Antifungal Activities and Mode of Action of Cymbopogon citratus, Thymus vulgraris, and Origanum heracleoticum Essential Oil Vapors against Botrytis cinerea and Their Potential Application to Control Postharvest Strawberry Gray Mold
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pathogen Isolate, Essential Oils, and Fruit Materials
2.2. Screening of Essential Oils
2.3. Analysis of Essential Oil Composition
2.4. Analysis of Botrytis cinerea Morphology and Ultrastructure
2.5. Analysis of Botrytis cinerea Membrane Integrity
2.6. Effects of Selected Essential Oils on Strawberries Inoculated with Botrytis cinerea
2.7. Effects of Selected Essential Oils on Fruit Natural Decayand Postharvest Quality
2.8. Statistical Analysis
3. Results
3.1. Screening of Essential Oils Based on Inhibition Efficiency
3.2. Composition of the Selected Essential Oils
3.3. Effects of Cc, Tv, and Oh on Botrytis cinerea Morphology and Ultrastructure
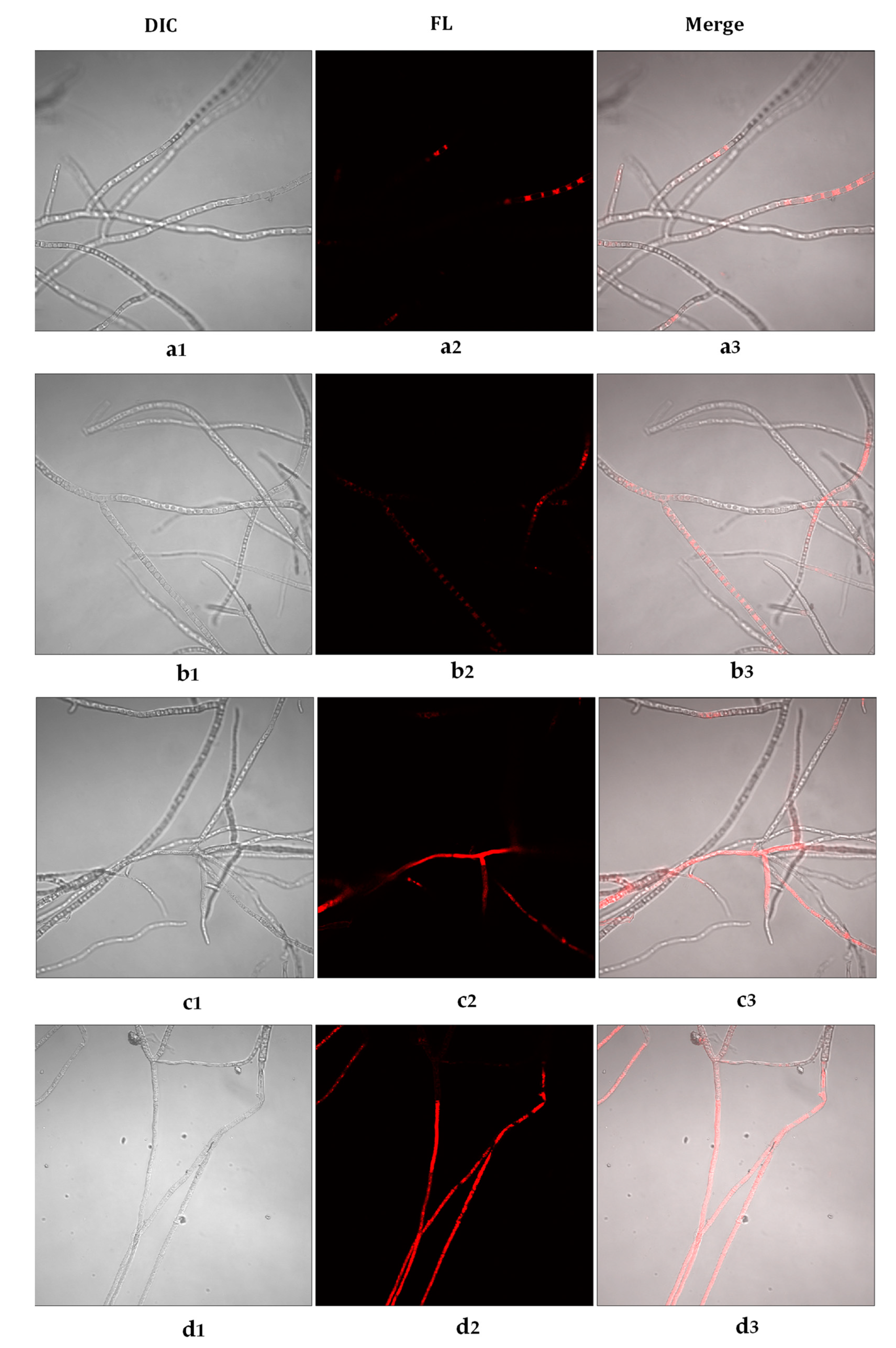
3.4. Effects of Cc, Tv, and Oh on Botrytis cinerea Membrane Integrity
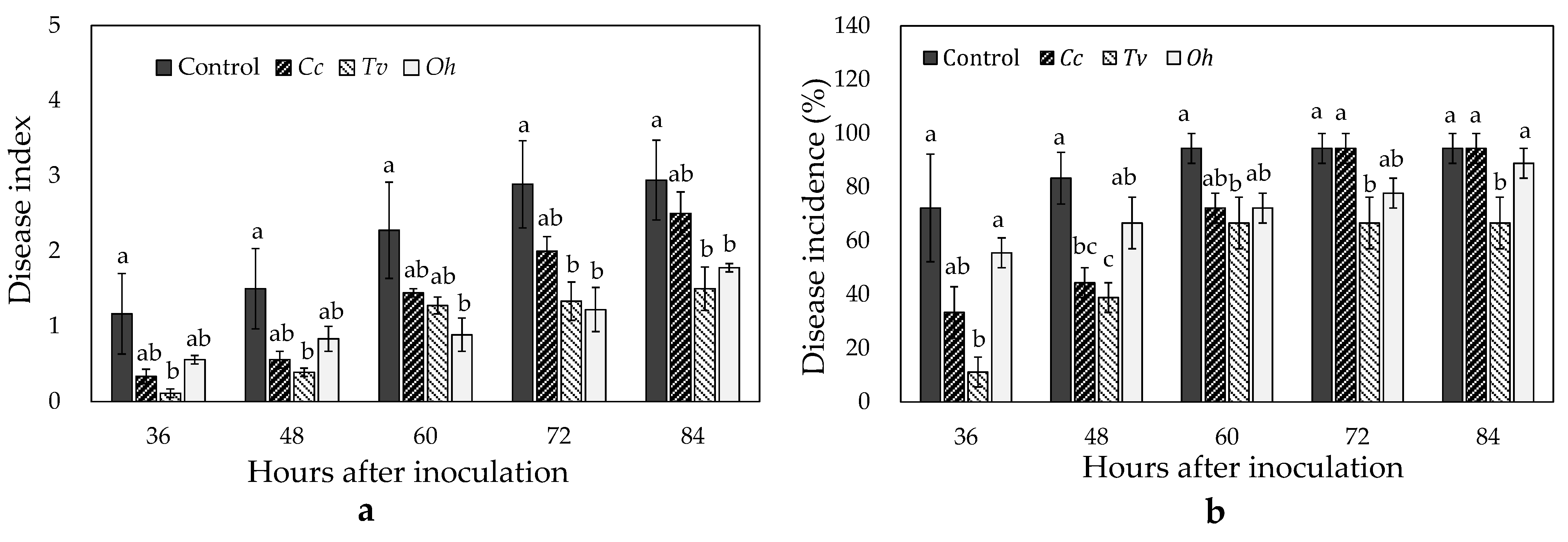
3.5. Effects of Cc, Tv, and Oh on Strawberries Inoculated with Botrytis cinerea
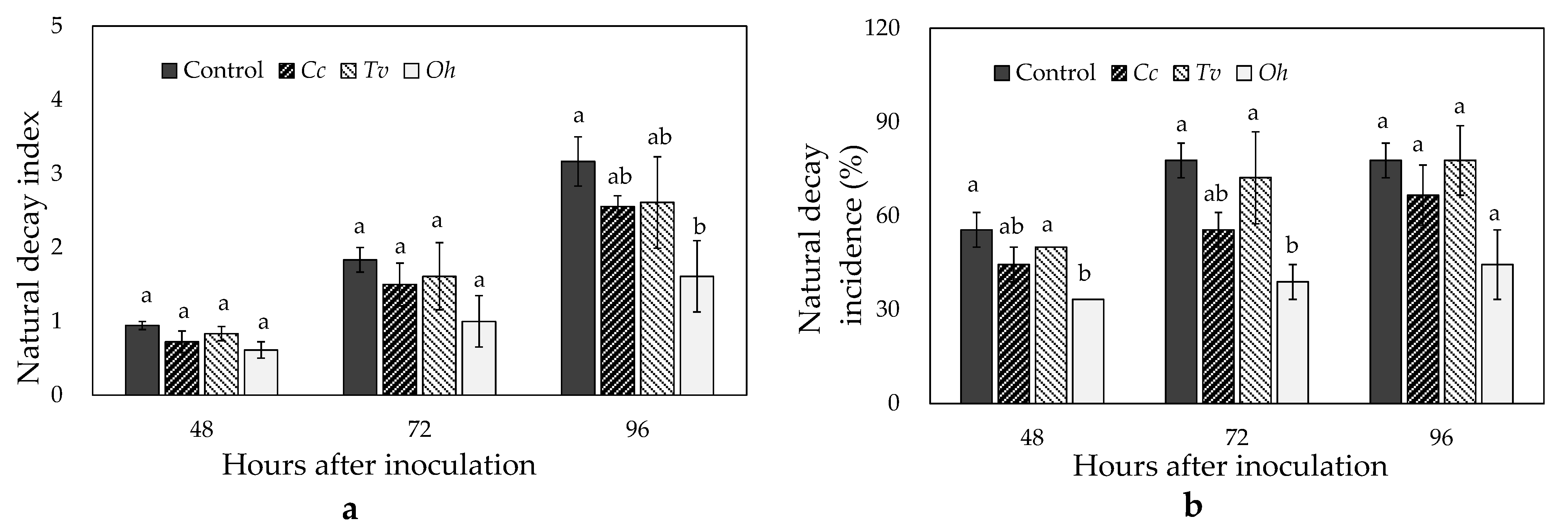
3.6. Effects of Cc, Tv, and Oh on Fruit Natural Decay and Postharvest Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lizalo, A.; Demirsoy, L. Several methods for extending the strawberry production season. In Proceedings of the 4th International Eurasian Agriculture and Natural Science Congress, Turkey, December 2020; Available online: https://www.researchgate.net/publication/347937037_Several_Methods_For_Extending_The_Strawberry_Production_Season (accessed on 23 June 2021).
- Petrasch, S.; Knapp, S.J.; Kan, J.; Blanco-Ulate, B. Grey mould of strawberry, a devastating disease caused by the ubiquitous necrotrophic fungal pathogen Botrytis cinerea. Mol. Plant Pathol. 2019, 20, 877–892. [Google Scholar] [CrossRef] [Green Version]
- Vanti, G.L.; Leshem, Y.; Masaphy, S. Resistance response enhancement and reduction of Botrytis cinerea infection in strawberry fruit by Morchella conica mycelial extract. Postharvest Biol. Technol. 2021, 175, 111470. [Google Scholar] [CrossRef]
