Novel Antioxidant and Hypoglycemic Water-Soluble Polysaccharides from Jasmine Tea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Jasmine Tea
2.3. Extraction, Isolation, and Purification of Crude Polysaccharides from Jasmine Tea
2.4. Functional Activities of JSP Sub-Fractions
2.4.1. Antioxidant Activities
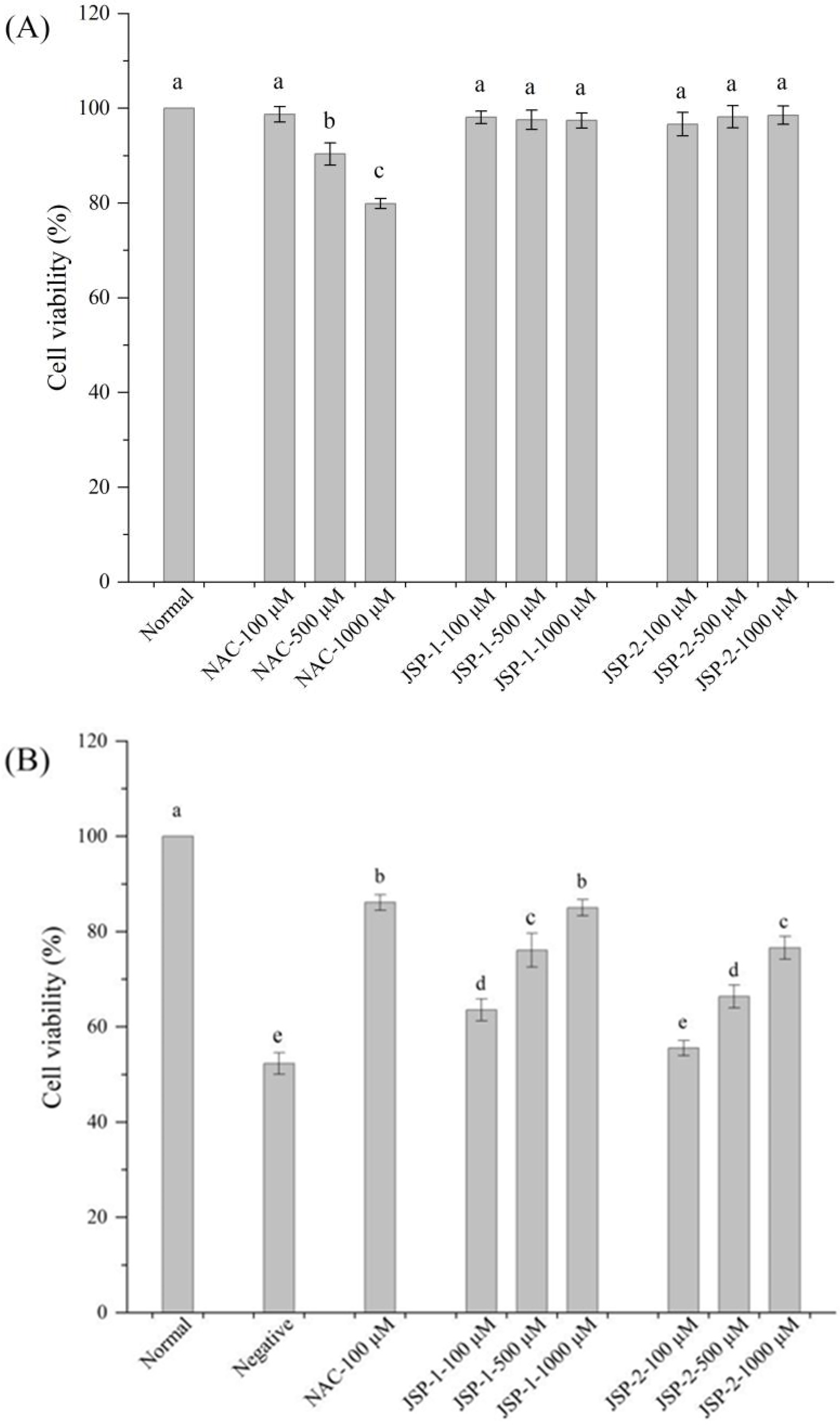
2.4.2. Hypoglycemic Effect
Cell Culture
Cytotoxicity Assay
Protective Effect on High-Glucose-Induced RIN-m5F Cell Injury
2.5. Primary Structure Analysis of JSP Sub-Fractions
2.5.1. Determination of Molecular Weight and Homogeneity
2.5.2. Analysis of Monosaccharide Composition
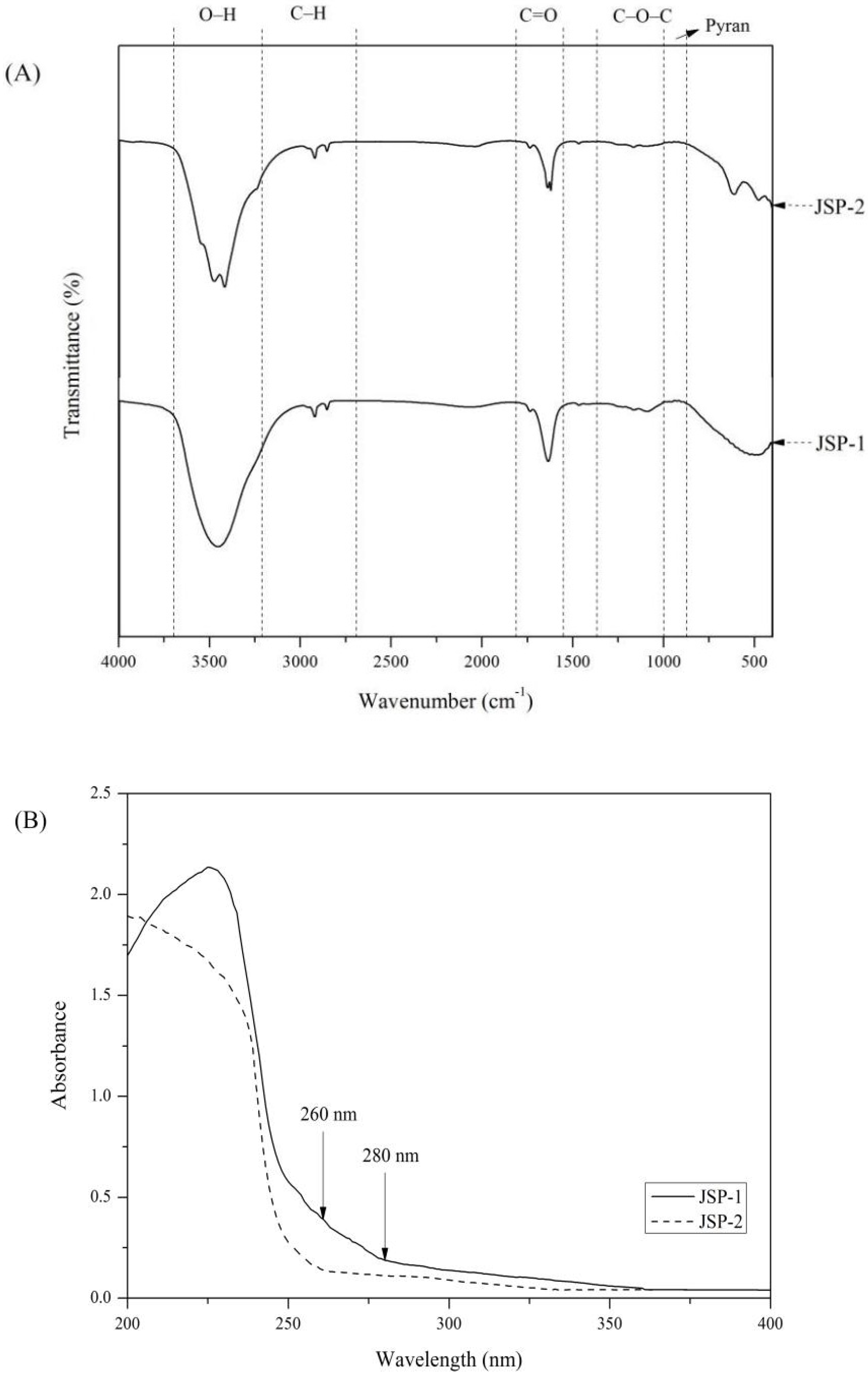
2.5.3. FTIR and UV Analysis
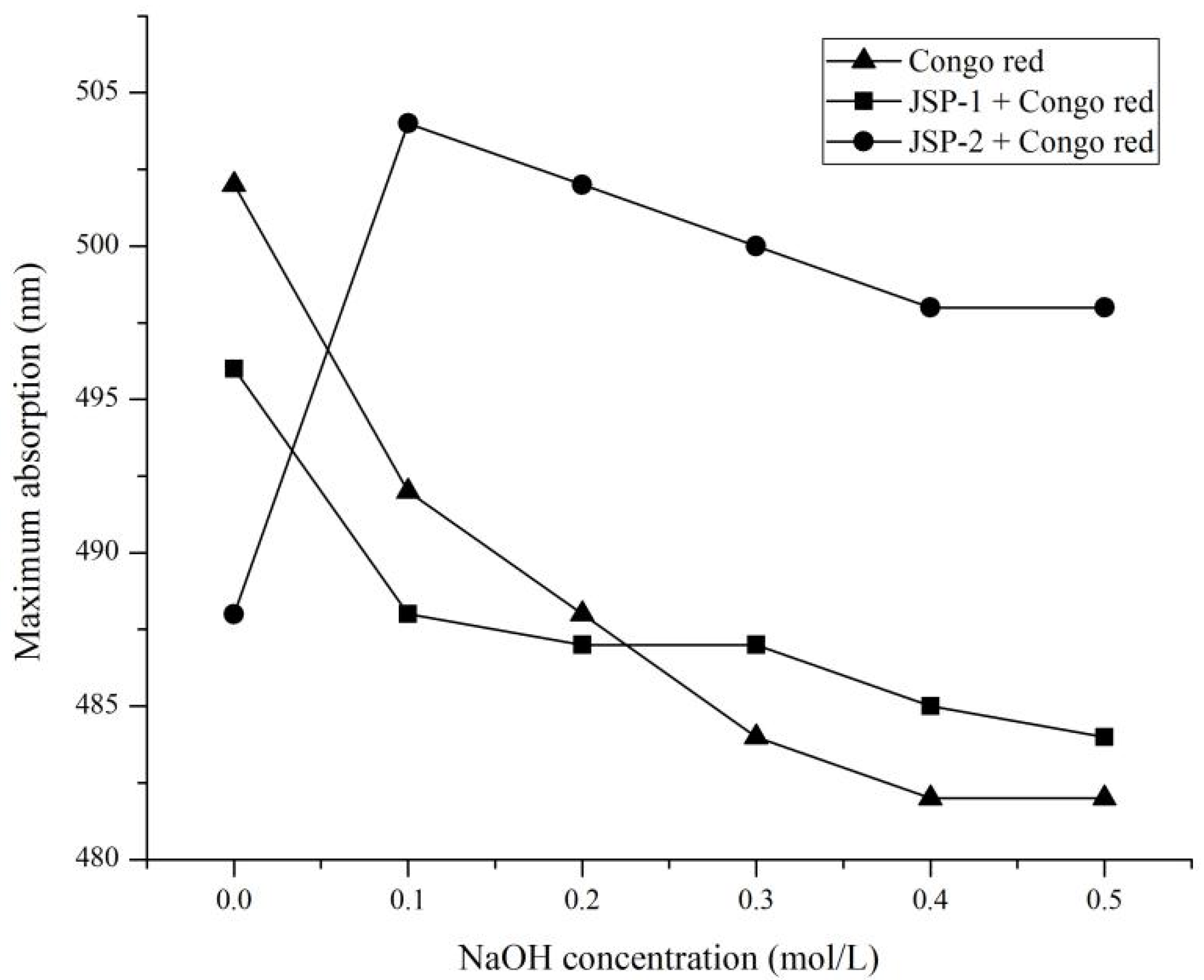
2.5.4. Congo Red Analysis
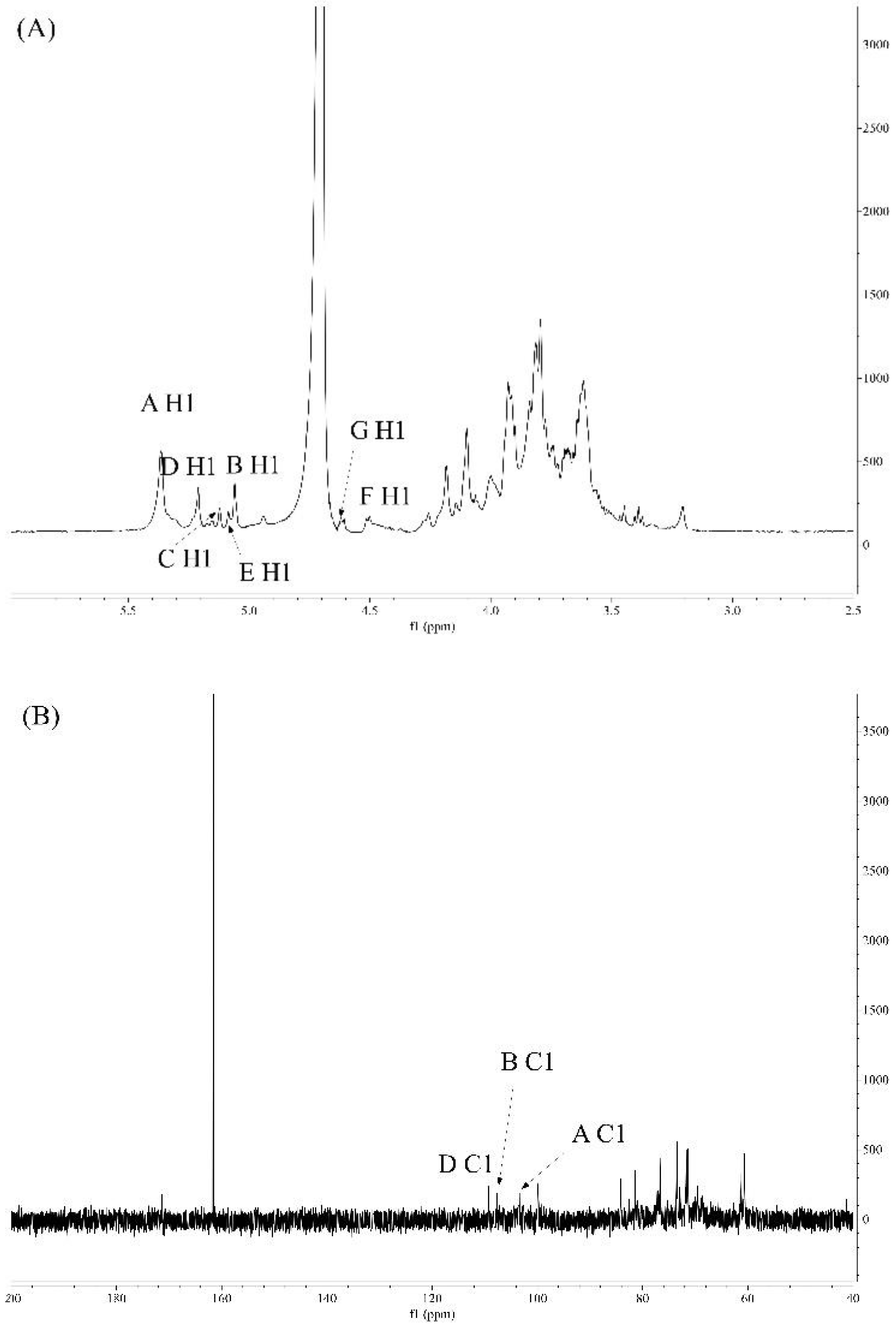
2.5.5. Analysis of NMR Spectroscopy
2.6. Statistical Analysis
3. Results and Discussion
3.1. Isolation and Purification of Crude JSP
3.2. Functional Activities of JSP Sub-Fractions
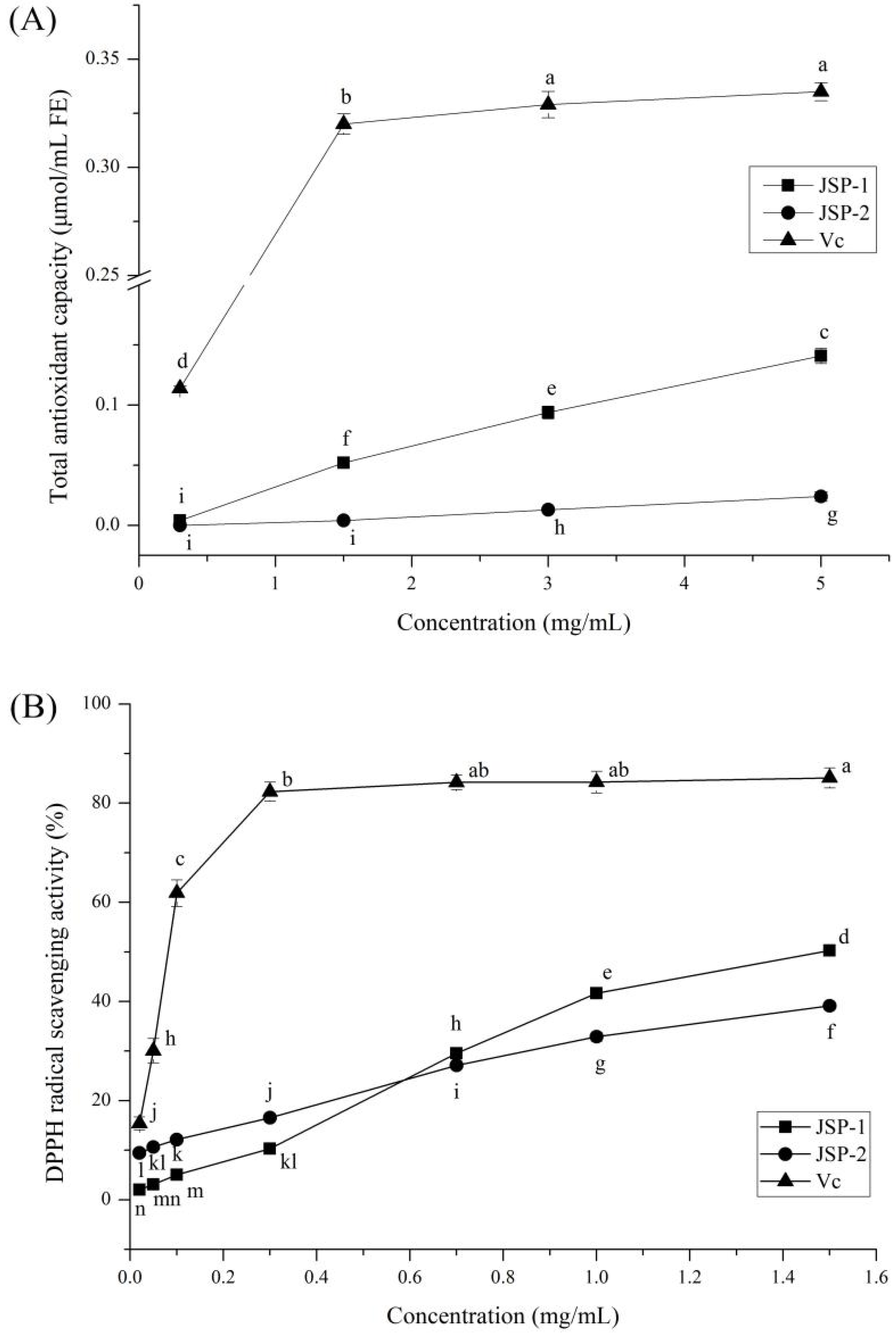
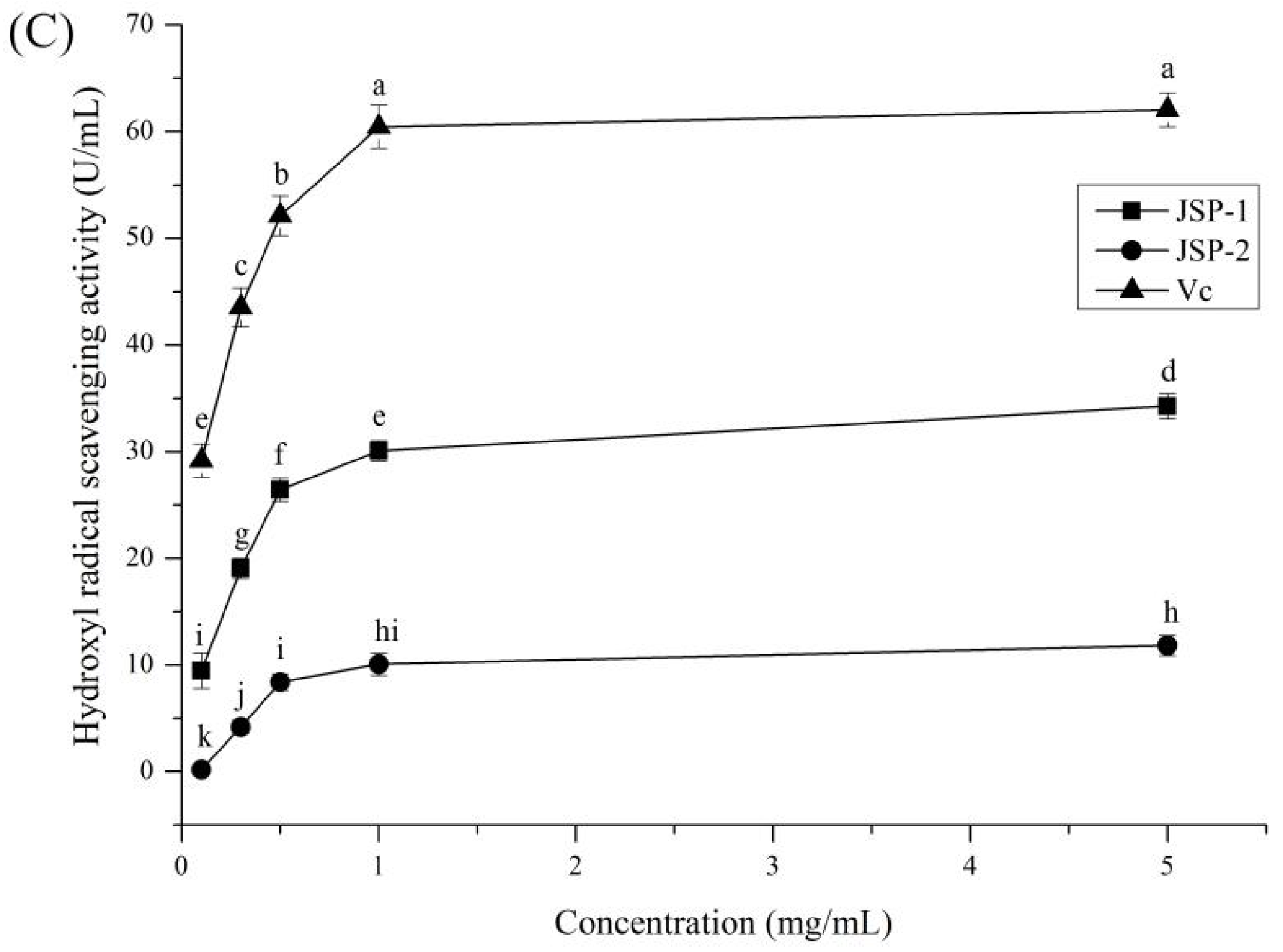
3.2.1. Antioxidant Activities
3.2.2. Hypoglycemic Activities
3.3. Structural Analysis of JSP Sub-Fractions
3.3.1. Monosaccharide Composition and Molecular Weight
3.3.2. FTIR and UV Analysis
3.3.3. Congo Red Test
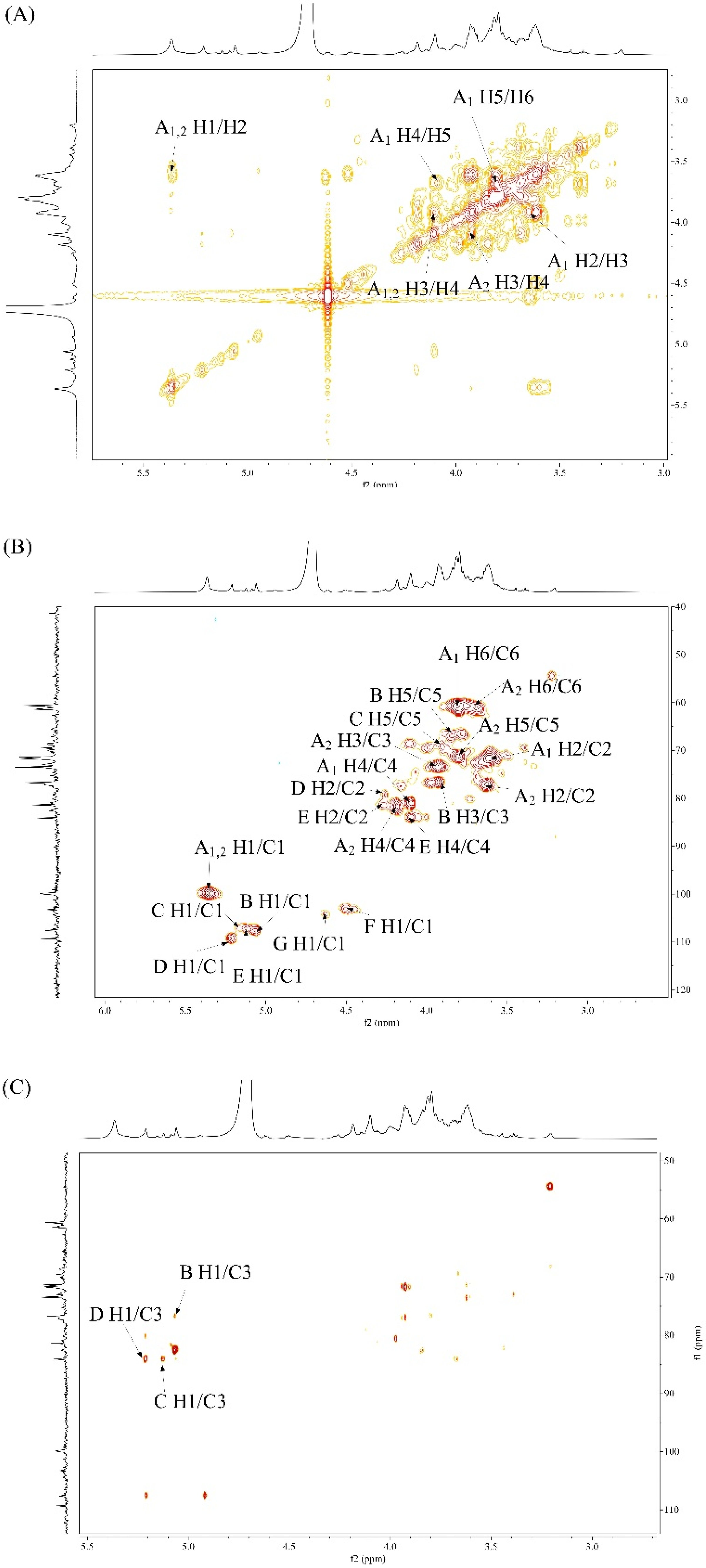
3.3.4. NMR Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Rhodes, C.J. Type 2 diabetes-a matter of β-cell life and death. Science 2005, 307, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.M.; Zhao, J.C.; Guo, Y.; Zhang, H.X.; Duan, M.Y.; Wu, H.X. Extraction, purification, emulsifying property, hypoglycemic activity, and antioxidant activity of polysaccharides from comfrey. Ind. Crop. Prod. 2020, 146, 1–12. [Google Scholar] [CrossRef]
