Genotype and Environment Affect the Grain Quality and Yield of Winter Oats (Avena sativa L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Field Sites and Plot Management
2.2. Climatic Conditions at Each Site
2.3. Yield and Grain Size, Shape and Quality
2.4. Grain Composition
2.5. Statistical Analysis
3. Results
3.1. Weather Conditions and Grain Yield
3.2. Grain Physical Quality Characters
3.3. Grain Composition
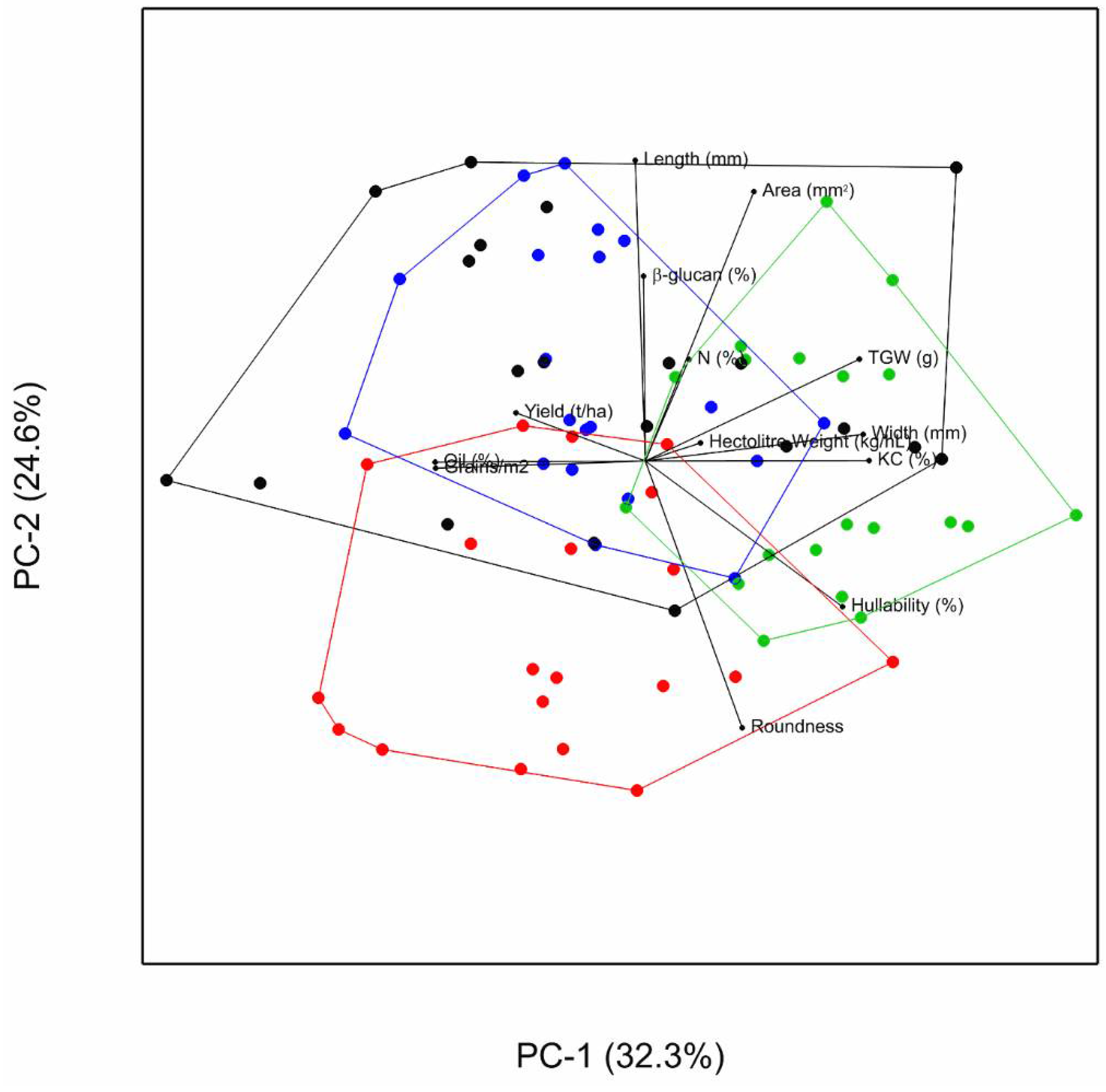
3.4. Association between Traits
3.5. Association between Traits and Environmental Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Marshall, A.H.; Cowan, S.; Edwards, S.; Griffiths, I.; Howarth, C.J.; Langdon, T.; White, E. Crops that feed the world 9. Oats- a cereal crop for human and livestock feed with industrial applications. Food Secur. 2013, 5, 13–33. [Google Scholar] [CrossRef]
- Andersson, A.A.A.; Börjesdottir, D. Effect of environment and variety on content and molecular weight of β-glucan in oats. J. Cereal Sci. 2011, 54, 122–128. [Google Scholar] [CrossRef]
- Stewart, D.; McDougall, G. Oat agriculture, cultivation and breeding targets: Implications for human nutrition and health. Brit. J. Nutr. 2014, 112, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Villaluenga, C.; Peñas, E. Health benefits of oat: Current evidence and molecular mechanisms. Curr. Opin. Food Sci. 2017, 14, 26–31. [Google Scholar] [CrossRef] [Green Version]
- Redaelli, R.; Del Frate, V.; Bellato, S.; Terracciano, G.; Ciccoritti, R.; Germeier, C.U.; De Stefanis, E.; Sgrulletta, D. Genetic and environmental variability in total and soluble β-glucan in European oat genotypes. J. Cereal Sci. 2013, 57, 193–199. [Google Scholar] [CrossRef]
- Doehlert, D.C.; McMullen, M.S.; Hammond, J.J. Genotype and environmental effects on grain yield and quality of oat grain in North Dakota. Crop Sci. 2001, 41, 1066–1072. [Google Scholar] [CrossRef]