- Dewey, F.M.; Grant-Downton, R. Botrytis—Biology, detection and quantification. In Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 17–34. [Google Scholar] [CrossRef]
- Ceredi, G.; Antoniacci, L.; Montuschi, C.; Paoli, E.D.; Mari, M.; Gengotti, S. Ten years of field trials on grey mold control on strawberries. Acta Hortic. 2009, 842, 327–330. [Google Scholar] [CrossRef]
- Li, W.; Yuan, S.; Sun, J.; Li, Q.; Jiang, W.; Cao, J. Ethyl p-coumarate exerts antifungal activity in vitro and in vivo against fruit Alternaria alternata via membrane-targeted mechanism. Int. J. Food Microbiol. 2018, 278, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Cameron, R.G.; Plotto, A.; Zhong, T.; Bai, J. The effect of controlled-release carvacrol on safety and quality of blueberries stored in perforated packaging. Foods 2021, 10, 1487. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wu, H.; Shi, F.; Wang, H.; Chen, K.; Feng, J.; Jia, W. Antifungal activity screening for mint and thyme essential oils against Rhizopus stolonifer and their application in postharvest preservation of strawberry and peach fruits. J. Appl. Microbiol. 2020, 130, 1993–2007. [Google Scholar] [CrossRef]
- Antunes, M.D.C.; Cavaco, A.M. The use of essential oils for postharvest decay control. Flavour Fragr. J. 2010, 25, 351–366. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, Z.; Li, M.; Xing, M.; Xian, T.; Tu, K. Carvacrol and eugenol effectively inhibit Rhizopus stolonifera and control postharvest soft rot decay in peaches. J. Appl. Microbiol. 2017, 124, 166–178. [Google Scholar] [CrossRef]
- Kawhena, T.G.; Opara, U.L.; Fawole, O.A. A comparative study of antimicrobial and antioxidant activities of plant essential oils and extracts as candidate ingredients for edible coatings to control decay in ‘Wonderful’ pomegranate. Molecules 2021, 26, 3367. [Google Scholar] [CrossRef]
- Wei, Y.; Wei, Y.; Xu, F.; Shao, X. The combined effects of tea tree oil and hot air treatment on the quality and sensory characteristics and decay of strawberry. Postharvest Biol. Technol. 2018, 136, 139–144. [Google Scholar] [CrossRef]
- Hudaib, M.; Speroni, E.; Pietra, A.M.D.; Cavrini, V. GC/MS evaluation of thyme (Thymus vulgaris L.) oil composition and variations during the vegetative cycle. J. Pharm. Biomed. Anal. 2002, 29, 691–700. [Google Scholar] [CrossRef]
