In Vitro Screening Potential Antibacterial Properties of the Greek Oregano Honey against Clinical Isolates of Helicobacter pylori
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbial Cultures and Antibiotic Sensitivity of H. pylori Strains
2.2. Determination of Antibacterial Activity of Native Oregano Honey
Osmotic Effect
2.3. Study Design for Determination In Vitro Anti-Helicobacter pylori Activity of Extracts of Crude Honey
2.3.1. Used Solvents
2.3.2. Extraction of Crude Honey
2.3.3. Screening the Antibacterial Efficacy of Honey Extracts
Antimicrobial Assay
Minimum Inhibitory Concentration (MIC)
2.4. Urease Inhibitory Effect
- (a)
- Crude honey
- (b)
- Dried honey extracts with the solvent diethyl ether
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
- All strains of H. pylori were susceptible to the action of oregano honey, although not all of them equally.
- The H. pylori strains were tested for their susceptibility against sixantibiotics that are used in clinical therapeutics to treat H. pylori infections, and the results indicate that, in general, the strains that were isolated from ulcers were more resistant than the ones isolated from gastritis cases.
- The extracts of oregano honey by the four different organic solvents showed significant differences in their antibacterial activity against H. pylori. Each of these solvents, for chemical reasons, extracts a different mixture of compounds, and this finding points to a synergistic effect of these compounds rather than to one super drastic compound with antibacterial effects.
- H. pylori was found to be resistant to artificial honey (sucrose 75% and 15%), implying resistance to the osmotic effect.
- Oregano honey inhibits the activity of urease secreted by H. pylori, rendering it vulnerable to the acidic pH of the stomach.
- Future perspectives include the chemical identification of the substances responsible for the antibacterial effect of oregano honey as well as conducting clinical trials for volunteers.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Israili, Z.H. Antimicrobial properties of honey. Am. J. Ther. 2014, 21, 304–323. [Google Scholar] [CrossRef] [PubMed]
- Skiadas, P.K.; Lascaratos, J.G. Dietetics in ancient Greek philosophy: Plato’s concepts of healthy diet. Eur. J. Clin. Nutr. 2001, 55, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.D.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sell, S.A.; Wolfe, P.S.; Spence, A.J.; Rodriguez, I.A.; McCool, J.M.; Petrella, R.L.; Garg, K.; Ericksen, J.J.; Bowlin, G.L. A preliminary study on the potential of manuka honey and platelet-rich plasma in wound healing. Int. J. Biomater. 2012. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.; Cooper, R. Improving antibiotic activity against wound pathogens with manuka honey in vitro. PLoS ONE 2012, 7, e45600. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Visavadia, B.G.; Honeysett, J.; Danford, M.H. Manuka honey dressing: An effective treatment for chronic wound infections. Br. J. Oral. Maxillofac. Surg. 2008, 46, 55–56. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.V.; Krishnan, K.T.; Salleh, N.; Gan, S.H. Biological and therapeutic effects of honey produced by honey bees and stingless bees: A comparative review. Rev. Bras. Farmacogn. 2016, 26, 657–664. [Google Scholar] [CrossRef]
- Tosi, E.A.; Ré, E.; Lucero, H.; Bulacio, L. Effect of honey high-temperature short-time heating on parameters related to quality, crystallization phenomena and fungal inhibition. Food Sci. Technol. 2004, 37, 669–678. [Google Scholar] [CrossRef]
- Hasan, S.H. Effect of Storage and Processing Temperatures on Honey Quality. J. Babylon Univ. Pure Appl. Sci. 2013, 6, 2244–2253. Available online: https://www.iasj.net/iasj/download/b52db327044ada1d (accessed on 28 June 2021).