- Lo, T.C.T.; Chang, C.A.; Chiu, K.H.; Tsay, P.K.; Jen, J.F. Correlation evaluation of antioxidant properties on the monosaccharide components and glycosyl linkages of polysaccharide with different measuring methods. Carbohyd. Polym. 2011, 86, 320–327. [Google Scholar] [CrossRef]
- Sabharwal, S.; Aggarwal, S.; Vats, M.; Sardana, S. Jasminum sambac (Linn) Ait: Preliminary phytochemical screening and wound healing investigation using total ethanol stems extract. Int. J. Pharm. Sci. Rev. Res. 2012, 17, 44–47. [Google Scholar]
- Anima, P.; Arun, M.; Satish, S. Scientific validation of wound healing potential of Jasminum sambac Ait. Afr. J. Bot. 2019, 121, 584–589. [Google Scholar] [CrossRef]
- Kalaiselvi, M.; Narmadha, R.; Ragavendran, P.; Vidya, B.; Gomathi, D.; Raj, C.A.; Starlinraj, T.; Gopalakrishnan, V.K.; Uma, C.; Kalaivani, K. Chemopreventive effect and HPTLC fingerprinting analysis of Jasminum sambac (L.) Ait. extract against DLA-induced lymphoma in experimental animals. Appl. Biochem. Biotechnol. 2013, 169, 1098–1108. [Google Scholar] [CrossRef]
- Sengar, N.; Joshi, A.; Prasad, S.K.; Hemalatha, S. Anti-inflammatory, analgesic and anti-pyretic activities of standardized root extract of Jasminum sambac. J. Ethnopharmacol. 2015, 160, 140–148. [Google Scholar] [CrossRef]
- Kalaiselvi, M.; Narmadha, R.; Ragavendran, P.; Raj, A.; Sophia, D.; Kumar, G.R.; Gomathi, D.; Uma, C.; Kalaivani, K. In vivo simulated in vitro model of Jasminum sambac (Linn.) using mammalian liver slice technique. Asian Pac. J. Trop. Biomed. 2011, 1, S216–S219. [Google Scholar] [CrossRef]