- Barbosa, L.C.A.; Pereira, U.A.; Martinazzo, A.P.; Maltha, C.R.Á.; Teixeira, R.R.; Melo, E.C. Evaluation of the chemical composition of brazilian commercial Cymbopogon citratus (D.C.) stapf samples. Molecules 2008, 13, 1864. [Google Scholar] [CrossRef] [Green Version]
- Brown, R.E.; Jarvis, K.L.; Hyland, K.J. Protein measurement using bicinchoninic acid: Elimination of interfering substances. Anal. Biochem. 1989, 180, 136–139. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.E. Involvement of carbohydrate, protein and phenylanine ammonia lyase in up-regulation of secondary metabolites in Labisia pumila under various CO2 and N2 level. Molecules 2011, 16, 4172–4190. [Google Scholar] [CrossRef]
- Zhong, T.; Zhang, J.X.; Sun, X.X.; Kou, J.J.; Zhang, Z.K.; Bai, J.H.; Ritenour, M.A. The potential of gaseous chlorine dioxide for the control of citrus postharvest stem-end rot caused by Lasiodiplodia theobromae. Plant Dis. 2021. [Google Scholar] [CrossRef]
- Sharma, A.; Rajendran, S.; Srivastava, A.; Sharma, S.; Kundu, B. Antifungal activities of selected essential oils against Fusarium oxysporum f. sp. lycopersici 1322, with emphasis on Syzygium aromaticum essential oil. J. Biosci. Bioeng. 2017, 123, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and thyme essential oil-new insights into selected therapeutic applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef]
- Azzouz, M.A.; Bullerman, L.B. Comparative antimycotic effects of selected herbs, spices, plant components and commercial antifungal agents. J. Food Prot. 1982, 45, 1298–1301. [Google Scholar] [CrossRef] [PubMed]
- Numpaque, M.A.; Oviedo, L.A.; Gil, J.H.; García, C.M.; Durango, D.L. Thymol and carvacrol: Biotransformation and antifungal activity against the plant pathogenic fungi Colletotrichum acutatum and Botryodiplodia theobromae. Trop. Plant Pathol. 2011, 36, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Lee, Y.S.; Lee, S.G.; Shin, S.C.; Park, I.K. Fumigant antifungal activity of plant essential oils and components from West Indian bay (Pimenta racemosa) and thyme (Thymus vulgaris) oils against two phytopathogenic fungi. Flavour Fragr. 2008, 23, 272–277. [Google Scholar] [CrossRef]