- Snowdon, J.A.; Cliver, D.O. Microorganisms in honey. Int. J. Food Microbiol. 1996, 31, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Tsekoura, F.; Alexopoulos, A.; Stefanis, C.; Papadopoulos, I.; Voidarou, C.; Bolkas, S.; Zoulis, N.; Giannakourou, M.; Gergopoulou, A.; Bezirtzoglou, E. Aerobic and anaerobic bacteriology of Greek honeys. In Proceedings of the International Congress of Bioprocessing in Food Industry (ICBF), Patra, Greece, 18–21 June 2006. [Google Scholar]
- Coccimiglio, J.; Alipour, M.; Jiang, Z.-H.; Gottardo, C.; Suntres, Z. Antioxidant, Antibacterial, and Cytotoxic Activities of the Ethanolic Origanum vulgare Extract and Its Major Constituents. Oxidative Med. Cell. Longev. 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; Jamal, A. Origanum compactum Benth: A Review on Phytochemistry and Pharmacological Properties. Med. Aromat. Plants 2016, 5. [Google Scholar] [CrossRef]
- Mcnulty, C.; Owen, R.; Tompkins, D.; Hawtin, P.; Mccoll, K.; Price, A.; Smith, G.; Teare, L. Helicobacter pylori susceptibility testing by disc diffusion. J. Antimicrob. Chemother. 2002, 49, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, P.; Axon, A.T.R. Is there a rationale for eradication of Helicobacter pylori? Cost-benefit: The case for. Br. Med. Bull. 1998, 54, 243–250. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ebrahimpour, S.; Esmaeili, H.; Ghadimi, R. Food bioactive compounds, a possible adjuvant for H.pylori eradication. Letter to Editor. Caspian J. Intern Med. 2017, 8, 131–132. [Google Scholar] [CrossRef]
- Eusebi, L.H.; Zagari, R.M.; Bazzoli, F. Epidemiology of Helicobacter pylori infection. Helicobacter 2014, 19 (Suppl. 1), 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sherif, M.; Mohran, Z.; Fathy, H.; Rockabrand, D.M.; Rozmajzl, P.J.; Frenck, R.W. Universal high-level primary metronidazole resistance in Helicobacter pylori isolated from children in Egypt. J. Clin. Microbiol. 2004, 42, 4832–4834. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Asrat, D.; Nilsson, I.; Mengistu, Y.; Ashenafi, S.; Ayenew, K.; Al-Soud, W.A.; Wadström, T.; Kassa, E. Prevalence of Helicobacter pylori infection among adult dyspeptic patients in Ethiopia. Ann. Trop. Med. Parasitol. 2004, 98, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Mégraud, F.; O’Morain, C.; Hungin, A.P.S.; Jones, R.; Axon, A.; Graham, D.Y.; Tytgat, G. Current concepts in the management of Helicobacter pylori infection-The Maastricht 2-2000 Consensus Report. Aliment. Pharmacol. Ther. 2002, 16, 167–180. [Google Scholar] [CrossRef]
- Hiyama, T.; Tanaka, S.; Masuda, H.; Shima, H.; Kose, K.; Tuncel, H.; Ito, M.; Kitadai, Y.; Sumii, M.; Uemura, N.; et al. Prevalence of Helicobacter pylori resistance to clarithromycin and metronidazole determined by23S ribosomal RNAandrdxAgene analyses in Hiroshima, Japan. J. Gastroenterol. Hepatol. 2003, 18, 1202–1207. [Google Scholar] [CrossRef]
- Crone, J.; Granditsch, G.; Huber, W.D.; Binder, C.; Innerhofer, A.; Amann, G.; Hirschl, A.M. Helicobacter pylori in children and adolescents: Increase of primary clarithromycin resistance, 1997–2000. J. Pediatr. Gastroenterol. Nutr. 2003, 36, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.T.; Chowdhury, M.N.; al Humayyd, M.S. Inhibitory effect of natural honey on Helicobacter pylori. Trop. Gastroenterol. 1991, 12, 139–143. [Google Scholar] [PubMed]
- Al Somal, N.; Coley, K.E.; Molan, P.C.; Hancock, B.M. Susceptibility of Helicobacter pylori to the antibacterial activity of manuka honey. J. R. Soc. Med. 1994, 87, 9–12. [Google Scholar] [PubMed] [PubMed Central]
- Mc-Govern, P.B.; Abbas, S.Z.; Vivian, G.; Dalton, H.R. Manuka honey against Helicobacter pylori. J. R. Soc. Med. 1999, 92, 439. [Google Scholar] [CrossRef] [PubMed]
- Mobley, H.L.T.; Mendz, G.L.; Hazell, S.L. (Eds.) Helicobacter pylori: Physiology and Genetics; ASM Press: Washington, DC, USA, 2001. [Google Scholar] [PubMed]
- Andersen, L.P.; Wadström, T. Basic Bacteriology and Culture. In Helicobacter pylori: Physiology and Genetics; Mobley, H.L.T., Mendz, G.L., Hazell, S.L., Eds.; ASM Press: Washington, DC, USA, 2001; Chapter 4. [Google Scholar] [PubMed]
- Rojas, M.A.B. Microbiological conditions for culturing Helicobacter pylori. Rev. Col. Gastroenterol. 2013, 28, 94–99. Available online: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0120-99572013000200002&lng=en&nrm=iso (accessed on 3 June 2021).
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters. 2013 Version 3.1. Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Consultation/EUCAST_clinical_breakpoints_for_Helicobacter_pylori.pdf (accessed on 3 June 2021).
- Smith, S.M. Antimicrobial susceptibility testing for Helicobacter pylori in times of increasing antibiotic resistance. World J. Gastroenterol. 2014, 20, 9912. [Google Scholar] [CrossRef]
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals: Approved Standards. Document M31-A. 2008. Available online: https://www.dbt.univr.it/documenti/OccorrenzaIns/matdid/matdid485539.pdf (accessed on 3 June 2021).