- Khidzir, K.M.; Cheng, S.F.; Chuah, C.H. Interspecies variation of chemical constituents and antioxidant capacity of extracts from Jasminum sambac and Jasminum multiflorum grown in Malaysia. Ind. Crop. Prod. 2015, 74, 635–641. [Google Scholar] [CrossRef]
- Rambabu, B.; Rao Patnaik, K.S.K. Anti diabetic and anti ulcer activity of ethanolic flower extract of Jasminum sambac in rats. Asian J. Res. Chem. 2014, 7, 580–585. [Google Scholar]
- Eakwaropas, P.; Wisidsri, N. Effect of Jasminum sambac flower extracts on fibroblast cell viability and film characteristics. Appl. Mech. Mater. 2019, 886, 27–33. [Google Scholar] [CrossRef]
- Borazjani, N.J.; Tabarsa, M.; You, S.; Rezaei, M. Purification, molecular properties, structural characterization, and immunomodulatory activities of water soluble polysaccharides from Sargassum angustifolium. Int. J. Biol. Macromol. 2018, 109, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Nowacka-Jechalke, N.; Nowak, R.; Juda, M.; Malm, A.; Lemieszek, M.; Rzeski, W.; Kaczyński, Z. New biological activity of the polysaccharide fraction from Cantharellus cibarius and its structural characterization. Food Chem. 2018, 268, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Y.; Ye, H.F.; Wu, D.D.; Shi, H.; Zhou, X.X. Chemoenzymatic quantification for monitoring unpurified polysaccharide in rich medium. Appl. Microbiol. Biotechnol. 2019, 103, 7635–7645. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-Y.; He, X.-M.; Sun, J.; Li, C.-B.; Li, L.; Sheng, J.-F.; Xin, M.; Li, Z.-C.; Zheng, F.-J.; Liu, G.-M.; et al. Polyphenols and alkaloids in by-products of longan fruits (Dimocarpus longan Lour.) and their bioactivities. Molecules 2019, 24, 1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Hawary, S.S.; EL-Hefnawy, H.M.; Osman, S.M.; Mostafa, E.S.; Mokhtar, F.A.; El-Raey, M.A. Chemical profile of two Jasminum sambac L. (Ait) cultivars cultivated in Egypt-their mediated silver nanoparticles synthesis and selective cytotoxicity. Int. J. Appl. Pharmaceut. 2019, 11, 154–164. [Google Scholar] [CrossRef]