- Wuryatmo, E.; Klieber, A.A.; Scott, E.S. Inhibition of citrus postharvest pathogens by vapor of citral and related compounds in culture. J. Agric. Food Chem. 2003, 51, 2637–2640. [Google Scholar] [CrossRef] [PubMed]
- Boukhatem, M.N.; Ferhat, M.A.; Kameli, A.; Saidi, F.; Kebir, H.T. Lemon grass (Cymbopogon citratus) essential oil as a potent anti-inflammatory and antifungal drugs. Libyan J. Med. 2014, 9, 25431. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Shao, Y.L.; Tang, Y.J.; Zhou, W.W. Antifungal activity of essential oil compounds (geraniol and citral) and inhibitory mechanisms on grain pathogens (Aspergillus flavus and Aspergillus ochraceus). Molecules 2018, 23, 2108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharon, A.; Finkelstein, A.; Shlezinger, N.; Hatam, I. Fungal apoptosis: Function, genes and gene function. Fems Microbiol. Rev. 2009, 33, 833–854. [Google Scholar] [CrossRef] [PubMed]
- Han, S.I.; Kim, Y.S.; Kim, T.H. Role of apoptotic and necrotic cell death under physiologic conditions. BMB Rep. 2008, 41, 1. [Google Scholar] [CrossRef] [Green Version]
- Hao, B.; Cheng, S.; Clancy, C.J.; Nguyen, M.H. Caspofungin kills Candida albicans by causing both cellular apoptosis and necrosis. Antimicrob. Agents Chemother. 2013, 57, 326–332. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Srivastava, S. Anti-Candida activity of two-peptide bacteriocins, plantaricins (Pln E/F and J/Kk) and their mode of action. Fungal Biol. 2014, 118, 264–275. [Google Scholar] [CrossRef]
- Han, Y.Z.; Zhao, J.J.; Zhang, B.; Shen, Q.; Shang, Q.M.; Li, P.L. Effect of a novel antifungal peptide P852 on cell morphology and membrane permeability of Fusarium oxysporum. Biomembranes 2019, 1861, 532–539. [Google Scholar] [CrossRef]
- Shemesh, R.; Krepker, M.; Nitzan, N.; Vaxman, A.; Segal, E. Active packaging containing encapsulated carvacrol for control of postharvest decay. Postharvest Biol. Technol. 2016, 118, 175–182. [Google Scholar] [CrossRef]
- Feliziani, E.; Romanazzi, G. Postharvest decay of strawberry fruit: Etiology, epidemiology, and disease management. J. Berry Res. 2016, 6, 47–63. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Gomez, A.; Ros-Chumillas, M.; Buendia-Moreno, L.; Navarro-Segura, L.; Martinez-Hernandez, G.B. Active cardboard box with smart internal lining based on encapsulated essential oils for enhancing the shelf life of fresh mandarins. Foods 2020, 9, 590. [Google Scholar] [CrossRef] [PubMed]