- Konturek, J.W. Discovery by Jaworski of Helicobacter pylori and its pathogenetic role in peptic ulcer, gastritis and gastric cancer. J. Physiol. Pharmacol. 2003, 54 (Suppl. 3), 23–41. [Google Scholar] [PubMed]
- Zaghloul, A.A.; el-Shattawy, H.H.; Kassem, A.A.; Ibrahim, E.A.; Reddy, I.K.; Khan, M.A. Honey, a prospective antibiotic: Extraction, formulation, and stability. Pharmazie 2001, 56, 643–647. [Google Scholar] [PubMed]
- Manyi-Loh, C.E.; Clarke, A.M.; Munzhelele, T.; Green, E.; Mkwetshana, N.F.; Ndip, R.N. Selected South African Honeys and Their Extracts Possess In Vitro Anti-Helicobacter pylori Activity. Arch. Med Res. 2010, 41, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Hamilton-Miller, J.M.; Gargan, R.A. Rapid screening for urease inhibitors. Investig. Urol. 1979, 16, 327–328. [Google Scholar] [PubMed]
- Mobley, H.L.; Cortesia, M.J.; Rosenthal, L.E.; Jones, B.D. Characterization of urease from Campylobacter pylori. J. Clin. Microbiol. 1988, 26, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Korona-Glowniak, I.; Glowniak-Lipa, A.; Ludwiczuk, A.; Baj, T.; Malm, A. The In Vitro Activity of Essential Oils against Helicobacter Pylori Growth and Urease Activity. Molecules 2020, 25, 586. [Google Scholar] [CrossRef] [PubMed]
- Oshowo, A.; Gillam, D.; Botha, A.; Tunio, M.; Holton, J.; Boulos, P.; Hobsley, M. Helicobacter pylori: The mouth, stomach, and gut axis. Ann. Periodontol. 1998, 3, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Akamatsu, T.; Sugiyama, A.; Ota, H.; Katsuyama, T. Helicobacter pylori and the Surface Mucous Gel Layer of the Human Stomach. Helicobacter 1996, 1, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Dunn, B.E.; Cohen, H.; Blaser, M.J. Helicobacter pylori. Clin. Microbiol. Rev. 1997, 10, 720–741. [Google Scholar] [CrossRef]
- De Reuse, H.; Vinella, D.; Cavazza, C. Common themes and unique proteins for the uptake and trafficking of nickel, a metal essential for the virulence of Helicobacter pylori. Front. Cell. Infect. Microbiol. 2013, 3. [Google Scholar] [CrossRef]
- Wroblewski, L.E.; Peek, R.M., Jr.; Wilson, K.T. Helicobacter pylori and gastric cancer: Factors that modulate disease risk. Clin. Microbiol. Rev. 2010, 23, 713–739. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, J. Caffeic acid derivatives in dried Lamiaceae and Echinacea purpurea products. J. Funct. Foods 2010, 2, 158–162. [Google Scholar] [CrossRef]