- Bei, W.; Wang, Y.; Chen, J.; Zhang, J.; Wang, L.; Gu, Z.; Hu, Y.; Huang, Y.; Xu, W.; Lei, Z.; et al. Chinese medicine FTZ recipe protects against high-glucose-induced beta cell injury through alleviating oxidative stress. Evid.-Based Complement. Altern. Med. 2019, 2019, 6378786. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.L.; Zhang, S.H.; Wang, Y.Q.; Li, M.X.; Ding, K. Isolation and structure characterization of a polysaccharide from Crataegus pinnatifida and its bioactivity on gut microbiota. Int. J. Biol. Macromol. 2020, 154, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.Y.; Jiang, Y.; Chen, G.C.; Li, S.S.; Yang, F.; Ma, Q. A sensitive and rapid UPLC-MS/MS method for determination of monosaccharides and anti-allergic effect of the polysaccharides extracted from Saposhnikoviae radix. Molecules 2018, 23, 1924. [Google Scholar] [CrossRef] [Green Version]
- Tian, W.; Xiao, N.; Yang, Y.; Xiao, J.; Zeng, R.; Xie, L.; Qiu, Z.; Li, P.; Du, B. Structure, antioxidant and immunomodulatory activity of a polysaccharide extracted from Sacha inchi seeds. Int. J. Biol. Macromol. 2020, 162, 116–126. [Google Scholar] [CrossRef]
- Gong, L.; Meng, F.; Hou, Y.; Liu, Y.; Xu, J.; Zhang, W.; Chen, Y. Purification, characterization, and bioactivity of two new polysaccharide fractions from Thelephora ganbajun mushroom. J. Food Biochem. 2020, 44, 1–9. [Google Scholar] [CrossRef]
