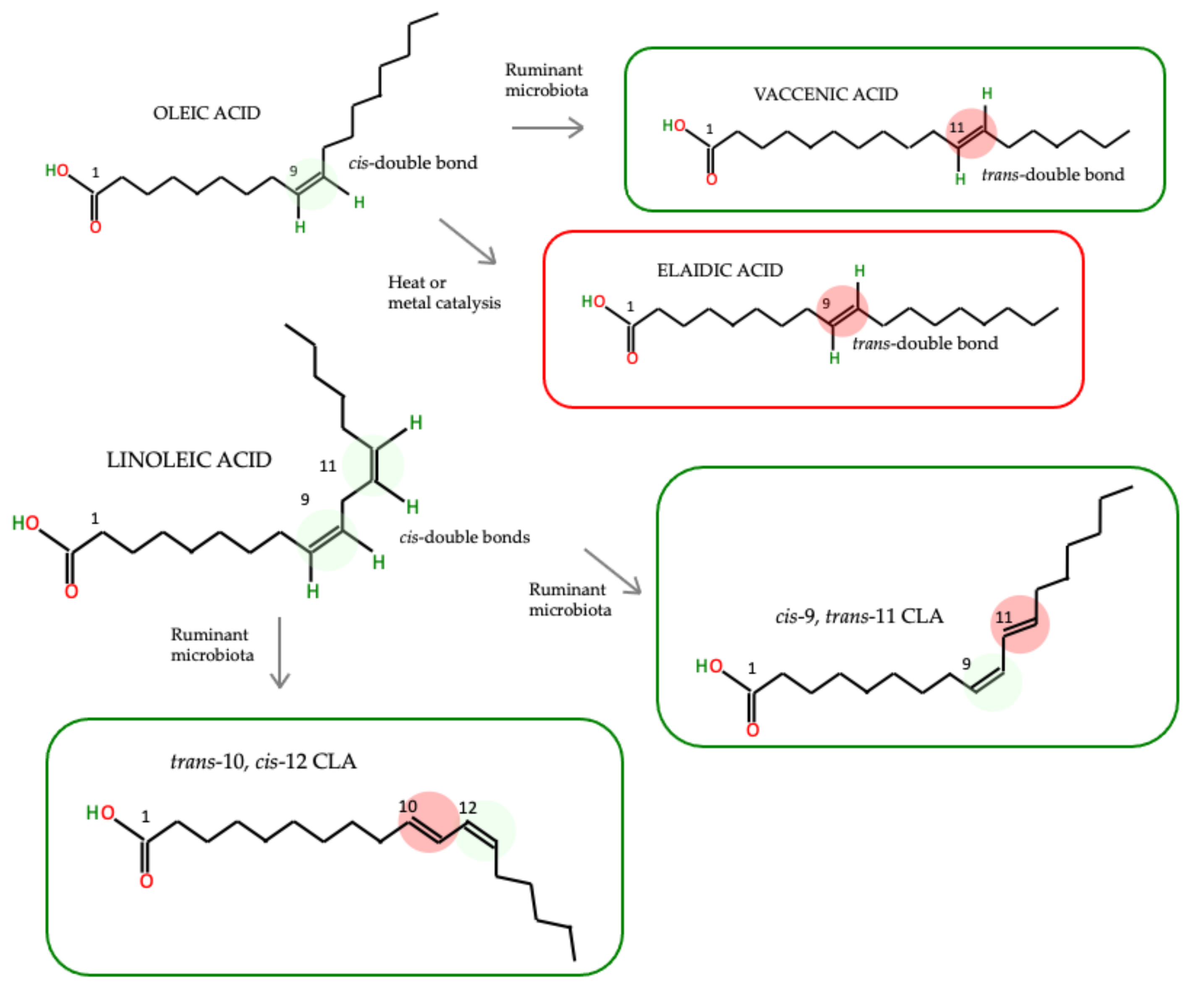
4. Do the Health Effects of TFAs Differ Depending on Their Origin?
5. nTFAs and iTFAs’ Impact on Gut Microbiota: Eubiotics or Xenobiotics?
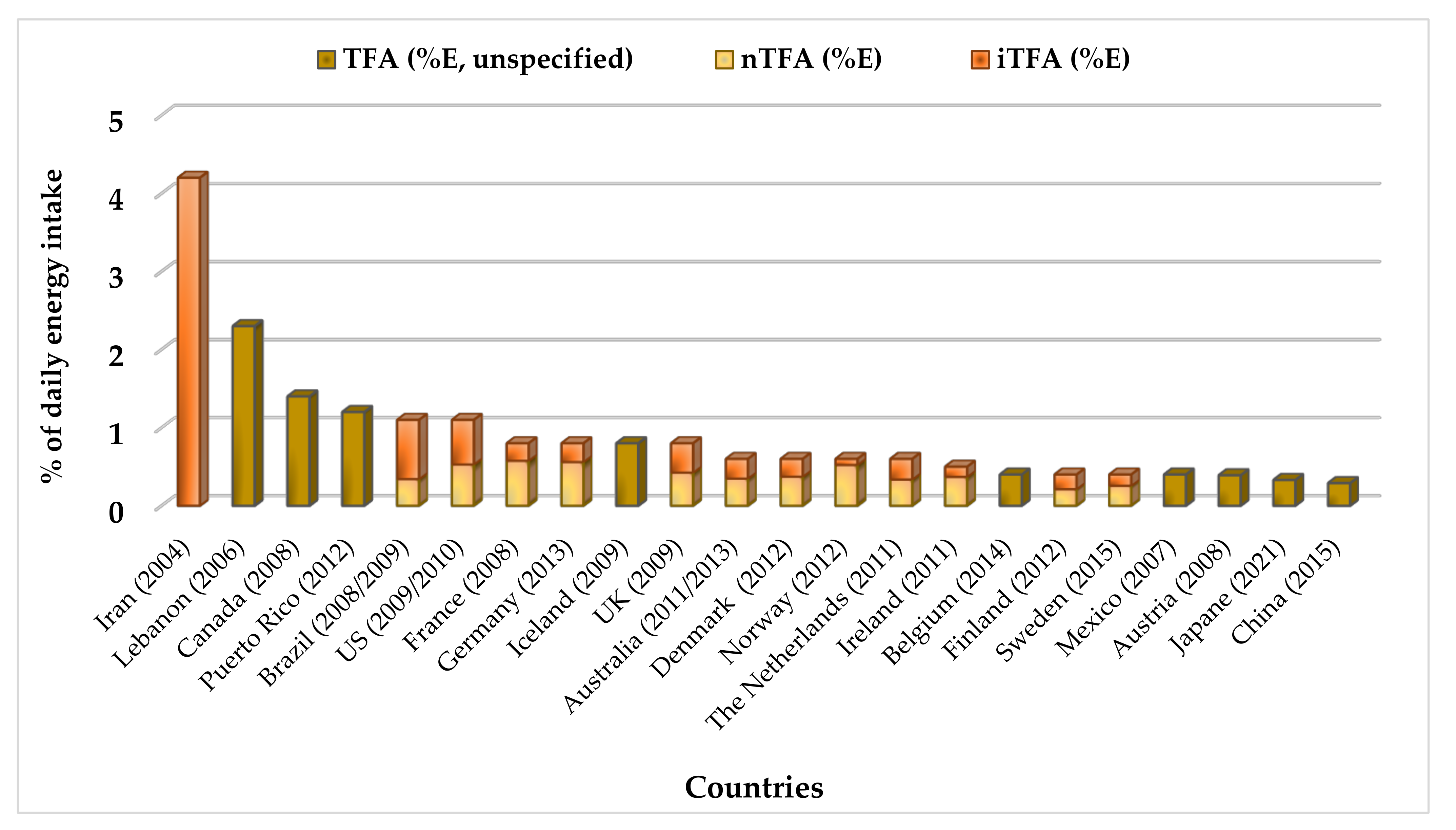
6. Comparison of the Regulations and Reduction Policies of TFAs Worldwide
Comparison of the Intake and Health Effects of TFAs
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Semma, M. Trans Fatty Acids: Properties, Benefits and Risks. J. Health Sci. 2002, 48, 7–13. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Autority (EFSA). Opinion of the Scientific Panel on Dietetic products, nutrition and allergies [NDA] related to the presence of trans fatty acids in foods and the effect on human health of the consumption of trans fatty acids. EFSA J. 2004, 2, 1–49. [Google Scholar] [CrossRef]
- Allott, E.H.; Arab, L.; Su, L.J.; Farnan, L.; Fontham, E.T.H.; Mohler, J.L.; Bensen, J.T.; Steck, S.E. Saturated fat intake and prostate cancer aggressiveness: Results from the population-based North Carolina-Louisiana Prostate Cancer Project. Prostate Cancer Prostatic Dis. 2017, 20, 48–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briggs, M.; Petersen, K.; Kris-Etherton, P. Saturated Fatty Acids and Cardiovascular Disease: Replacements for Saturated Fat to Reduce Cardiovascular Risk. Healthcare 2017, 5, 29. [Google Scholar] [CrossRef] [Green Version]
- Nettleton, J.A.; Brouwer, I.A.; Geleijnse, J.M.; Hornstra, G. Saturated Fat Consumption and Risk of Coronary Heart Disease and Ischemic Stroke: A Science Update. Ann. Nutr. Metab. 2017, 70, 26–33. [Google Scholar] [CrossRef]
- Parks, E.; Yki-Järvinen, H.; Hawkins, M. Out of the frying pan: Dietary saturated fat influences nonalcoholic fatty liver disease. J. Clin. Investig. 2017, 127, 454–456. [Google Scholar] [CrossRef] [Green Version]
- Aldai, N.; de Renobales, M.; Barron, L.J.R.; Kramer, J.K.G. What are the trans fatty acids issues in foods after discontinuation of industrially produced trans fats? Ruminant products, vegetable oils, and synthetic supplements. Eur. J. Lipid Sci. Technol. 2013, 115, 1378–1401. [Google Scholar] [CrossRef]
- Valenzuela, C.A.; Baker, E.J.; Miles, E.A.; Calder, P.C. Eighteen-carbon trans fatty acids and inflammation in the context of atherosclerosis. Prog. Lipid Res. 2019, 76. [Google Scholar] [CrossRef]
- Kim, E.J.; Huws, S.A.; Lee, M.R.F.; Scollan, N.D. Dietary transformation of lipid in the rumen microbial ecosystem. Asian-Australas. J. Anim. Sci. 2009, 22, 1341–1350. [Google Scholar] [CrossRef]
- Wanders, A.J.; Zock, P.L.; Brouwer, I.A. Trans fat intake and its dietary sources in general populations worldwide: A systematic review. Nutrients 2017, 9, 840. [Google Scholar] [CrossRef] [PubMed]
- Puligundla, P.; Variyar, P.S.; Ko, S.; Obulam, V.S.R. Emerging trends in modification of dietary oils and fats, and health implications—A review. Sains Malays. 2012, 41, 871–877. [Google Scholar]
- Vargas-bello-pérez, E.; Garnsworthy, P.C. Trans Fatty Acids And Their Role In The Milk Of Dairy Cows. Cien. Inv. Agr. 2013, 40, 449–473. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Cobb, L.K.; Vesper, H.W.; Asma, S. Global surveillance of trans-fatty acids. Prev. Chronic Dis. 2019, 16, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Federico, E.; Jones, A.; Wu, J.H.Y. Presence of trans fatty acids containing ingredients in pre-packaged foods in Australia in 2018. Aust. N. Z. J. Public Health 2020, 44, 419–420. [Google Scholar] [CrossRef] [PubMed]
- Dominguez Rodríguez, L.; Barbagallo, M. Not all fats are unhealthy. In The Prevention of Cardiovascular Disease through the Mediterranean Diet; Sánchez-Villegas, A., Sánchez-Tainta, A., Eds.; Elsevier: London, UK, 2018; pp. 35–58. [Google Scholar]
- Chen, Y.; Yang, Y.; Nie, S.; Yang, X.; Wang, Y.; Yang, M.; Li, C.; Xie, M. The analysis of trans fatty acid profiles in deep frying palm oil and chicken fillets with an improved gas chromatography method. Food Control 2014, 44, 191–197. [Google Scholar] [CrossRef]
- Dhaka, V.; Gulia, N.; Ahlawat, K.S.; Khatkar, B.S. Trans fats-sources, health risks and alternative approach—A review. J. Food Sci. Technol. 2011, 48, 534–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loor, J.J.; Lin, X.; Herbein, J.H. Effects of dietary cis 9, trans 11–18: 2, trans 10, cis 12–18: 2, or vaccenic acid (trans 11–18: 1) during lactation on body composition, tissue fatty acid profiles, and litter growth in mice. Br. J. Nutr. 2003, 90, 1039–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brouwer, I.A.; Wanders, A.J.; Katan, M.B. Effect of animal and industrial Trans fatty acids on HDL and LDL cholesterol levels in humans—A quantitative review. PLoS ONE 2010, 5, e9434. [Google Scholar] [CrossRef]