- Goodwin, C.S.; Armstrong, J.A. Microbiological aspects of Helicobacter pylori (Campylobacter pylori). Eur. J. Clin. Microbiol. Infect. Dis. 1990, 9, 1–13. [Google Scholar] [CrossRef]
- Georgopoulos, S.D.; Papastergiou, V.; Karatapanis, S. Helicobacter pylori Eradication Therapies in the Era of Increasing Antibiotic Resistance: A Paradigm Shift to Improved Efficacy. Gastroenterol. Res. Pract. 2012, 757926. [Google Scholar] [CrossRef]
- Graham, D.Y.; Fischbach, L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 2010, 59, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Megraud, F.; Coenen, S.; Versporten, A.; Kist, M.; Lopez-Brea, M.; Hirschl, A.M.; Andersen, L.P.; Goossens, H.; Glupczynski, Y. Study Group participants. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut 2013, 62, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Vilegas, W.; Sanommiya, M.; Rastrelli, L.; Pizza, C. Isolation and structure elucidation of two new flavonoid glycosides from the infusion of maytenus aquifolium leaves. Evaluation of the antiulcer activity of the infusion. J. Agric. Food Chem. 1999, 47, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Kanaze, F.I.; Gabrieli, C.; Kokkalou, E.; Georgarakis, M.; Niopas, I. Simultaneous reversed-phase high-performance liquid chromatographic method for the determination of diosmin, hesperidin and naringin in different citrus fruit juices and pharmaceutical formulations. J. Pharm. Biomed. Anal. 2003, 33, 243–249. [Google Scholar] [CrossRef]
- Borrelli, F.; Izzo, A.A. The plant kingdom as a source of anti-ulcer remedies. Phytother. Res. 2000, 14, 581–591. [Google Scholar] [CrossRef]
- Zhu, M.; Lew, K.T.; Leung, P.-L. Protective effect of a plant formula on ethanol-induced gastric lesions in rats. Phytother. Res. 2002, 16, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Al-Howiriny, T.; Al-Sohaibani, M.; El Tahir, K.; Syed, R. Prevention of experimentally-induced gastric ulcers in rats by an ethanolic extract of parsley Petroselinum crispum. Am. J. Chin. Med. 2003, 31, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Osadebe, P.O.; Okoye, F.B.C. Anti-inflammatory effects of crude methanolic extract and fractions of Alchornea cordifolia leaves. J. Ethnopharmacol. 2003, 89, 19–24. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J.A. Functional Properties of Honey, Propolis, and Royal Jelly. J. Food Sci. 2008, 73, R117–R124. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, M.C.; Ferreres, F.; GarcíA-Viguera, C.; Bankova, V.S.; De Castro, S.L.; Dantas, A.P.; Valente, P.H.M.; Paulino, N. Phenolic compounds from Brazilian propolis with pharmacological activities. J. Ethnopharmacol. 2001, 74, 105–112. [Google Scholar] [CrossRef]