- Ker, Y.-B.; Chen, K.-C.; Chyau, C.-C.; Chen, C.-C.; Guo, J.-H.; Hsieh, C.-L.; Wang, H.-E.; Peng, C.-C.; Chang, C.-H.; Peng, R.Y. Antioxidant capability of polysaccharides fractionated from submerge-cultured Agaricus blazei Mycelia. J. Agric. Food Chem. 2005, 53, 7052–7058. [Google Scholar] [CrossRef]
- Cui, K.; Zhang, S.B.; Jiang, X.; Xie, W.D. Novel synergic antidiabetic effects of Astragalus polysaccharides combined with Crataegus flavonoids via improvement of islet function and liver metabolism. Mol. Med. Rep. 2016, 13, 4737–4744. [Google Scholar] [CrossRef] [Green Version]
- Watson, J.D. Type 2 diabetes as a redox disease. Lancet 2014, 383, 841–843. [Google Scholar] [CrossRef]
- Chen, S.; Khan, B.M.; Cheong, K.L.; Liu, Y. Pumpkin polysaccharides: Purification, characterization and hypoglycemic potential. Int. J. Biol. Macromol. 2019, 139, 842–849. [Google Scholar] [CrossRef]
- Li, Z.M.; Nie, K.Y.; Wang, Z.J.; Luo, D.H. Quantitative structure activity relationship models for the antioxidant activity of polysaccharides. PLoS ONE 2016, 11, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Guo, Z.; Zeng, X.; Hao, C.; Zhang, Y.; Zhang, M.; Liu, Y.; Li, H.; Li, J.; Zhang, L. Chemical, biochemical, preclinical and clinical studies of Ganoderma lucidum polysaccharide as an approved drug for treating myopathy and other diseases in China. J. Cell. Mol. Med. 2018, 22, 3278–3297. [Google Scholar] [CrossRef] [Green Version]
- Yi, Y.; Lamikanra, O.; Sun, J.; Wang, L.M.; Min, T.; Wang, H.X. Activity diversity structure−activity relationship of polysaccharides from lotus root varieties. Carbohyd. Polym. 2018, 190, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.L.; Sun, H.Q.; Zhang, X.J.; Wu, L.R.; Zhu, Z.Y. A novel acid polysaccharide from fermented broth of Pleurotus citrinopileatus: Hypoglycemic activity in vitro and chemical structure. J. Mol. Struct. 2020, 1220, 1–13. [Google Scholar] [CrossRef]