- Qiu, B.; Wang, Q.; Liu, W.; Xu, T.C.; Liu, L.N.; Zong, A.Z.; Jia, M.; Li, J.; Du, F.L. Biological effects of trans fatty acids and their possible roles in the lipid rafts in apoptosis regulation. Cell Biol. Int. 2018, 42, 904–912. [Google Scholar] [CrossRef]
- World Health Organisation (WHO). International Agency for Research on Cancer (IARC). The Global Burden of Cancer. In World Cancer Report; IARC Press: Lion, France, 2003; Volume 22. [Google Scholar]
- Stender, S.; Dyerberg, J. Influence of trans fatty acids on health. Ann. Nutr. Metab. 2004, 48, 61–66. [Google Scholar] [CrossRef]
- Fuke, G.; Nornberg, J.L. Systematic evaluation on the effectiveness of conjugated linoleic acid in human health. Crit. Rev. Food Sci. Nutr. 2017, 51, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, S.; Prakasan, P.; Sreedharan, S.; Wright, A.G.; Spener, F. Pros and cons of CLA consumption an insight. Nutr. Metab. 2015, 12, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferlay, A.; Bernard, L.; Meynadier, A.; Malpuech-Brugère, C. Production of trans and conjugated fatty acids in dairy ruminants and their putative effects on human health: A review. Biochimie 2017, 141, 107–120. [Google Scholar] [CrossRef]
- Lehnen, T.E.; da Silva, M.R.; Camacho, A.; Marcadenti, A.; Lehnen, A.M. A review on effects of conjugated linoleic fatty acid (CLA) upon body composition and energetic metabolism. J. Int. Soc. Sports Nutr. 2015, 12, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oteng, A.B.; Kersten, S. Mechanisms of Action of trans Fatty Acids. Adv. Nutr. 2020, 11, 697–708. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Rimm, E.B.; King, I.B.; Lawler, R.L.; McDonald, G.B.; Levy, W.C. Trans fatty acids and systemic inflammation in heart failure. Am. J. Clin. Nutr. 2004, 80, 1521–1525. [Google Scholar] [CrossRef]
- Wang, Q.; Afshin, A.; Yakoob, M.Y.; Singh, G.M.; Rehm, C.D.; Khatibzadeh, S.; Micha, R.; Shi, P.; Mozaffarian, D.; Ezzati, M.; et al. Impact of nonoptimal intakes of saturated, polyunsaturated, and trans fat on global burdens of coronary heart disease. J. Am. Heart Assoc. 2016, 5, e002891. [Google Scholar] [CrossRef] [Green Version]
- Gaullier, J.M.; Halse, J.; Høye, K.; Kristiansen, K.; Fagertun, H.; Vik, H.; Gudmundsen, O. Conjugated linoleic acid supplementation for 1 y reduces body fat mass in healthy overweight humans. Am. J. Clin. Nutr. 2004, 79, 1118–1125. [Google Scholar] [CrossRef] [Green Version]
- Blankson, H.; Stakkestad, J.A.; Fagertun, H.; Thom, E.; Wadstein, J.; Gudmundsen, O. Conjugated linoleic acid reduces body fat mass in overweight and obese humans. J. Nutr. 2000, 130, 2943–2948. [Google Scholar] [CrossRef]
- Chen, S.C.; Lin, Y.H.; Huang, H.P.; Hsu, W.L.; Houng, J.Y.; Huang, C.K. Effect of conjugated linoleic acid supplementation on weight loss and body fat composition in a Chinese population. Nutrition 2012, 28, 559–565. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, J.; Ruth, M.R.; Goruk, S.D.; Reaney, M.J.; Glimm, D.R.; Vine, D.F.; Field, C.J.; Proctor, S.D. Trans-11 vaccenic acid dietary supplementation induces hypolipidemic effects in JCR:LA-cp rats. J. Nutr. 2008, 138, 2117–2122. [Google Scholar] [CrossRef] [Green Version]
- Jacome-Sosa, M.M.; Borthwick, F.; Mangat, R.; Uwiera, R.; Reaney, M.J.; Shen, J.; Quiroga, A.D.; Jacobs, R.L.; Lehner, R.; Proctor, S.D. Diets enriched in trans-11 vaccenic acid alleviate ectopic lipid accumulation in a rat model of NAFLD and metabolic syndrome. J. Nutr. Biochem. 2014, 25, 692–701. [Google Scholar] [CrossRef]
- Mohankumar, S.K.; Hanke, D.; Siemens, L.; Cattini, A.; Enns, J.; Shen, J.; Reaney, M.; Zahradka, P.; Taylor, C.G. Dietary supplementation of trans-11-vaccenic acid reduces adipocyte size but neither aggravates nor attenuates obesity-mediated metabolic abnormalities in fa/fa Zucker rats. Br. J. Nutr. 2013, 109, 1628–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, S.V.; Jacques, H.; Plourde, M.; Mitchell, P.L.; McLeod, R.S.; Jones, P.J.H. Conjugated linoleic acid supplementation for 8 weeks does not affect body composition, lipid profile, or safety biomarkers in overweight, hyperlipidemic men. J. Nutr. 2011, 141, 1286–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chajès, V.; Biessy, C.; Ferrari, P.; Romieu, I.; Freisling, H.; Huybrechts, I.; Scalbert, A.; Bueno de Mesquita, B.; Romaguera, D.; Gunter, M.J.; et al. Plasma Elaidic Acid Level as Biomarker of Industrial Trans Fatty Acids and Risk of Weight Change: Report from the EPIC Study. PLoS ONE 2015, 10, e0118206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholz, A.; Navarrete-Muñoz, E.M.; García-de-la-Hera, M.; Fernandez-Somoano, A.; Tardon, A.; Santa-Marina, L.; Pereda-Pereda, E.; Romaguera, D.; Guxens, M.; Beneito, A.; et al. Association between Trans Fatty Acid Intake and Overweight Including Obesity in 4 to 5-year-old Children from the INMA Study. Pediatr. Obes. 2019, 14, e12528. [Google Scholar] [CrossRef] [PubMed]