- White, E.; Watson, S. An investigation of the relationship between hullability and morphological features in grains of four oat varieties. Ann. Appl. Biol. 2010, 156, 281–295. [Google Scholar] [CrossRef]
- Burke, J.I.; Browne, R.A.; White, E.M. Factors affecting yield and quality of oats. Teagasc, Crop Research Centre, Oak Park Carlow. Proj. Rep. 2001, 32, 4493. [Google Scholar]
- Yan, W.; Fregeau-Reid, J.; Pageau, D.; Martin, R. Genotype by environment interaction and trait associations in two genetic populations of oat. Crop Sci. 2016, 56, 1136–1145. [Google Scholar] [CrossRef]
- Yan, W.; Fregeau-Reid, J.; Ma, B.; Pageau, D.; Vera, C. Nitrogen fertilizer complements breeding in improving yield and quality of milling oat. Crop Sci. 2017, 57, 3291–3302. [Google Scholar] [CrossRef]
- Ahmad, M.; Zaffar, G.; Razvi, S.M.; Mir, S.D.; Rather, M.A. Stability properties of certain oats (Avena sativa L.) genotypes for major grain yielding characteristics. Int. J. Plant Breed. Genet. 2013, 7, 182–187. [Google Scholar] [CrossRef] [Green Version]
- Becker, H.C.; Leon, J. Stability Analysis in Plant Breeding. Plant Breed. 1988, 101, 1–23. [Google Scholar] [CrossRef]
- Subira, J.; Álvaro, F.; Garcĺa del Moral, L.F.; Royo, C. Breeding effects on the cultivar x environment interaction of durum wheat yield. Eur. J. Agron. 2015, 68, 78–88. [Google Scholar] [CrossRef] [Green Version]
- Hongyu, K.; García-Peña, M.; AraÚjo, L.B.; Dias, C.T.S. Statistical analysis of yield trials by AMMI analysis of genotype x environment interaction. Biom. Lett. 2014, 51, 89–102. [Google Scholar] [CrossRef] [Green Version]
- Saastamoinen, M.; Plaami, S.; Kumpulainen, J. Genetic and environmental variation in β-glucan content of oats cultivated in Finland. J. Cereal Sci. 1992, 16, 279–290. [Google Scholar] [CrossRef]
- AHDB Cereal Trials Protocol. Available online: https://ahdb.org.uk/ahdb-recommended-lists-for-cereals-and-oilseeds-2016-2021 (accessed on 20 August 2021).