- Takaisi, N.B.; Scjoncjer, H. Electron microscopy and microcalorimetric investigations of the possible mechanism of the antibacterial action of a defined propole provenance. Planta Med. 1994, 60, 222–227. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Detection of galangin-induced cytoplasmic membrane damage in Staphylococcus aureus by measuring potassium loss. J. Ethnopharmacol. 2005, 101, 243–248. [Google Scholar] [CrossRef]
- Mirzoeva, O.K.; Grishanin, R.N.; Calder, P.C. Antimicrobial action of propolis and some of its components: The effects on growth, membrane potential and motility of bacteria. Microbiol. Res. 1997, 152, 239–246. [Google Scholar] [CrossRef]
- Manyi-Loh, C.E.; Clarke, A.M.; Ndip, R.N. An overview of honey: Therapeutic properties and contribution in nutrition and human health. Afr. J. Microbiol. Res. 2011, 5, 844–852. [Google Scholar] [CrossRef]
- Tan, H.T.; Rahman, R.A.; Gan, S.H.; Halim, A.S.; Hassan, S.A.; Sulaiman, S.A.; Bs, K.-K. The antibacterial properties of Malaysian tualang honey against wound and enteric microorganisms in comparison to manuka honey. BMC Complementary Altern. Med. 2009, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-L.; Guo, Y.-S.; Wang, C.-H.; Li, G.-Q.; Xu, J.-J.; Chung, H.Y.; Ye, W.-C.; Li, Y.-L.; Wang, G.-C. Phenolic compounds from Origanum vulgare and their antioxidant and antiviral activities. Food Chem. 2014, 152, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K. Honey as an Ecological Reservoir of Antibacterial Compounds Produced by Antagonistic Microbial Interactions in Plant Nectars, Honey and Honey Bee. Antibiotics 2021, 10, 551. [Google Scholar] [CrossRef] [PubMed]
- Singletary, K. Oregano: Overview of the literature on health benefits. Nutr. Today 2010, 45, 129–138. [Google Scholar] [CrossRef]
- Brdjanin, S.; Bogdanovic, N.; Kolundzic, M.; Milenkovic, M.; Golic, N.; Kojic, M.; Kundakovic, T. Antimicrobial activity of oregano (Origanum vulgare L.): And basil (Ocimum basilicum L.): Extracts. Adv. Technol. 2015, 4, 5–10. [Google Scholar] [CrossRef]
- Almasaudi, S. The antibacterial activities of honey. Saudi J. Biol. Sci. 2021, 28, 2188–2196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Venegas, A.; Hantar Touma, J.; Bravo, J.; Perez-Perez, G. Progress in Use of Natural Products and Their Active Components against Helicobacter pylori. Adv. Microb. 2016, 6, 1091–1129. [Google Scholar] [CrossRef][Green Version]