- Honicky, M.; Cardoso, S.M.; Lima, L.R.A.; Ozcariz, S.G.I.; Vieira, F.G.K.; Carlos Back, I.; Moreno, Y.M.F. Added Sugar and Trans Fatty Acid Intake and Sedentary Behavior Were Associated with Excess Total-body and Central Adiposity in Children and Adolescents with Congenital Heart Disease. Pediatr. Obes. 2020, 15, e12623. [Google Scholar] [CrossRef]
- Koochakpour, G.; Esfandiar, Z.; Hosseini-Esfahani, F.; Mirmiran, P.; Daneshpour, M.S.; Sedaghati-Khayat, B.; Azizi, F. Evaluating the Interaction of Common FTO Genetic Variants, Added Sugar, and Trans-Fatty Acid Intakes in Altering Obesity Phenotypes. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 474–480. [Google Scholar] [CrossRef]
- Risérus, U. Trans fatty acids, insulin sensitivity and type 2 diabetes. Scand. J. Food Nutr. 2006, 50, 161–165. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Aro, A.; Willett, W.C. Health effects of trans-fatty acids: Experimental and observational evidence. Eur. J. Clin. Nutr. 2009, 63, S5–S21. [Google Scholar] [CrossRef] [Green Version]
- Itcho, K.; Yoshii, Y.; Ohno, H.; Oki, K.; Shinohara, M.; Irino, Y.; Toh, R.; Ishida, T.; Hirata, K.; Yoneda, M. Association between Serum Elaidic Acid Concentration and Insulin Resistance in Two Japanese Cohorts with Different Lifestyles. J. Atheroscler. Thromb. 2017, 24, 1206–1214. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Sun, Y.; Snetselaar, L.G.; Sun, Q.; Yang, Q.; Zhang, Z.; Liu, L.; Hu, F.B.; Bao, W. Association between Plasma Trans-Fatty Acid Concentrations and Diabetes in a Nationally Representative Sample of US Adults. J. Diabetes 2018, 10, 653–664. [Google Scholar] [CrossRef]
- Angelieri, C.T.; Barros, C.R.; Siqueira-Catania, A.; Ferreira, S.R.G. Trans fatty acid intake is associated with insulin sensitivity but independently of inflammation. Brazilian J. Med. Biol. Res. 2012, 45, 625–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishibashi, K.; Nehashi, K.; Oshima, T.; Ohkura, N.; Atsumi, G.-I. Differentiation with Elaidate Tends to Impair Insulin-Dependent Glucose Uptake and GLUT4 Translocation in 3T3-L1 Adipocytes. Int. J. Food Sci. Nutr. 2016, 67, 99–110. [Google Scholar] [CrossRef]
- Zhao, X.; Shen, C.; Zhu, H.; Wang, C.; Liu, X.; Sun, X.; Han, S.; Wang, P.; Dong, Z.; Ma, X.; et al. Trans-Fatty Acids Aggravate Obesity, Insulin Resistance and Hepatic Steatosis in C57BL/6 Mice, Possibly by Suppressing the IRS1 Dependent Pathway. Molecules 2016, 21, 705. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Niu, X.; Wang, M.; Li, Z.; Jiang, H.-K.; Li, C.; Caton, S.J.; Bai, Y. Endoplasmic Reticulum Stress May Be Involved in Insulin Resistance and Lipid Metabolism Disorders of the White Adipose Tissues Induced by High-Fat Diet Containing Industrial Trans-Fatty Acids. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 1625–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zulyniak, M.A.; Ralston, J.C.; Tucker, A.J.; MacKay, K.A.; Hillyer, L.M.; McNicholas, P.D.; Graham, T.E.; Robinson, L.E.; Duncan, A.M.; Ma, D.W.L.; et al. Vaccenic acid in serum triglycerides is associated with markers of insulin resistance in men. Appl. Physiol. Nutr. Metab. 2012, 37, 1003–1007. [Google Scholar] [CrossRef]
- Weir, N.L.; Steffen, B.T.; Guan, W.; Johnson, L.M.; Djousse, L.; Mukamal, K.J.; Tsai, M.Y. Circulating omega-7 fatty acids are differentially related to metabolic dysfunction and incident type II diabetes: The Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Metab. 2020, 46, 319–325. [Google Scholar] [CrossRef]
- Larsen, T.M.; Toubro, S.; Gudmundsen, O.; Astrup, A. Conjugated linoleic acid supplementation for 1 y does not prevent weight or body fat regain. Am. J. Clin. Nutr. 2006, 83, 606–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, E.V.; Goedecke, J.H.; Bluett, K.; Heggie, K.; Claassen, A.; Rae, D.E.; West, S.; Dugas, J.; Dugas, L.; Meltzer, S.; et al. Conjugated linoleic acid versus high-oleic acid sunflower oil: Effects on energy metabolism, glucose tolerance, blood lipids, appetite and body composition in regularly exercising individuals. Br. J. Nutr. 2007, 97, 1001–1011. [Google Scholar] [CrossRef]
- Ryder, J.W.; Portocarrero, C.P.; Song, X.M.; Cui, L.; Yu, M.; Combatsiaris, T.; Galuska, D.; Bauman, D.E.; Barbano, D.M.; Charron, M.J.; et al. Isomer-Specific Antidiabetic Properties of Conjugated Linoleic Acid. Diabetes 2001, 50, 1149–1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Micha, R.; Mozaffarian, D. Trans fatty acids: Effects on cardiometabolic health and implications for policy. Prostaglandins Leukot. Essent. Fat. Acids 2008, 79, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Kummerow, F.A.; Zhou, Q.; Mahfouz, M.M.; Smiricky, M.R.; Grieshop, C.M.; Schaeffer, D.J. Trans fatty acids in hydrogenated fat inhibited the synthesis of the polyunsaturated fatty acids in the phospholipid of arterial cells. Life Sci. 2004, 74, 2707–2723. [Google Scholar] [CrossRef]