- Fradgley, N.S.; Creissen, H.E.; Pearce, H.; Howlett, S.A.; Pearce, B.D.; Döring, T.F.; Girling, R.D. Weed Suppression and Tolerance in Winter Oats. Weed Technol. 2017, 31, 740–751. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.metoffice.gov.uk/datapoint/product/historical-station-obs (accessed on 20 August 2021).
- Available online: https://power.larc.nasa.gov/data-access-viewer/ (accessed on 20 August 2021).
- Mosse, J. Nitrogen-to-protein conversion factor for ten cereals and six legumes or oilseeds. A reappraisal of its definition and determination. Variation according to species and to seed protein content. J. Agric. Food Chem. 1990, 38, 18–24. [Google Scholar] [CrossRef]
- Shenk, J.S.; Westerhaus, M.O. Population definition, sample selection, and calibration procedures for near infrared spectroscopy. Crop Sci. 1991, 31, 469–474. [Google Scholar] [CrossRef]
- Sanderson, R.; Lister, S.J.; Dhanoa, M.S.; Barnes, R.J.; Thomas, C. Use of near infrared reflectance spectroscopy to predict and compare the composition of carcass samples from young steers. Anim. Sci. 1997, 65, 45–54. [Google Scholar] [CrossRef]
- Barnes, R.J.; Dhanoa, M.S.; Lister, S.J. Standard normal variate transformation and de-trending of near-infrared diffuse reflectance spectra. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- McCleary, B.V.; Mugford, D.C. Determination of β-Glucan in Barley and Oats by Streamlined Enzymic Method: Summary of Collaborative Study. J. AOAC Int. 1997, 80, 580–583. [Google Scholar] [CrossRef] [Green Version]
- Baird, D.; Murray, D.; Payne, R.; Soutar, D. Introduction to Genstat® for WindowsTM, 19th ed.; VSN International: Hemel Hempstead, UK, 2017. [Google Scholar]
- Digby, P.G.N. Modified joint regression analysis for incomplete variety × environment data. J. Agric. Sci. 1979, 93, 81–86. [Google Scholar] [CrossRef]
- Gabriel, K.R. The biplot graphic display of matrices with application to principal component analysis. Biometrika 1971, 58, 453–467. [Google Scholar] [CrossRef]
- Yan, W.; Tinker, N. An integrated biplot analysis system for displaying, interpreting and exploring genotype x environment interaction. Crop Sci. 2005, 45, 1004–1016. [Google Scholar] [CrossRef]
- Van Ittersum, M.K.; Cassman, K.G.; Grassini, P.; Wolf, J.; Tittonell, P.; Hochman, Z. Yield gap analysis with local to global relevance-a review. Field Crop. Res. 2013, 143, 4–17. [Google Scholar] [CrossRef] [Green Version]
- Finlay, K.W.; Wilkinson, G.N. The analysis of adaptation in a plant breeding programme. Aust. J. Agric. Res. 1963, 14, 742–754. [Google Scholar] [CrossRef] [Green Version]
- Hinton-Jones, M.; Marshall, A.H.; Thomas, I.D.; Humphreys, M.O.; Marum, P.; Ševčíkováv, M.; Šrámek, P.; De Sousa, M.M.T.; Nielson, N.C.; Dhanoa, M.S. Environmental effects on seed yield and costs of temperate forages during regeneration. Eur. J. Agron. 2007, 26, 235–248. [Google Scholar] [CrossRef]
- Rasheed, A.; Xia, X.; Ogbonnaya, F.; Mahmood, T.; Zhang, Z.; Mujeeb-Kazi, A.; He, Z. Genome-wide association for grain morphology in synthetic hexaploid wheats using digital imaging analysis. BMC Plant Biol. 2014, 14, 128. [Google Scholar] [CrossRef] [Green Version]