- Kan, L.B.; Chai, Y.Y.; Li, X.Y.; Zhao, M. Structural analysis and potential anti-tumor activity of Sporisorium reilianum (Fries) polysaccharide. Int. J. Biol. Macromol. 2020, 153, 986–994. [Google Scholar] [CrossRef]
- Cai, W.; Xu, H.; Xie, L.; Sun, J.; Sun, T.; Wu, X.; Fu, Q. Purification, characterization and in vitro anticoagulant activity of polysaccharides from Gentiana scabra Bunge roots. Carbohyd. Polym. 2016, 140, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhu, Z.Y.; Liu, Y.; Sun, H.Q.; Song, Q.Y.; Zhang, Y.M. The chemical structure and anti-aging bioactivity of an acid polysaccharide obtained from rose buds. Food Funct. 2018, 9, 2300–2312. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.K.; Hua, D.H.; Huang, D.; Zhang, Q.; Yan, C.Y. Characterization of a new heteropolysaccharide from green guava and its application as an α-glucosidase inhibitor for the treatment of type II diabetes. Food Funct. 2018, 9, 3997–4007. [Google Scholar] [CrossRef]
- Gandolfi-Donadío, L.; Gallo-Rodriguez, C.; de Lederkremer, R.M. Syntheses of β-D-Galf-(1→6)-β-D-Galf-(1→5)-D-Galf and β-D-Galf-(1→5)-β-D-Galf-(1→6)-D- Galf, trisaccharide units in the galactan of Mycobacterium tuberculosis. J. Org. Chem. 2003, 68, 6928–6934. [Google Scholar] [CrossRef]
- Qu, H.; Gao, X.; Cheng, C.L.; Zhao, H.T.; Wang, Z.Y.; Yi, J.J. Hepatoprotection mechanism against alcohol-induced liver injury in vivo and structural characterization of Pinus koraiensis pine nut polysaccharide. Int. J. Biol. Macromol. 2020, 154, 1007–1021. [Google Scholar] [CrossRef]