- Revin, V.V.; Gromova, N.V.; Revina, E.S.; Martynova, M.I.; Seikina, A.I.; Revina, N.V.; Imarova, O.G.; Solomadin, I.N.; Tychkov, A.Y.; Zhelev, N. Role of membrane lipids in the regulation of erythrocytic oxygen-transport function in cardiovascular diseases. Biomed. Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khaw, K.T.; Friesen, M.D.; Riboli, E.; Luben, R.; Wareham, N. Plasma phospholipid fatty acid concentration and incident coronary heart disease in men and women: The EPIC-Norfolk prospective study. PLoS Med. 2012, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flock, M.R.; Kris-Etherton, P.M. Diverse physiological effects of long-chain saturated fatty acids: Implications for cardiovascular disease. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Gillman, M.W.; Cupples, L.A.; Gagnon, D.; Millen, B.E.; Ellison, R.C.; Castelli, W.P. Margarine intake and subsequent coronary heart disease in men. Epidemiology 1997, 8, 144–149. [Google Scholar] [CrossRef]
- Lindmark Månsson, H. Fatty acids in bovine milk fat. Food Nutr. Res. 2008, 52, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Harvey, K.A.; Walker, C.L.; Xu, Z.D.; Whitley, P.; Siddiqui, R.A. Trans fatty acids: Induction of a proinflammatory phenotype in endothelial cells. Lipids 2012, 47, 647–657. [Google Scholar] [CrossRef]
- Li, J.; Rao, H.; Bin, Q.; Fan, Y.W.; Li, H.Y.; Deng, Z.Y. Linolelaidic acid induces apoptosis, cell cycle arrest and inflammation stronger than elaidic acid in human umbilical vein endothelial cells through lipid rafts. Eur. J. Lipid Sci. Technol. 2017, 119, 1600374. [Google Scholar] [CrossRef]
- Flock, M.R.; Kris-Etherton, P.M. Dietary guidelines for americans 2010: Implications for cardiovascular disease. Curr. Atheroscler. Rep. 2011, 13, 499–507. [Google Scholar] [CrossRef]
- Takeuchi, H.; Ito, E.; Tomioka, T.; Tabuchi, E.; Fuhshuku, K.I.; Asano, Y. Trans fatty acid intake and serum cholesterol levels in young japanese women. Biosci. Biotechnol. Biochem. 2012, 76, 1627–1632. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization (WHO). Replace Trans Fat: Frequently Asked Questions; World Health Organisation: Geneva, Switzerland, 2018. [Google Scholar]
- Stender, S.; Astrup, A.; Dyerberg, J. Ruminant and industrially produced trans fatty acids: Health aspects. Food Nutr. Res. 2008, 52, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baer, D.J.; Judd, J.T.; Clevidence, B.A.; Tracy, R.P. Dietary fatty acids affect plasma markers of inflammation in healthy men fed controlled diets: A randomized crossover study. Am. J. Clin. Nutr. 2004, 79, 969–973. [Google Scholar] [CrossRef] [Green Version]
- Oie, E.; Ueland, T.; Dahl, C.P.; Bohov, P.; Berge, C.; Yndestad, A.; Gullestad, L.; Aukrust, P.; Berge, R.K. Fatty acid composition in chronic heart failure: Low circulating levels of eicosatetraenoic acid and high levels of vaccenic acid are associated with disease severity and mortality. J. Intern. Med. 2011, 270, 263–272. [Google Scholar] [CrossRef]
- Stampfer, W.C.W.M.J.; Manson, J.E.; Speizer, G.A.C.F.E.; Rosner, B.A.; Hennekens, L.A.S.C.H. Intake of trans fatty acids and risk of coronary heart disease among women. Lancet 1993, 341, 581–585. [Google Scholar] [CrossRef]
- Pietinen, P.; Ascherio, A.; Korhonen, P.; Hartman, A.M.; Willett, W.C.; Albanes, D.; Virtamo, J. Intake of fatty acids and risk of coronary heart disease in a cohort of Finnish men. The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am. J. Epidemiol. 1997, 145, 876–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassett, C.M.C.; Edel, A.L.; Patenaude, A.F.; McCullough, R.S.; Blackwood, D.P.; Chouinard, P.Y.; Paquin, P.; Lamarche, B.; Pierce, G.N. Dietary vaccenic acid has antiatherogenic effects in LDLr-/- mice. J. Nutr. 2010, 140, 18–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Jeong, C.H.; Lee, H.G.; Han, S.G. Comparison of trans-fatty acids on proliferation and migration of vascular smooth muscle cells. Food Sci. Biotechnol. 2017, 26, 501–505. [Google Scholar] [CrossRef]
- Reynolds, C.M.; Loscher, C.E.; Moloney, A.P.; Roche, H.M. Cis-9, trans-11-conjugated linoleic acid but not its precursor trans-vaccenic acid attenuate inflammatory markers in the human colonic epithelial cell line Caco-2. Br. J. Nutr. 2008, 100, 13–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derakhshande-Rishehri, S.M.; Mansourian, M.; Kelishadi, R.; Heidari-Beni, M. Association of foods enriched in conjugated linoleic acid (CLA) and CLA supplements with lipid profile in human studies: A systematic review and meta-analysis. Public Health Nutr. 2015, 18, 2041–2054. [Google Scholar] [CrossRef] [Green Version]