- Ball, D.W. The Chemical Composition of Honey. J. Chem. Educ. 2007, 84, 1643–1646. [Google Scholar] [CrossRef]
- Nolan, V.C.; Harrison, J.; Cox, J.A.G. Dissecting the Antimicrobial Composition of Honey. Antibiotics 2019, 5, 251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, Y.T.; Kwon, Y.I.; Labbe, R.G.; Shetty, K. Inhibition of Helicobacter pylori and Associated Urease by Oregano and Cranberry Phytochemical Synergies. Appl. Environ. Microbiol. 2005, 71, 8558–8564. [Google Scholar] [CrossRef] [PubMed]
- Ndip, R.N.; Takang, A.E.; Echakachi, C.M.; Malongue, A.; Akoachere, J.F.; Ndip, L.M.; Luma, H.N. In-vitro antimicrobial activity of selected honeys on clinical isolates of Helicobacter pylori. Afr. Health Sci. 2007, 7, 228–2321. [Google Scholar] [PubMed] [PubMed Central]
- Nzeako, B.C.; Al-Namaani, F. The antibacterial activity of honey on helicobacter pylori. Sultan Qaboos Univ. Med. J. 2006, 6, 71–76. [Google Scholar] [PubMed] [PubMed Central]
- Goodwin, C.S.; Worsley, B.W. Helicobacter pylori Biology and Clinical Practice. N. Engl. J. Med. 1994, 330, 1625–1626. [Google Scholar] [CrossRef]
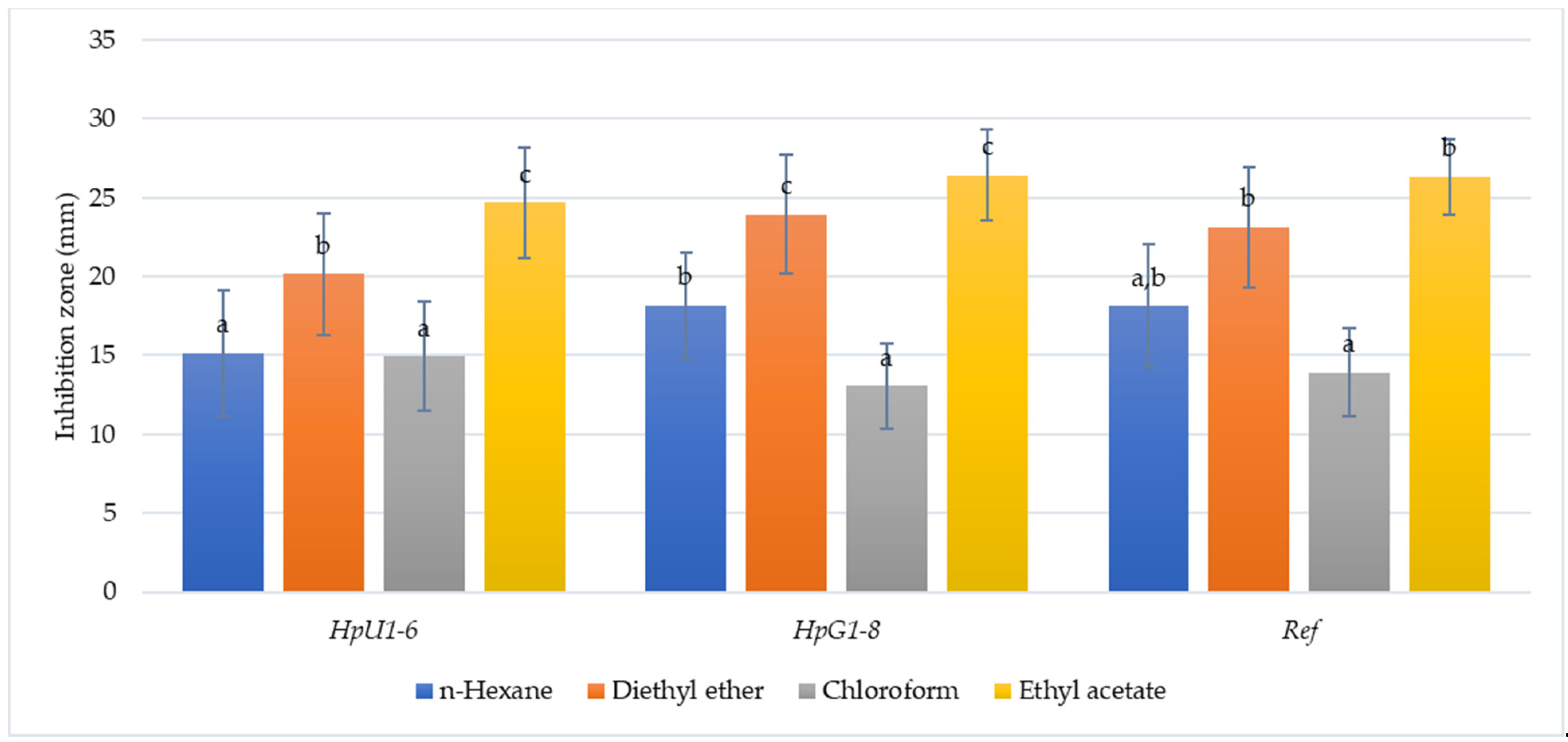
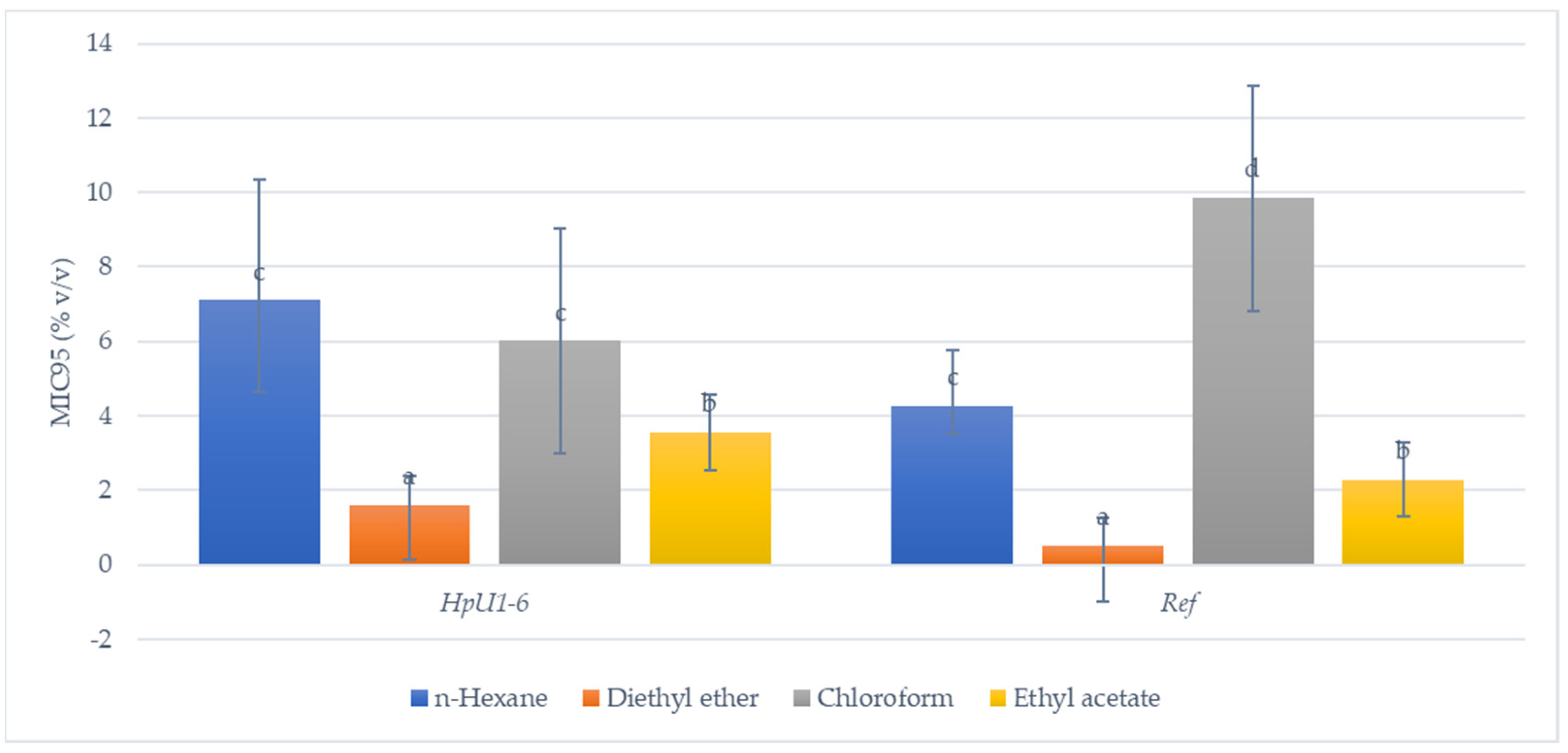
| Strain | Concentration of Honey (% v/v) | ||||
|---|---|---|---|---|---|
| 75 | 50 | 25 | 12.5 | 6 | |
| HpU1 | 27.26 ± 6.4 ab | 22.28 ± 6.3 abc | 15.2 ± 6.9 ab | 6.56 ± 3.4 a | 3.16 ± 1.7 a |
| HpU2 | 21.45 ± 5.1 ab | 18.63 ± 4.14 ab | 13.8 ± 3.9 a | 7.7 ± 3.1 a | 2.41 ± 1.28 a |
| HpU3 | 15.38 ± 3.3 a | 14.36 ± 3.9 a | 12.18 ± 2.9 a | 9.18 ± 2.2 ab | 5.14 ± 2.1 ab |
| HpU4 | 22.48 ± 4.81 ab | 18.16 ± 3.56 ab | 13.3 ± 3.45 a | 9.19 ± 3.39 ab | 3.18 ± 1.2 a |
| HpU5 | 29.1 ± 5.1 ab | 21.45 ± 3.1 abc | 18.13 ± 4.3 abc | 5.48 ± 4.1 a | 3.5 ± 1.8 a |
| HpU6 | 28.78 ± 4.18 ab | 27.15 ± 4.0 bcd | 21.45 ± 3.6 abc | 18.13 ± 1.3 cd | 12.14 ± 2.4 bc |
| HpG1 | 47.7 ± 4.5 cd | 43.5 ± 4.7 h | 36.26 ± 3.7 d | 30.44 ± 1.6 e | 20.1 ± 2.9 d |
| HpG2 | 50.28 ± 4.2 d | 45.88 ± 4.0 h | 26.66 ± 4.6 bcd | 15.7 ± 3.9 abc | 5.48 ± 5.4 ab |
| HpG3 | 53.44 ± 3.8 d | 32.13 ± 3.9 cdeg | 14.44 ± 2.14 a | 9.90 ± 1.6 ab | 3.78 ± 2 a |
| HpG4 | 35.16 ± 4.13 abc | 28.76 ± 3.28 bcde | 18.90 ± 4.2 ac | 13.12 ± 2.45 abc | 4.13 ± 1.1 a |
| HpG5 | 44.6 ± 5.51 cd | 42.42 ± 4.5 egh | 34.66 ± 3.9 bd | 27.24 ± 4.4 de | 18.00 ± 4.0 acd |
| HpG6 | 48.84 ± 5.3 cd | 33.18 ± 3.64 cdeg | 29.88 ± 4.18 cd | 22.14 ± 4.4 ce | 15.70 ± 3.15 cd |