- Liu, H.; He, P.; He, L.; Li, Q.; Cheng, J.; Wang, Y.; Yang, G.; Yang, B. Structure characterization and hypoglycemic activity of an arabinogalactan from Phyllostachys heterocycla bamboo shoot shell. Carbohydr. Polym. 2018, 201, 189–200. [Google Scholar] [CrossRef]
- Zhang, Z.; Kong, F.; Ni, H.; Mo, Z.; Wan, J.-B.; Hua, D.; Yan, C. Structural characterization, α-glucosidase inhibitory and DPPH scavenging activities of polysaccharides from guava. Carbohydr. Polym. 2016, 144, 106–114. [Google Scholar] [CrossRef]
- Zhang, S.; An, L.; Li, Z.; Wang, H.; Shi, L.; Zhang, J.; Li, Y.; Jin, D.-Q.; Tuerhong, M.; Ohizumi, Y.; et al. An active heteropolysaccharide from the rinds of Garcinia mangostana Linn.: Structural characterization and immunomodulation activity evaluation. Carbohydr. Polym. 2020, 235, 115929. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Chen, G.T.; Meng, G.L.; Xu, J.L. Characterization of pumpkin polysaccharides and protective effects on streptozotocin-damaged islet cells. Chin. J. Nat. Med. 2015, 13, 199–207. [Google Scholar] [CrossRef]
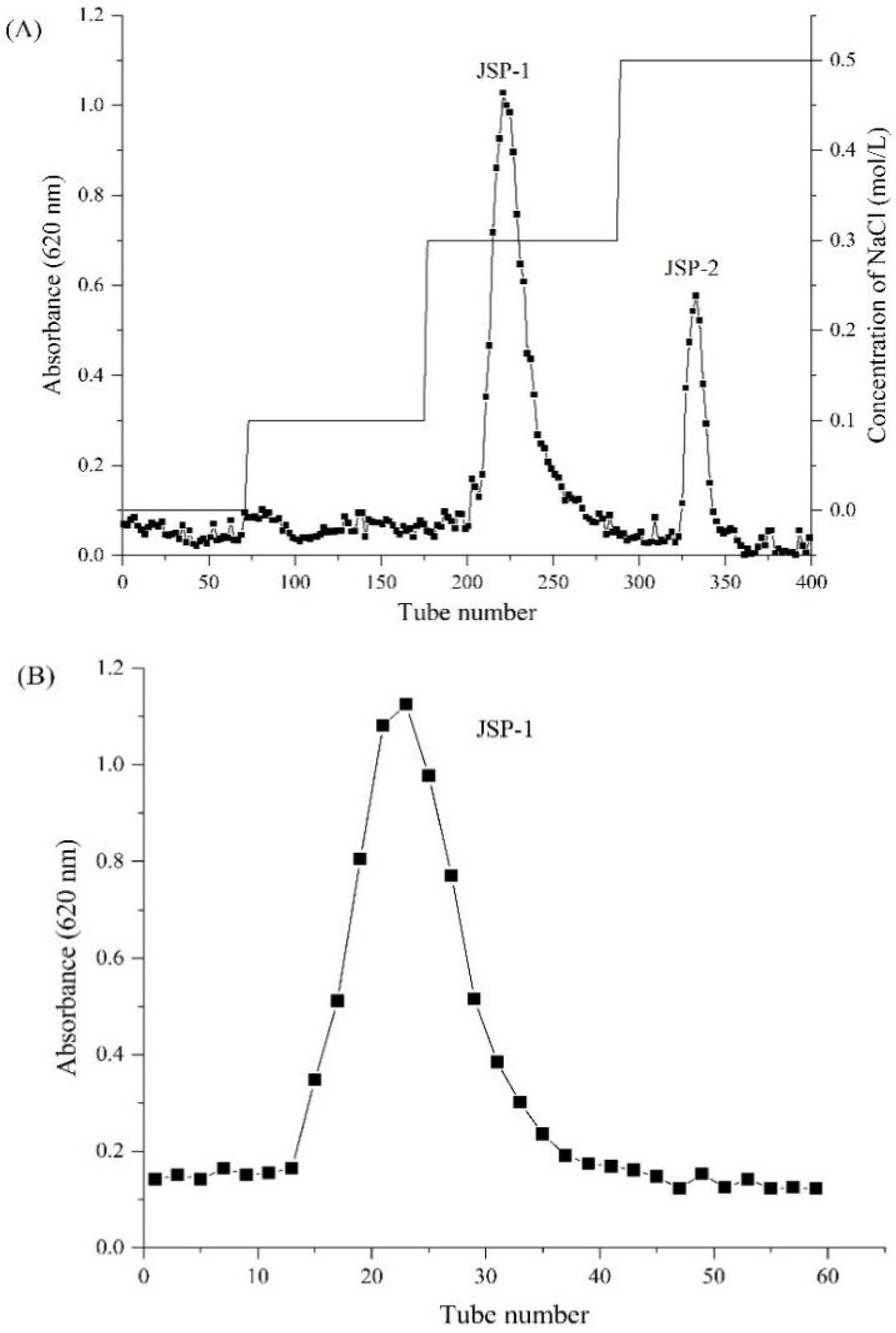
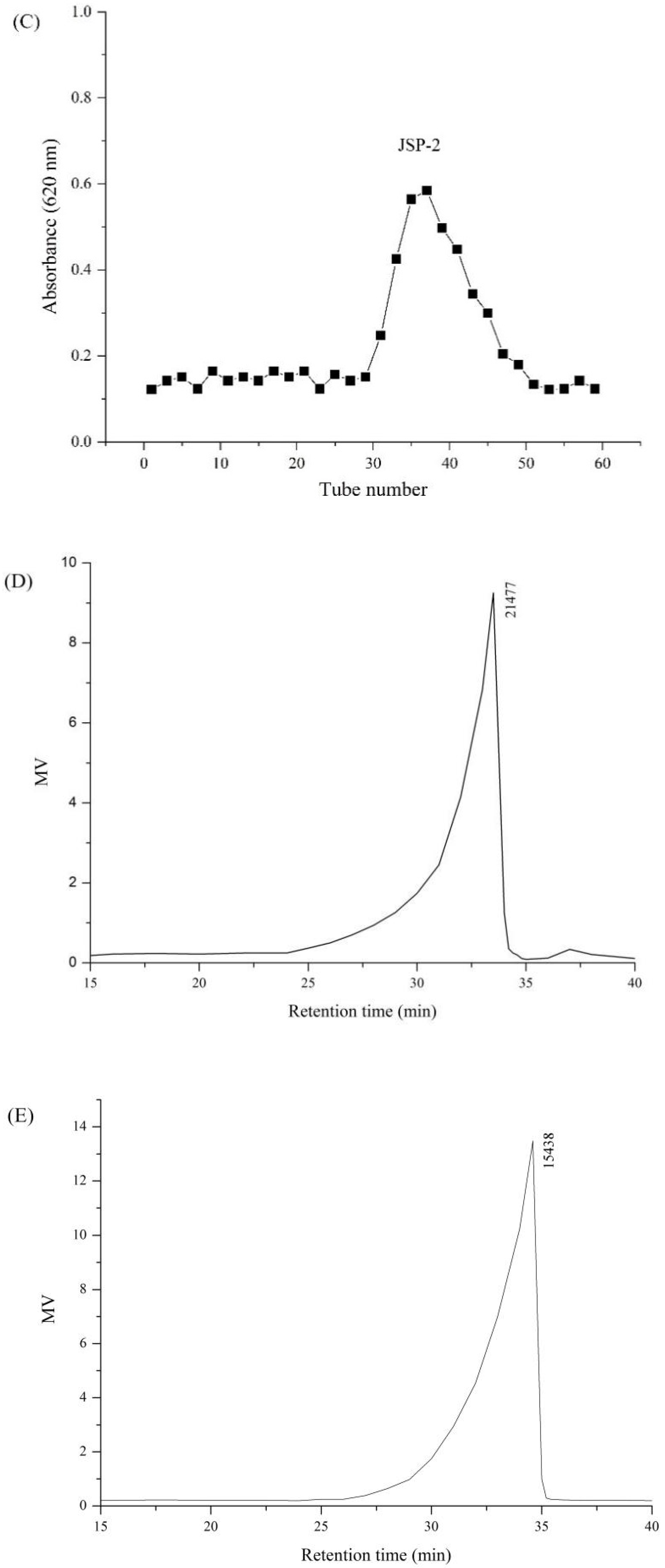
| Sample | Molecular Weight (kDa) | Monosaccharide Composition (Molar Ratio) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Man | Glu | Gal | Rha | Fuc | Xyl | Ara | GlcA | GalA | ||
| JSP-1 | 18.4 | 0.06 | 1.14 | 4.69 | 1.00 | 0.18 | 9.92 | 13.79 | 0.10 | 4.09 |
| JSP-2 | 14.1 | 0.29 | - | 3.80 | 1.00 | 0.17 | 8.27 | 11.85 | 0.14 | 5.05 |
| Sugar Residues | 1 | 2 | 3 | 4 | 5 | 6 | ||
|---|---|---|---|---|---|---|---|---|
| A1 | 1,3-linked α-Galf | H | 5.36 | 3.61 | 3.93 | 4.10 | 3.69 | 3.81 |
| C | 99.75 | 71.64 | 76.75 | 80.87 | 72.85 | 60.66 | ||
| A2 | 1,2-linked α-Galf | H | 5.36 | 3.63 | 3.93 | 4.19 | 3.80 | 3.69 |
| C | 99.78 | 77.07 | 73.39 | 81.79 | 71.21 | 61.43 | ||