- Ardisson Korat, A.V.; Chiu, Y.H.; Bertrand, K.A.; Zhang, S.; Epstein, M.M.; Rosner, B.A.; Chiuve, S.; Campos, H.; Giovannucci, E.L.; Chavarro, J.E.; et al. Red blood cell membrane trans fatty acid levels and risk of non-Hodgkin lymphoma: A prospective nested case-control study. Am. J. Clin. Nutr. 2020, 112, 1576–1583. [Google Scholar] [CrossRef]
- Yammine, S.; Huybrechts, I.; Biessy, C.; Dossus, L.; Aglago, E.K.; Naudin, S.; Ferrari, P.; Weiderpass, E.; Tjønneland, A.; Louise Hansen, L.; et al. Dietary and Circulating Fatty Acids and Ovarian Cancer Risk in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1739–1749. [Google Scholar] [CrossRef]
- Aglago, E.K.; Murphy, N.; Huybrechts, I.; Nicolas, G.; Casagrande, C.; Fedirko, V.; Weiderpass, E.; Rothwell, J.A.; Dahm, C.C.; Olsen, A.; et al. Dietary intake and plasma phospholipid concentrations of saturated, monounsaturated and trans fatty acids and colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition cohort. Int. J. Cancer. 2021, 149, 865–882. [Google Scholar] [CrossRef]
- Matta, M.; Huybrechts, I.; Biessy, C.; Casagrande, C.; Yammine, S.; Agnès Fournier, A.; Olsen, K.S.; Lukic, M.; Gram, I.T.; Ardanaz, E.; et al. Dietary intake of trans fatty acids and breast cancer risk in 9 European countries. BMC Med. 2021, 19, 81. [Google Scholar] [CrossRef]
- Pickens, C.A.; Lane-Elliot, A.; Comstock, S.S.; Fenton, J.I. Altered saturated and monounsaturated plasma phospholipid fatty acid profiles in adult males with colon adenomas. Cancer Epidemiol. Biomark. Prev. 2016, 25, 498–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohmori, H.; Fujii, K.; Kadochi, Y.; Mori, S.; Nishiguchi, Y.; Fujiwara, R.; Kishi, S.; Sasaki, T.; Kuniyasu, H. Elaidic Acid, a Trans -Fatty Acid, Enhances the Metastasis of Colorectal Cancer. Cells Pathobiol. 2017, 84, 144–151. [Google Scholar] [CrossRef]
- Voorrips, L.E.; Brants, H.A.M.; Kardinaal, A.F.M.; Hiddink, G.J.; van den Brandt, P.A.; Alexandra Goldbohm, R. Intake of conjugated linoleic acid, fat, and other fatty acids in relation to postmenopausal breast cancer: The Netherlands Cohort Study on Diet and Cancer. Am. J. Clin. Nutr. 2002, 76, 873–882. [Google Scholar] [CrossRef] [Green Version]
- Benjamin, S.; Spener, F. Conjugated linoleic acids as functional food: An insight into their health benefits. Nutr. Metab. 2009, 6, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corl, B.A.; Barbano, D.M.; Bauman, D.E.; Ip, C. cis-9, trans-11 CLA derived endogenously from trans-11 18:1 reduces cancer risk in rats. J. Nutr. 2003, 133, 2893–2900. [Google Scholar] [CrossRef] [Green Version]
- Lock, A.L.; Corl, B.A.; Barbano, D.M.; Bauman, D.E.; Ip, C. The anticarcinogenic effect of trans-11 18:1 is dependent on its conversion to cis-9, trans-11 CLA by Δ9-desaturase in rats. J. Nutr. 2004, 134, 2698–2704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banni, S.; Angioni, E.; Murru, E.; Carta, G.; Melis, M.P.; Bauman, D.; Dong, Y.; Ip, C. Vaccenic acid feeding increases tissue levels of conjugated linoleic acid and suppresses development of premalignant lesions in rat mammary gland. Nutr. Cancer 2001, 41, 91–97. [Google Scholar] [CrossRef]
- Lampen, A.; Leifheit, M.; Voss, J.; Nau, H. Molecular and cellular effects of cis-9, trans-11-conjugated linoleic acid in enterocytes: Effects on proliferation, differentiation, and gene expression. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2005, 1735, 30–40. [Google Scholar] [CrossRef]
- Oh, J.J.; Lee, J.S.; Lim, J.N.; Wang, T.; Kim, S.H.; Lee, H.G. Trans vaccenic acid (trans-11 18:1), a precursor of cis-9, trans-11-conjugated linoleic acid, exerts a direct anti-carcinogenic function in T47D breast carcinoma cells. Food Sci. Biotechnol. 2014, 23, 641–646. [Google Scholar] [CrossRef]
- Lim, J.N.; Oh, J.J.; Wang, T.; Lee, J.S.; Kim, S.H.; Kim, Y.J.; Lee, H.G. Trans-11 18:1 vaccenic acid (TVA) has a direct anti-carcinogenic effect on MCF-7 human mammary adenocarcinoma cells. Nutrients 2014, 6, 627–636. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Wang, Y.; Fan, X.; Wu, H.; Han, J.; Yang, M.; Lu, L.; Nie, G. Trans-vaccenic acid inhibits proliferation and induces apoptosis of human nasopharyngeal carcinoma cells via a mitochondrial-mediated apoptosis pathway. Lipids Health Dis. 2019, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund/American Institute for Cancer Research Worlwide Cancer Data: Global Cancer Statistics for the Most Common Cancers. Available online: https://www.wcrf.org/dietandcancer/worldwide-cancer-data/ (accessed on 14 October 2021).