| HpG7 | 51.51 ± 5.8 cd | 40.28 ± 4.6 egh | 27.80 ± 4.3 cd | 12.90 ± 3.45 abcd | 4.15 ± 2.1 a |
| HpG8 | 47.62 ± 3.9 ad | 35.66 ± 4.9 degh | 29.22 ± 4.3 cd | 15.78 ± 3.5 abc | 4.6 ± 1.8 ab |
| Ref | 51.24 ± 2.4 d | 30.24 ± 1.5 bcdeg | 15.4 ± 1.1 ab | 10.8 ± 1.3 abc | 1.34 ± 1.1 a |
| Antibiotic | HpU1 | HpU2 | HpU3 | HpU4 | HpU5 | HpU6 | HpG1 | HpG2 | HpG3 | HpG4 | HpG5 | HpG6 | HpG7 | HpG8 | HpRef | Clinical Breakpoints (mg/L) * | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Susceptible | Resistant | ||||||||||||||||
| Amoxicillin | R | R | S | R | R | R | S | S | S | S | R | S | R | S | S | ≤0.12 | >0.12 |
| Clarithromycin | R | S | R | S | R | R | R | S | S | S | S | S | S | S | S | ≤0.25 | >0.5 |
| Metronidazole | S | R | R | S | S | R | S | S | R | R | S | S | R | R | S | ≤8 | >8 |
| Tetracycline | R | S | S | R | R | S | S | S | S | R | R | R | R | S | S | ≤1 | >1 |
| Levofloxacin | R | R | R | S | R | S | S | R | R | S | R | S | S | S | S | ≤1 | >1 |
| Rifampicin ** | R | S | R | R | S | R | S | S | R | S | R | S | S | S | S | ≤1 | >1 |
| Honey Extract (75% v/v) | HpU1 * | HpU2 | HpU3 | HpU4 | HpU5 | HpU6 | HpG1 | HpG2 | HpG3 | HpG4 | HpG5 | HpG6 | HpG7 | HpG8 | H. pylori DSM21031 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n-Hexane | 14.35 ± 3.5 a | 12.85 ± 6.4 a | 14.89 ± 5.3 a | 16.41 ± 3.8 a | 15.74 ± 3.8 a | 16.81 ± 3.25 a | 17.38 ± 3.8 ab | 18.71 ± 4.5 ab | 17.99 ± 3.7 ab | 16.9 ± 3.5 ab | 18.45 ± 4.3 ab | 17.85 ± 3.6.b | 18.4 ± 3.8 ab | 19.7 ± 4.2 ab | 18.13 ± 3.9 ab |
| Diethyl ether | 22.1 ± 5.7 a | 19.5 ± 3.7 a | 19.8 ± 4.7 a | 19.88 ± 5.3 a | 19.8 ± 2.9 a | 19.87 ± 3.7 ab | 23.7 ± 5.4 ab | 25.1 ± 4.7 b | 22.35 ± 4.7 ab | 25.8 ± 4.7 bc | 26.4 ± 3.1 b | 22.7 ± 2.8 ab | 22.78 ± 3.8 b | 22.74 ± 2.7 b | 23.12 ± 3.8 b |
| Chloroform | 14.7 ± 5.7 a | 15.4 ± 2.8 a | 14.18 ± 2.9 a | 15.1 ± 3.9 a | 15.78 ± 5.2 a | 14.5 ± 2.8 a | 12.1 ± 2.1 a | 13.24 ± 3.4 a | 12.7 ± 3.8 a | 13.5 ± 3.8 a | 12.8 ± 2.8 b | 13.7 ± 3.8 a | 12.75 ± 2.8 a | 13.7 ± 2.8 a | 13.9 ± 2.8 a |
| Ethyl acetate | 25.42 ± 4.7 a | 23.78 ± 4.7 a | 24.58 ± 3.5 a | 23.85 ± 4.8 a | 25.45 ± 3.8 a | 25.05 ± 2.7 b | 24.78 ± 6.1 b | 27.35 ± 2.1 b | 25.78 ± 2.5 b | 27.8 ± 1.8 c | 25.78 ± 3.2 b | 26.58 ± 3.5 b | 26.85 ± 2.4 b | 26.65 ± 2.5 b | 26.35 ± 2.4 b |
| Solvent | N * | HpU1 | N * | HpU2 | N * | HpU3 | N * | HpU4 | N * | HpU5 | N * | HpU6 | N * | Hp DSM21031 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n-hexane | 32 | 10.23 ± 2.25 4c | 27 | 6.42 ± 4.16 3,b | 7 | 7.78 ± 3.55 3,bc | 11 | 5.97 ± 3.29 3,ab | 6 | 5.83 ± 3.41 2,3,ab | 9 | 6.38 ± 2.82 3,ab | 21 | 4.28 ± 2.45 3,a |