| Essential Oil Concentrations | ||||
|---|---|---|---|---|
| Essential Oil | Origin | 150 µL L−1 | 100 µL L−1 | 50 µL L−1 |
| Pinus sylvestris | Bosnia | 10.71 ± 3.61 a | -- | -- |
| Artemisia indica | Nepal | 43.21 ± 2.95 b | -- | -- |
| Pinus nigra | Bosnia | 60.70 ± 5.97 c | -- | -- |
| Melaleuca viridiflora | Madagascar | 64.41 ± 9.04 cd | -- | -- |
| Melaleuca leucadendron | Indonesia | 70.96 ± 13.85 d | -- | -- |
| Rosmarinus officinalis | Spain | 72.30 ± 1.70 de | -- | -- |
| Cymbopogon khasans | India | 73.41 ± 1.97 de | -- | -- |
| Ocimum sanctum | India | 81.55 ± 0.47 ef | -- | -- |
| Lavandula angustifolia | Bulgaria | 81.74 ± 3.07 ef | -- | -- |
| Ocimum basilicum | Egypt | 82.53 ± 3.67 ef | -- | -- |
| Lavandula latifolia | Spain | 85.92 ± 0.20 f | -- | -- |
| Syzygium aromaticum | Indonesia | 86.78 ± 1.35 f | -- | -- |
| Mentha spicata | India | 97.01 ± 0.66 g | -- | -- |
| Thymus zygis | Spain | 100 ± 0.00 g | 21.62 ± 2.72 a | -- |
| Melaleuca ericifolia | Australia | 100 ± 0.00 g | 74.42 ± 4.52 b | -- |
| Pelargonium graveolens | Morocco | 100 ± 0.00 g | 84.85 ± 5.12 c | -- |
| Mentha piperita | India | 100 ± 0.00 g | 85.84 ± 4.16 c | -- |
| Thymus satureoides | Spain | 100 ± 0.00 g | 89.62 ± 1.39 cd | -- |
| Cymbopogon martinii | India | 100 ± 0.00 g | 97.23 ± 2.77 de | -- |
| Cymbopogon citratus (Cc) | India | 100 ± 0.00 g | 100 ± 0.00 e | 72.06 ± 6.47 a |
| Thymus vulgraris (Tv) | Spain | 100 ± 0.00 g | 100 ± 0.00 e | 98.69 ± 0.33 b |
| Origanum heracleoticum (Oh) | France | 100 ± 0.00 g | 100 ± 0.00 e | 99.32 ± 0.19 b |
| Compounds | Retention Index (RI) a | Retention Index (RI) b | Percentage (%) c | ||
|---|---|---|---|---|---|
| Origanum heracleoticum | Thymus vulgraris | Cymbopogon citratus | |||
| α-Thujene | 1025 | 1023 | 0.74 | 0.57 | -- |
| α-Pinene | 1037 | 1026 | 1.69 | 1.59 | -- |
| Camphene | 1081 | 1071 | 0.56 | 2.25 | 2.06 |
| β-Pinene | 1122 | 1114 | 0.29 | 1.97 | 0.2 |
| 3-Carene | 1135 | 1130 | 0.19 | -- | -- |
| β-Myrcene | 1152 | 1164 | 3.34 | -- | -- |
| L-Limonene | 1181 | 1196 | 0.55 | 0.69 | 2.92 |
| β-Phellandrene | 1214 | 1164 | 0.40 | 0.27 | -- |
| Eucalyptol | 1216 | 1211 | -- | 2.95 | -- |