- Cho, E.; Smith-Warner, S.A.; Spiegelman, D.; Beeson, W.L.; van den Brandt, P.A.; Colditz, G.A.; Folsom, A.R.; Fraser, G.E.; Freudenheim, J.L.; Giovannucci, E.; et al. Dairy foods, calcium, and colorectal cancer: A pooled analysis of 10 cohort studies. J. Natl. Cancer Inst. 2004, 96, 1015–1022. [Google Scholar] [CrossRef] [Green Version]
- Mohammadzadeh, M.; Faramarzi, E.; Mahdavi, R.; Nasirimotlagh, B.; Asghari Jafarabadi, M. Effect of conjugated linoleic acid supplementation on inflammatory factors and matrix metalloproteinase enzymes in rectal cancer patients undergoing chemoradiotherapy. Integr. Cancer Ther. 2013, 12, 496–502. [Google Scholar] [CrossRef]
- Aro, A.; Männistö, S.; Salminen, I.; Ovaskainen, M.L.; Kataja, V.; Uusitupa, M. Inverse association between dietary and serum conjugated linoleic acid and risk of breast cancer in postmenopausal women. Nutr. Cancer 2000, 38, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Y.; Zhang, L.; He, K.; Qin, L.Q. Dairy consumption and risk of breast cancer: A meta-analysis of prospective cohort studies. Breast Cancer Res. Treat. 2011, 127, 23–31. [Google Scholar] [CrossRef]
- den Hartigh, L.J. Conjugated linoleic acid effects on cancer, obesity, and atherosclerosis: A review of pre-clinical and human trials with current perspectives. Nutrients 2018, 11, 370. [Google Scholar] [CrossRef] [Green Version]
- Han, S.N.; Leka, L.S.; Lichtenstein, A.H.; Ausman, L.M.; Schaefer, E.J.; Meydani, S.N. Effect of hydrogenated and saturated, relative to polyunsaturated, fat on immune and inflammatory responses of adults with moderate hypercholesterolemia. J. Lipid Res. 2002, 43, 445–452. [Google Scholar] [CrossRef]
- López-Plaza, B.; Bermejo, L.M.; Weber, T.K.; Parra, P.; Serra, F.; Hernández, M.; Milla, S.P.; Gómez-Candela, C. Efecto de una suplementación láctea con ácido linoleico conjugado sobre el control de peso y la composición corporal de personas sanas con sobrepeso. Nutr. Hosp. 2013, 28, 2090–2098. [Google Scholar] [CrossRef]
- Steck, S.E.; Chalecki, A.M.; Miller, P.; Conway, J.; Austin, G.L.; Hardin, J.W.; Albright, C.D.; Thuillier, P. Conjugated linoleic acid supplementation for twelve weeks increases lean body mass in obese humans. J. Nutr. 2007, 137, 1188–1193. [Google Scholar] [CrossRef] [Green Version]
- Hirata, Y.; Takahashi, M.; Kudoh, Y.; Kano, K.; Kawana, H.; Makide, K.; Shinoda, Y.; Yabuki, Y.; Fukunaga, K.; Aoki, J.; et al. Trans-Fatty acids promote proinflammatory signaling and cell death by stimulating the apoptosis signal-regulating kinase 1 (ASK1)-p38 pathway. J. Biol. Chem. 2017, 292, 8174–8185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazidi, M.; Gao, H.K.; Shivappa, N.; Wirth, M.D.; Hebert, J.R.; Kengne, A.P. The relationship of plasma Trans fatty acids with dietary inflammatory index among US adults. Lipids Health Dis. 2017, 16, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higashimura, Y.; Tanaka, Y.; Takagi, T.; Uchiyama, K.; Mizushima, K.; Niki, E.; Naito, Y. Trans-unsaturated fatty acid activates NLRP3 inflammasome in macrophages and exacerbates intestinal inflammation in mice. Biochem. Biophys. Res. Commun. 2020, 529, 243–250. [Google Scholar] [CrossRef]
- Monguchi, T.; Hara, T.; Hasokawa, M.; Nakajima, H.; Mori, K.; Toh, R.; Irino, Y.; Ishida, T.; Hirata, K.I.; Shinohara, M. Excessive intake of trans fatty acid accelerates atherosclerosis through promoting inflammation and oxidative stress in a mouse model of hyperlipidemia. J. Cardiol. 2017, 70, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.B.; Zou, Q.; Lv, X.; Zhou, R.L.; Niu, X.; Weng, C.; Chen, F.; Fan, Y.W.; Deng, Z.Y.; Li, J. 9t18:1 and 11t18:1 activate the MAPK pathway to regulate the expression of PLA2 and cause inflammation in HUVECs. Food Funct. 2020, 11, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Smit, L.A.; Katan, M.B.; Wanders, A.J.; Basu, S.; Brouwer, I.A. A high intake of trans fatty acids has little effect on markers of inflammation and oxidative stress in humans. J. Nutr. 2011, 141, 1673–1678. [Google Scholar] [CrossRef] [Green Version]
- Gebauer, S.K.; Destaillats, F.; Dionisi, F.; Krauss, R.M.; Baer, D.J. Vaccenic acid and trans fatty acid isomers from partially hydrogenated oil both adversely affect LDL cholesterol: A double-blind, randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 1339–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.S.; Lim, J.N.; Wang, T.; Lee, S.B.; Hwang, J.H.; Jung, U.S.; Kim, M.J.; Choi, S.H.; Ishizuka, S.; Lee, H.G. Physiological concentrations of trans-11 18:1 vaccenic acid suppress pro-inflammatory markers under acute inflammation in isolated ICR mice splenocytes. Food Sci. Biotechnol. 2016, 25, 275–281. [Google Scholar] [CrossRef]
- Da Silva, M.S.; Julien, P.; Bilodeau, J.F.; Barbier, O.; Rudkowska, I. Trans Fatty Acids Suppress TNF-α-Induced Inflammatory Gene Expression in Endothelial (HUVEC) and Hepatocellular Carcinoma (HepG2) Cells. Lipids 2017, 52, 315–325. [Google Scholar] [CrossRef]
- Da Silva, M.S.; Bilodeau, J.F.; Larose, J.; Greffard, K.; Julien, P.; Barbier, O.; Rudkowska, I. Modulation of the biomarkers of inflammation and oxidative stress by ruminant trans fatty acids and dairy proteins in vascular endothelial cells (HUVEC). Prostaglandins Leukot. Essent. Fat. Acids 2017, 126, 64–71. [Google Scholar] [CrossRef]
- Jaudszus, A.; Jahreis, G.; Schlörmann, W.; Fischer, J.; Kramer, R.; Degen, C.; Rohrer, C.; Roth, A.; Gabriel, H.; Barz, D.; et al. Vaccenic acid-mediated reduction in cytokine production is independent of c9,t11-CLA in human peripheral blood mononuclear cells. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2012, 1821, 1316–1322. [Google Scholar] [CrossRef]
- Li, J.; Hu, S.B.; He, Y.M.; Zhuo, C.F.; Zhou, R.L.; Chen, F.; Li, H.Y.; Deng, Z.Y. 9c11tCLA modulates 11t18:1 and 9t18:1 induced inflammations differently in human umbilical vein endothelial cells. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Pranger, I.G.; Muskiet, F.A.J.; Kema, I.P.; Singh-Povel, C.; Bakker, S.J.L. Potential biomarkers for fat from dairy and fish and their association with cardiovascular risk factors: Cross-sectional data from the LifeLines Biobank and Cohort Study. Nutrients 2019, 11, 1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verneque, B.J.F.; Machado, A.M.; de Abreu Silva, L.; Lopes, A.C.S.; Duarte, C.K. Ruminant and industrial trans-fatty acids consumption and cardiometabolic risk markers: A systematic review. Crit. Rev. Food Sci. Nutr. 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Aminot-Gilchrist, D.V.; Anderson, H.D.I. Insulin resistance-associated cardiovascular disease: Potential benefits of conjugated linoleic acid. Am. J. Clin. Nutr. 2004, 79, 1159–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chichlowski, M.W.; Schroeder, J.W.; Park, C.S.; Keller, W.L.; Schimek, D.E. Altering the fatty acids in milk fat by including canola seed in dairy cattle diets. J. Dairy Sci. 2005, 88, 3084–3094. [Google Scholar] [CrossRef] [Green Version]