| B | 1,5-linked α-Araf | H | 5.06 | 4.10 | 3.99 | 4.19 | 3.85 | - |
| C | 107.54 | 80.96 | 76.55 | 82.25 | 67.60 | - | ||
| C | 1,3,5-linked α-Araf | H | 5.12 | - | 4.26 | 4.06 | 3.90 | - |
| C | 107.23 | - | 84.21 | 79.44 | 69.75 | - | ||
| D | 1,3-linked α-Araf | H | 5.21 | 4.19 | 4.10 | 4.24 | - | - |
| C | 109.27 | 80.88 | 84.12 | 81.36 | - | - | ||
| E | T-linked α-Araf | H | 5.08 | 4.27 | 4.19 | 4.00 | 3.69 | - |
| C | 107.57 | 81.32 | 81.52 | 83.70 | 61.38 | - | ||
| F | 1,2-linked β-Xylp | H | 4.51 | 3.62 | 3.70 | 3.39 | 3.84 | - |
| C | 103.26 | 71.44 | 75.23 | 69.46 | 60.66 | - | ||
| G | 1,3-linked β-Xylp | H | 4.62 | 3.63 | 3.75 | 4.07 | 3.84 | - |
| C | 104.42 | 77.23 | 80.01 | 74.54 | 60.66 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Sheng, J.; He, X.; Sun, J.; Wei, Z.; Liu, G.; Li, C.; Lin, B.; Li, L. Novel Antioxidant and Hypoglycemic Water-Soluble Polysaccharides from Jasmine Tea. Foods 2021, 10, 2375. https://doi.org/10.3390/foods10102375
Tang Y, Sheng J, He X, Sun J, Wei Z, Liu G, Li C, Lin B, Li L. Novel Antioxidant and Hypoglycemic Water-Soluble Polysaccharides from Jasmine Tea. Foods. 2021; 10(10):2375. https://doi.org/10.3390/foods10102375
Chicago/Turabian StyleTang, Yayuan, Jinfeng Sheng, Xuemei He, Jian Sun, Zhen Wei, Guoming Liu, Changbao Li, Bo Lin, and Li Li. 2021. "Novel Antioxidant and Hypoglycemic Water-Soluble Polysaccharides from Jasmine Tea" Foods 10, no. 10: 2375. https://doi.org/10.3390/foods10102375
APA StyleTang, Y., Sheng, J., He, X., Sun, J., Wei, Z., Liu, G., Li, C., Lin, B., & Li, L. (2021). Novel Antioxidant and Hypoglycemic Water-Soluble Polysaccharides from Jasmine Tea. Foods, 10(10), 2375. https://doi.org/10.3390/foods10102375