| γ-Terpinene | 1240 | 1230 | 7.80 | 11.47 | -- |
| trans-β-Ocimene | 1242 | 1245 | 0.33 | -- | -- |
| p-Cymene | 1265 | 1273 | 21.51 | 20.43 | -- |
| Terpinolene | 1275 | 1270 | 2.15 | 2.47 | -- |
| Linalool | 1542 | 1540 | 1.2 | 5.81 | 3.52 |
| (+)-2-Bornanone | 1544 | 1539 | -- | 4.05 | -- |
| cis-Verbenol | 1546 | 1551 | -- | -- | 0.64 |
| α-Caryophyllene | 1577 | 1593 | 0.34 | 0.14 | -- |
| Terpinen-4-ol | 1595 | 1584 | -- | 2.65 | -- |
| β-Caryophyllene | 1599 | 1565 | 2.6 | 3.11 | 2.27 |
| Bornyl acetate | 1603 | 1595 | -- | 0.31 | -- |
| Menthol | 1630 | 1626 | 0.39 | -- | -- |
| 4-Terpinenyl acetate | 1631 | 1613 | 0.32 | -- | -- |
| trans-Dihydrocarvone | 1632 | 1642 | 0.22 | -- | -- |
| Levomenthol | 1633 | 1626 | 0.21 | 0.14 | -- |
| Alloaromadendrene | 1643 | 1639 | -- | 0.16 | -- |
| cis-β-Farnesene | 1654 | 1648 | 0.21 | -- | -- |
| Borneol | 1680 | 1705 | 2.09 | 6.11 | -- |
| γ-Muurolene | 1683 | 1681 | 0.32 | -- | -- |
| Geraniol formate | 1689 | 1667 | -- | -- | 0.62 |
| α-Terpineol | 1691 | 1680 | 2.23 | 1.43 | 2.36 |
| β-Citral | 1694 | 1689 | -- | -- | 29.51 |
| Nerol acetate | 1698 | 1700 | 0.20 | -- | -- |
| β-Bisabolene | 1700 | 1717 | 3.03 | -- | -- |
| Verbenone | 1701 | 1699 | -- | 0.17 | -- |
| α-Citral | 1742 | 1737 | -- | -- | 38.34 |
| δ-Cadinene | 1753 | 1757 | 0.6 | 0.44 | -- |
| γ-Cadinene | 1756 | 1766 | 0.28 | 0.23 | 0.35 |
| Geraniol acetate | 1760 | 1770 | 0.43 | -- | 6.36 |
| cis-Carveol | 1850 | 1848 | -- | -- | 0.17 |
| Carvacryl acetate | 1896 | 1890 | 0.25 | -- | -- |
| cis-Geraniol | 1900 | 1884 | -- | -- | 0.75 |
| Lemonol | 1956 | 1921 | -- | -- | 6.41 |
| Caryophyllene oxide | 1967 | 2002 | 2.47 | 0.80 | 1.75 |
| Epiglobulol | 2001 | 2000 | -- | 0.54 | -- |
| Spathulenol | 2133 | 2129 | -- | 0.32 | -- |
| Thymol | 2159 | 2152 | 5.32 | 22.71 | -- |
| 16-Kaurene | 2162 | 2167 | -- | -- | 0.44 |
| Carvacrol | 2166 | 2161 | 37.47 | 6.22 | 1.32 |
| β-Guaiene | 2170 | 2155 | 0.26 | -- | -- |
| Storage Day | Treatment | Weight Loss (%) | Firmness (g) | SSC |
|---|---|---|---|---|
| 0 | Control | 0.00 ± 0.00 | 68.3 ± 5.51 | 13.03 ± 0.07 |
| 12 | Control | 1.54 ± 0.19 a | 68.13 ± 6.93 a | 10.70 ± 0.10 a |
| Cc | 1.40 ± 0.30 a | 75.57 ± 5.87 a | 11.20 ± 0.32 ab | |
| Tv | 1.54 ± 0.19 a | 76.48 ± 8.54 a | 11.53 ± 0.09 bc | |