- Belury, M.A. Dietary conjugated linoleic acid in health: Physiological effects and mechanisms of action. Annu. Rev. Nutr. 2002, 22, 505–531. [Google Scholar] [CrossRef]
- Mazidi, M.; Banach, M.; Kengne, A.P. Association between plasma trans fatty acids concentrations and leucocyte telomere length in US adults. Eur. J. Clin. Nutr. 2018, 72, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tian, W.; Liu, Y.; Yan, G.; Fang, S.; Wang, Y.; Yu, B. Temporal trend of circulating trans-fatty acids and risk of long-term mortality in general population. Clin. Nutr. 2021, 40, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Howard, F.A.C.; Henderson, C. Hydrogenation of polyunsaturated fatty acids by human colonic bacteria. Lett. Appl. Microbiol. 1999, 29, 193–196. [Google Scholar] [CrossRef]
- Farnworth, E.R.; Chouinard, Y.P.; Jacques, H.; Venkatramanan, S.; Maf, A.A.; Defnoun, S.; Jones, P.J.H. The effect of drinking milk containing conjugated linoleic acid on fecal microbiological profile, enzymatic activity, and fecal characteristics in humans. Nutr. J. 2007, 6, 1–9. [Google Scholar] [CrossRef]
- den Hartigh, L.J.; Gao, Z.; Goodspeed, L.; Wang, S.; Das, A.K.; Burant, C.F.; Chait, A.; Blaser, M.J. Obese mice losing weight due to trans-10,cis-12 conjugated linoleic acid supplementation or food restriction harbor distinct gut microbiota. J. Nutr. 2018, 148, 562–572. [Google Scholar] [CrossRef]
- Li, H.; Zhuang, P.; Zhang, Y.; Shou, Q.; Lu, Y.; Wang, G.; Qiu, J.; Wang, J.; He, L.; Chen, J.; et al. Mixed Conjugated Linoleic Acid Sex-dependently Reverses High-fat Diet-induced Insulin Resistance via the Gut-adipose Axis. FASEB J. 2021, 35, e21466. [Google Scholar] [CrossRef]
- Marques, T.M.; Wall, R.; O’Sullivan, O.; Fitzgerald, G.F.; Shanahan, F.; Quigley, E.M.; Cotter, P.D.; Cryan, J.F.; Dinan, T.G.; Ross, R.P.; et al. Dietary trans-10, cis-12-conjugated linoleic acid alters fatty acid metabolism and microbiota composition in mice. Br. J. Nutr. 2015, 113, 728–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reeves, A.E.; Koenigsknecht, M.J.; Bergin, I.L.; Young, V.B. Suppression of Clostridium difficile in the gastrointestinal tracts of germfree mice inoculated with a murine isolate from the family Lachnospiraceae. Infect. Immun. 2012, 80, 3786–3794. [Google Scholar] [CrossRef] [Green Version]
- Bajaj, J.S.; Ridlon, J.M.; Hylemon, P.B.; Thacker, L.R.; Heuman, D.M.; Smith, S.; Sikaroodi, M.; Gillevet, P.M. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am. J. Physiol.-Gastrointest. Liver Physiol. 2012, 302, 168–175. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Zhang, M.; Wang, S.; Han, R.; Cao, Y.; Hua, W.; Mao, Y.; Zhang, X.; Pang, X.; Wei, C.; et al. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J. 2010, 4, 232–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Yang, B.; Ross, R.P.; Jin, Y.; Stanton, C.; Zhao, J.; Zhang, H.; Chen, W. Orally Administered CLA Ameliorates DSS-Induced Colitis in Mice via Intestinal Barrier Improvement, Oxidative Stress Reduction, and Inflammatory Cytokine and Gut Microbiota Modulation. J. Agric. Food Chem. 2019, 67, 13282–13298. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Liu, W.; Tao, H.; Zhang, Y.; Liu, L.; Liu, Z.; Qiu, B.; Xu, T. Effect of industrial trans-fatty acids-enriched diet on gut microbiota of C57BL/6 mice. Eur. J. Nutr. 2019, 58, 2625–2638. [Google Scholar] [CrossRef] [PubMed]
- Ijssennagger, N.; van der Meer, R.; van Mil, S.W.C. Sulfide as a Mucus Barrier-Breaker in Inflammatory Bowel Disease? Trends Mol. Med. 2016, 22, 190–199. [Google Scholar] [CrossRef]
- Del Chierico, F.; Nobili, V.; Vernocchi, P.; Russo, A.; De Stefanis, C.; Gnani, D.; Furlanello, C.; Zandonà, A.; Paci, P.; Capuani, G.; et al. Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology 2017, 65, 451–464. [Google Scholar] [CrossRef]
- Zeng, H.; Liu, J.; Jackson, M.I.; Zhao, F.Q.; Yan, L.; Combs, G.F. Fatty liver accompanies an increase in lactobacillus species in the hind gut of C57BL/6 mice fed a high-fat diet. J. Nutr. 2013, 143, 627–631. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zhang, Y.; Ge, Y.; Qiu, B.; Zhang, D.; Wang, X.; Liu, W.; Tao, H. Comparative transcriptome and microbiota analyses provide new insights into the adverse effects of industrial trans fatty acids on the small intestine of C57BL/6 mice. Eur. J. Nutr. 2021, 60, 975–987. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, G.C.B.C.; Moura, C.S.; Roquetto, A.R.; Barrera-Arellano, D.; Yamada, A.T.; dos Santos, A.; Saad, M.J.A.; Amaya-Farfan, J. Impact of Trans-Fats on Heat-Shock Protein Expression and the Gut Microbiota Profile of Mice. J. Food Sci. 2018, 83, 489–498. [Google Scholar] [CrossRef]
- Awadel-Kariem, F.M.; Patel, P.; Kapoor, J.; Brazier, J.S.; Goldstein, E.J.C. First report of Parabacteroides goldsteinii bacteraemia in a patient with complicated intra-abdominal infection. Anaerobe 2010, 16, 223–225. [Google Scholar] [CrossRef]
- Ulger Toprak, N.; Bozan, T.; Birkan, Y.; Isbir, S.; Soyletir, G. Butyricimonas virosa: The first clinical case of bacteraemia. New Microbes New Infect. 2015, 4, 7–8. [Google Scholar] [CrossRef] [Green Version]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Fan, R.; Zhao, L.; Tong, C.; Qian, X.; Zhang, M.; Xiao, R.; Ma, W. Trans -Fatty Acids Alter the Gut Microbiota in High-Fat-Diet-Induced Obese Rats. Br. J. Nutr. 2020, 124, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Okamura, T.; Hashimoto, Y.; Majima, S.; Senmaru, T.; Ushigome, E.; Nakanishi, N.; Asano, M.; Yamazaki, M.; Takakuwa, H.; Hamaguchi, M.; et al. Trans Fatty Acid Intake Induces Intestinal Inflammation and Impaired Glucose Tolerance. Front. Immunol. 2021, 12, 669672. [Google Scholar] [CrossRef] [PubMed]
- Stender, S.; Astrup, A.; Dyerberg, J. Tracing artificial trans fat in popular foods in Europe: A market basket investigation. BMJ Open 2014, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- European Heart Network European Cardiovascular Disease Statistics: 2017 Edition. Available online: https://ehnheart.org/cvd-statistics/cvd-statistics-2017.html (accessed on 14 October 2021).
- World Health Organisation (WHO); International Agency for Research on Cancer (IARC). REPLACE Trans Fat: An Action Package to Eliminate Industrially Produced Trans-Fatty Acids: Module 1: Review; World Health Organisation: Geneva, Switzerland, 2020. [Google Scholar]
- European Food Safety Autority (EFSA). Scientific and technical assistance on trans fatty acids. EFSA Support. Publ. 2018, 15, 1433e. [Google Scholar] [CrossRef] [Green Version]
- Institute of Medicine (IOM). Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids; The National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- PAHO/WHO. Plan of Action for The Elimination of Industrially Produced Trans-Fatty Acids: PAHO 2020–2025; Pan American Health Organization: Washington DC, USA, 2020; Volume 2017. [Google Scholar]
- World Health Organization (WHO). Global database on the Implementation of Nutrition Action (GINA). TFA Country Score Card. Available online: https://extranet.who.int/nutrition/gina/en/scorecard/TFA (accessed on 14 October 2021).