| Diethyl ether | 36 | 3.11 ± 2.84 1,d | 39 | 0.93 ± 0.96 1,ab | 41 | 2.13 ± 2.26 1,c | 29 | 0.96 ± 0.65 1,ab | 37 | 1.51 ± 1.58 1,bc | 46 | 1.02 ± 0.64 1,ab | 48 | 0.52 ± 0.76 1,a |
| Chloroform | 25 | 7.3 ± 2.78 3,b | 22 | 4.26 ± 2.36 2,a | 5 | 5.0 ± 1.76 2,3,ab | 12 | 6.83 ± 3.48 3,b | 13 | 6.21 ± 3.68 3,ab | 17 | 6.45 ± 4.07 3,b | 20 | 9.85 ± 2.27 4,c |
| Ethyl acetate | 28 | 4.82 ± 2.03 2,ab | 38 | 2.96 ± 2.51 2 | 34 | 3.46 ± 3.44 2,abc | 33 | 3.35 ± 3.27 2,ab | 32 | 3.99 ± 3.24 2,bc | 50 | 2.70 ± 2.80 2,a | 36 | 2.29 ± 1.25 2,a |
| H. pylori Strain | Oregano Honey | N * | Effective Concentration (mg/mL) | ||
|---|---|---|---|---|---|
| Mean ± SD | Range | Median | |||
| HpU1 | Pure | 43 | 91.3 ± 55.8 ** | 12.5–200.0 | 100.0 |
| Diethyl ether extract | 50 | 22.0 ± 18.8 | 3.0–100.0 | 12.5 | |
| HpU2 | Pure | 49 | 57.5 ± 40.5 ** | 6.25–200.0 | 50.0 |
| Diethyl ether extract | 50 | 5.93 ± 3.32 | 1.56–12.5 | 6.25 | |
| DSM21031 | Pure | 49 | 28.3 ± 18.3 ** | 12.5–100.0 | 25.0 |
| Diethyl ether extract | 50 | 4.43 ± 2.33 | 1.56–12.5 | 3.125 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voidarou, C.; Rozos, G.; Alexopoulos, A.; Plessas, S.; Mantzourani, I.; Stavropoulou, E.; Tzora, A.; Bezirtzoglou, E. In Vitro Screening Potential Antibacterial Properties of the Greek Oregano Honey against Clinical Isolates of Helicobacter pylori. Foods 2021, 10, 1568. https://doi.org/10.3390/foods10071568
Voidarou C, Rozos G, Alexopoulos A, Plessas S, Mantzourani I, Stavropoulou E, Tzora A, Bezirtzoglou E. In Vitro Screening Potential Antibacterial Properties of the Greek Oregano Honey against Clinical Isolates of Helicobacter pylori. Foods. 2021; 10(7):1568. https://doi.org/10.3390/foods10071568
Chicago/Turabian StyleVoidarou, Chrysoula (Chrysa), Georgios Rozos, Athanasios Alexopoulos, Stavros Plessas, Ioanna Mantzourani, Elisavet Stavropoulou, Athina Tzora, and Eugenia Bezirtzoglou. 2021. "In Vitro Screening Potential Antibacterial Properties of the Greek Oregano Honey against Clinical Isolates of Helicobacter pylori" Foods 10, no. 7: 1568. https://doi.org/10.3390/foods10071568
APA StyleVoidarou, C., Rozos, G., Alexopoulos, A., Plessas, S., Mantzourani, I., Stavropoulou, E., Tzora, A., & Bezirtzoglou, E. (2021). In Vitro Screening Potential Antibacterial Properties of the Greek Oregano Honey against Clinical Isolates of Helicobacter pylori. Foods, 10(7), 1568. https://doi.org/10.3390/foods10071568