- Hughes, N.; Oliveira, H.; Fradgley, N.; Corke, F.; Cockram, J.; Doonan, J.; Nobre Nibau, C. CT trait analysis reveals morphometric differences between domesticated temperate small grain cereals and their wild relatives. Plant J. 2019, 99, 98–111. [Google Scholar] [CrossRef] [Green Version]
- Acreche, M.; Slafer, G. Grain weight response to increases in number of grains in wheat in a Mediterranean area. Field Crop. Res. 2006, 98, 52–59. [Google Scholar] [CrossRef]
- Doehlert, D.C.; McMullen, M.S.; Jannick, J.L.; Panigrahi, S.; Gu, H.; Riveland, N.R. Evaluation of oat kernel size uniformity. Crop Sci. 2004, 44, 1178–1186. [Google Scholar] [CrossRef] [Green Version]
- Browne, R.; White, E.; Burke, J. Responses of developmental yield formation processes in oats to variety, nitrogen, seed rate and plant growth regulator and their relationship to quality. J. Agric. Sci. 2006, 144, 533–545. [Google Scholar] [CrossRef]
- Gegas, V.C.; Nazari, A.; Griffiths, S.; Simmonds, J.; Fish, L.; Orford, S.; Sayers, L.; Doonan, J.H.; Snape, J.W. Genetic framework for grain size and shape variation in wheat. Plant Cell 2010, 22, 1046–1056. [Google Scholar] [CrossRef] [Green Version]
- Xie, Q.; Mayes, S.; Sparkes, D.L. Carpel size, grain filling, and morphology determine individual grain weight in wheat. J. Exp. Bot. 2015, 66, 6715–6730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderini, D.F.; Abeledo, L.G.; Savin, R.; Slafer, G.A. Final grain weight in wheat as affected by short periods of high temperature during pre-and post-anthesis under field conditions. Aust. J. Plant Physiol. 1999, 26, 453–458. [Google Scholar]
- Tiwari, U.; Cummins, E. Simulation of the factors affecting β-glucan levels during the cultivation of oats. J. Cereal Sci. 2009, 50, 175–183. [Google Scholar] [CrossRef]
- Peterson, D.M.; Wesenberg, D.M.; Burrup, D.E. β-Glucan Content and Its Relationship to Agronomic Characteristics in Elite Oat Germplasm. Crop Sci. 1995, 35, 965–970. [Google Scholar] [CrossRef]
- Chappell, A.; Scott, K.P.; Griffiths, I.A.; Cowan, A.A.; Hawes, C.; Wishart, J.; Martin, P. The agronomic performance and nutritional content of oat and barley varieties grown in a northern maritime environment depends on variety and growing conditions. J. Cereal Sci. 2017, 74, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Gales, K. Yield variation of wheat and barley in Britain in relation to crop growth and soil conditions- a review. J. Sci. Food Agric. 1983, 34, 1085–1104. [Google Scholar] [CrossRef]
- Finnan, J.; Spink, J. Identification of yield limiting phenological phases of oats to improve crop performance. J. Agric. Sci. 2017, 155, 1–17. [Google Scholar] [CrossRef]
- Allwood, J.W.; Martinez-Martin, P.; Xu, Y.; Cowan, A.; Pont, S.; Griffiths, I.; Sungurtas, J.; Clarke, S.; Goodacre, R.; Marshall, A.; et al. Assessing the impact of nitrogen supplementation in oats across multiple growth locations and years with targeted phenotyping and high-resolution metabolite profiling approaches. Food Chem. 2021, 355, 129585. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, J.L.; Wright-Morton, L.; Hall, B. Vulnerability of grain crops and croplands in the Midwest to climatic variability and adaptation strategies. Clim. Chang. 2018, 146, 263–275. [Google Scholar] [CrossRef] [Green Version]