- Customs Union. Technical Regulation on Fat and Oil Products; Custom Union: Moscow, Russia, 2013. [Google Scholar]
- European Commission (EU). Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011. Off. J. Eur. Union 2011, 304/18, 46. [Google Scholar] [CrossRef]
- European Commission (EU). Commission Regulation Amending Annex III to Regulation (EC) No 1925/2006 of the European Parliament and of the Council as Regards Trans Fat, Other than Trans Fat Naturally Occurring in Fat of Animal Origin; Official Journal of the European Union: Brussels, Belgium, 2019. [Google Scholar]
- Astiasarán, I.; Abella, E.; Gatta, G.; Ansorena, D. Margarines and fast-food French fries: Low content of trans fatty acids. Nutrients 2017, 9, 662. [Google Scholar] [CrossRef] [Green Version]
- Menaa, F.; Menaa, A.; Tréton, J.; Menaa, B. Technological approaches to minimize industrial trans fatty acids in foods. J. Food Sci. 2013, 78, 377–386. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Eliminating Trans Fats in Europe: A Policy Brief; WHO Regional Office for Europe: Copenhagen, Denmark, 2015. [Google Scholar]
- World Health Organization (WHO). Salt and Trans-Fats Assessed in Kyrgyz Republic with WHO Support. Available online: https://www.euro.who.int/en/countries/kyrgyzstan/news/news/2016/11/salt-and-trans-fats-assessed-in-kyrgyz-republic-with-who-support (accessed on 14 October 2021).
- Stender, S.; Dyerberg, J.; Bysted, A.; Leth, T.; Astrup, A. A trans world journey. Atheroscler. Suppl. 2006, 7, 47–52. [Google Scholar] [CrossRef]
| Score | Countries | N of Countries |
|---|---|---|
| 1. National policy commitment to eliminate TFA: National policies, strategies, or action plans that express a commitment to reduce iTFA in the food supply | Afghanistan, Albania, Algeria, Antigua and Barbuda, Bahamas, Bangladesh, Barbados, Belize, Benin, Bhutan, Bosnia and Herzegovina, Botswana, Cambodia, Chad, Costa Rica, Côte d’Ivoire, Djibouti, Dominican Republic, Egypt, Eswatini, French Polynesia, Ghana, Grenada, Guatemala, Guyana, Indonesia, Jamaica, Kenya, Lao People’s Democratic Republic, Lebanon, Maldives, Mauritania, Mauritius, Mongolia, Morocco, Myanmar, Namibia, Nauru, Nepal, Nigeria, North Macedonia, Papua New Guinea, Qatar, Saint Kitts and Nevis, Saint Lucia, Saint Vincent and the Grenadines, Samoa, Seychelles, Sri Lanka, Suriname, Timor-Leste, Trinidad and Tobago, Turkmenistan, Ukraine, United Arab Emirates, Vanuatu, Venezuela (Bolivarian Republic of), West Bank and Gaza Strip, Zambia | 59 |
| 2. Other complementary measures: Legislative or other measures that encourage consumers to make healthier choices about iTFA or mandatory limits on iTFA in foods in specific settings | Bolivia (Plurinational State of), Brazil, Brunei Darussalam, Cape Verde, China, El Salvador, Ethiopia, Fiji, Israel, Jordan, Mexico, Oman, Pakistan, Paraguay, Philippines, Republic of Korea, Republic of Moldova, Tajikistan, Tunisia | 20 |
| 3. Less restrictive TFA limits: Legislative or regulatory measures that limit iTFA in foods in all settings, but are less restrictive than the recommended approach | Argentina, Armenia, Bahrain, Belarus, Colombia, Ecuador, Georgia, India, Iran, Kazakhstan, Kuwait, Kyrgyzstan, Peru, Russian Federation, Singapore, Switzerland, Uruguay, Uzbekistan | 18 |
| 4. Best-practice TFA policy: Legislative or regulatory measures that limit industrially produced TFA in foods in all settings, and are in line with the recommended approach | Austria, Belgium, Bulgaria, Canada, Chile, Croatia, Cyprus, Czechia, Denmark, Estonia, Finland, France, Germany, Greece, Guam, Hungary, Iceland, Ireland, Italy, Latvia, Liechtenstein, Lithuania, Luxembourg, Malta, Netherlands, Northern Mariana Islands, Norway, Poland, Portugal, Romania, Saudi Arabia, Slovakia, Slovenia, South Africa, Spain, Sweden, Thailand, United Kingdom of Great Britain and Northern Ireland, US | 40 |
| Best practice TFA policy passed but not yet in effect | Brazil, India, Paraguay, Peru, Singapore, Uruguay | 6 |
| Monitoring mechanism for mandatory TFA limits | Argentina, Armenia, Austria, Belarus, Canada, Chile, Colombia, Denmark, Ecuador, Georgia, Hungary, Iceland, India, Kazakhstan, Kyrgyzstan, Latvia, Lithuania, Norway, Peru, Russian Federation, Saudi Arabia, Singapore, South Africa, Switzerland, Thailand, US, Uruguay | 27 |
| EU | EAEU | ||||
|---|---|---|---|---|---|
| Country | Proportion of CHD Death (%) Due to TFA Intake | Country | Proportion of CHD Death (%) Due to TFA Intake (>0.5% Energy) | Country | Proportion of CHD Death (%) Due to TFA Intake (>0.5% Energy) |
| Netherlands | 14.4 | Denmark | 4.4 | Belarus | 5.8 |
| Slovenia | 7 | Estonia | 4.3 | Kazakhstan | 4.4 |
| Austria | 6.6 | France | 4.1 | Russian Federation | 4.3 |
| Latvia | 6.2 | Croatia | 4.1 | Armenia | 4.1 |
| Czech Republic | 5.9 | Bulgaria | 4 | Kyrgyzstan | 3.3 |
| Germany | 5.6 | Belgium | 4 | ||
| Poland | 5.6 | Ireland | 3.9 | ||
| Lithuania | 5.5 | Romania | 3.7 | ||
| Hungary | 5.2 | Malta | 3.3 | ||
| Slovakia | 5 | Italy | 3 | ||
| Luxembourg | 4.8 | Sweden | 2.3 | ||
| Spain | 4.7 | Finland | 2.7 | ||
| Portugal | 4.6 | Cyprus | 1 | ||
| Greece | 4.6 | ||||
| Average | Average | ||||
| 6.12 | 4.38 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pipoyan, D.; Stepanyan, S.; Stepanyan, S.; Beglaryan, M.; Costantini, L.; Molinari, R.; Merendino, N. The Effect of Trans Fatty Acids on Human Health: Regulation and Consumption Patterns. Foods 2021, 10, 2452. https://doi.org/10.3390/foods10102452
Pipoyan D, Stepanyan S, Stepanyan S, Beglaryan M, Costantini L, Molinari R, Merendino N. The Effect of Trans Fatty Acids on Human Health: Regulation and Consumption Patterns. Foods. 2021; 10(10):2452. https://doi.org/10.3390/foods10102452
Chicago/Turabian StylePipoyan, Davit, Stella Stepanyan, Seda Stepanyan, Meline Beglaryan, Lara Costantini, Romina Molinari, and Nicolò Merendino. 2021. "The Effect of Trans Fatty Acids on Human Health: Regulation and Consumption Patterns" Foods 10, no. 10: 2452. https://doi.org/10.3390/foods10102452
APA StylePipoyan, D., Stepanyan, S., Stepanyan, S., Beglaryan, M., Costantini, L., Molinari, R., & Merendino, N. (2021). The Effect of Trans Fatty Acids on Human Health: Regulation and Consumption Patterns. Foods, 10(10), 2452. https://doi.org/10.3390/foods10102452