| Ov | 0.89 ± 0.43 b | 69.78 ± 3.05 a | 11.83 ± 0.07 c | |
| 24 | Control | 3.26 ± 0.24 a | 63.47 ± 4.61 a | 10.87 ± 0.23 a |
| Cc | 2.91 ± 0.29 a | 72.85 ± 4.93 a | 11.03 ± 0.47 a | |
| Tv | 2.93 ± 0.22 a | 73.42 ± 6.38 a | 12.27 ± 0.12 b | |
| Ov | 1.75 ± 0.26 b | 71.26 ± 3.50 a | 13.13 ± 0.03 b | |
| 36 | Control | 4.66 ± 0.36 a | 60.93 ± 3.86 a | 11.87 ± 0.13 a |
| Cc | 4.37 ± 0.35 a | 66.53 ± 7.72 a | 11.80 ± 0.12 a | |
| Tv | 4.60 ± 0.21 a | 66.50 ± 3.09 a | 12.53 ± 0.15 b | |
| Ov | 2.72 ± 0.22 b | 65.97 ± 2.62 a | 12.23 ± 0.28 ab | |
| 48 | Control | 5.95 ± 0.49 a | 60.78 ± 3.85 a | 10.33 ± 0.12 a |
| Cc | 5.54 ± 0.42 a | 64.47 ± 8.03 a | 11.60 ± 0.12 b | |
| Tv | 5.95 ± 0.19 a | 62.58 ± 5.11 a | 11.47 ± 0.63 b | |
| Ov | 3.57 ± 0.12 b | 66.37 ± 4.35 a | 11.53 ± 0.17 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Wu, H.; Chen, K.; Feng, J.; Zhang, Y. Antifungal Activities and Mode of Action of Cymbopogon citratus, Thymus vulgraris, and Origanum heracleoticum Essential Oil Vapors against Botrytis cinerea and Their Potential Application to Control Postharvest Strawberry Gray Mold. Foods 2021, 10, 2451. https://doi.org/10.3390/foods10102451
Yan J, Wu H, Chen K, Feng J, Zhang Y. Antifungal Activities and Mode of Action of Cymbopogon citratus, Thymus vulgraris, and Origanum heracleoticum Essential Oil Vapors against Botrytis cinerea and Their Potential Application to Control Postharvest Strawberry Gray Mold. Foods. 2021; 10(10):2451. https://doi.org/10.3390/foods10102451
Chicago/Turabian StyleYan, Jiaqi, Hua Wu, Keying Chen, Jiajun Feng, and Yansong Zhang. 2021. "Antifungal Activities and Mode of Action of Cymbopogon citratus, Thymus vulgraris, and Origanum heracleoticum Essential Oil Vapors against Botrytis cinerea and Their Potential Application to Control Postharvest Strawberry Gray Mold" Foods 10, no. 10: 2451. https://doi.org/10.3390/foods10102451
APA StyleYan, J., Wu, H., Chen, K., Feng, J., & Zhang, Y. (2021). Antifungal Activities and Mode of Action of Cymbopogon citratus, Thymus vulgraris, and Origanum heracleoticum Essential Oil Vapors against Botrytis cinerea and Their Potential Application to Control Postharvest Strawberry Gray Mold. Foods, 10(10), 2451. https://doi.org/10.3390/foods10102451