- Boyer, J.S. Plant productivity and environment. Science 1982, 218, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.A. Number of kernels in wheat crops and the influence of solar radiation and temperature. J. Agric. Sci. 1985, 105, 447–461. [Google Scholar] [CrossRef]
| Environment | Site | Latitude (N, °.′) | Longitude (E, °.′) | Sowing Date | Harvest Date | Rainfall Dec to April (mm) | Rainfall June + July (mm) | Mean March Temperature (°C) | Mean July Temperature (°C) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Gogerddan | 52.43 | −4.02 | 28/09/11 | 09/08/12 | 387 | 273 | 8.0 | 13.9 |
| 2 | Gogerddan | 52.43 | −4.02 | 23/10/12 | 18/08/13 | 426 | 91 | 2.9 | 16.6 |
| 3 | Gogerddan | 52.43 | −4.02 | 25/09/13 | 24/07/14 | 574 | 96 | 6.8 | 15.7 |
| 4 | Lydbury | 52.45 | −2.94 | 30/09/11 | 22/08/12 | 270 | 211 | 7.9 | 14.3 |
| 5 | Lydbury | 52.45 | −2.94 | 08/10/13 | 20/08/14 | 316 | 84 | 6.4 | 16.2 |
| 6 | Bidney | 52.20 | −2.87 | 15/10/10 | 17/08/11 | 145 | 105 | 6.1 | 14.4 |
| 7 | Rosemaund | 52.08 | −2.39 | 06/10/11 | 05/09/12 | 325 | 249 | 8.1 | 14.8 |
| 8 | Rosemaund | 51.97 | −2.62 | 06/02/13 | 03/09/13 | 56 | 2.4 | 17.9 | |
| 9 | Rosemaund | 51.98 | −2.60 | 30/09/13 | 31/07/14 | 555 | 88 | 6.9 | 16.9 |
| 10 | ADAS Rosemaund | 52.09 | −2.39 | 28/09/10 | 13/08/11 | 149 | 93 | 6.6 | 15.0 |
| 11 | ADAS Rosemaund | 52.09 | −2.39 | 27/09/11 | 06/09/12 | 325 | 249 | 8.1 | 14.8 |
| 12 | ORC Elm Farm | 52.36 | 1.35 | 19/10/10 | 03/08/11 | 131 | 126 | 6.4 | 15.3 |
| 13 | ORC Elm Farm | 52.36 | 1.35 | 12/10/11 | 22/08/12 | 275 | 168 | 8.1 | 16.0 |
| 14 | ORC Elm Farm | 52.36 | 1.35 | 16/10/12 | 24/08/13 | 292 | 35 | 2.7 | 17.6 |
| 15 | Glenrothes | 56.19 | −3.11 | 28/09/11 | 24/08/12 | 205 | 260 | 7.0 | 12.5 |
| 16 | Glenrothes | 56.19 | −3.11 | 02/10/12 | 14/08/13 | 317 | 90 | 1.4 | 15.4 |
| 17 | Glenrothes | 56.19 | −3.11 | 26/09/13 | 04/08/14 | 360 | 105 | 5.2 | 14.8 |
| 18 | Devon | 50.27 | −3.76 | 03/10/11 | 28/08/12 | 420 | 275 | 9.0 | 15.0 |
| 19 | Devon | 50.27 | −3.76 | 20/10/12 | 13/08/13 | 442 | 43 | 5.3 | 17.2 |
| 20 | Devon | 50.27 | −3.76 | 07/10/13 | 31/07/14 | 565 | 120 | 8.3 | 17.2 |
| 21 | Essex | 51.58 | 0.41 | 06/10/11 | 16/08/12 | 218 | 193 | 8.5 | 16.0 |
| 22 | Essex | 51.58 | 0.41 | 05/10/13 | 22/07/14 | 232 | 94 | 8.0 | 17.7 |
| Environment | Yield (t·ha−1) | Grain Number (1000 m−2) | Groat Content (%) | Hullability (%) | Hectoliter Weight (kg·hL−1) | TGW (g) |
|---|---|---|---|---|---|---|
| 1 | 6.03 | 16.03 | 68.65 | 90.07 | 43.14 | 37.65 |
| 2 | 8.31 | 19.90 | 73.45 | 77.46 | 50.17 | 41.89 |
| 3 | 9.31 | 24.83 | 68.88 | 83.44 | 50.26 | 37.92 |
| 4 | 8.08 | 18.20 | 75.49 | 93.60 | 46.01 | 44.96 |
| 5 | 7.92 | 16.56 | 75.48 | 91.36 | 53.66 | 48.18 |
| 6 | 10.02 | 22.06 | 75.23 | 88.32 | 55.35 | 45.27 |
| 7 | 6.77 | 16.78 | 73.42 | 98.50 | 44.47 | 40.41 |
| 8 | 4.96 | 12.38 | 75.73 | 93.03 | 50.89 | 41.40 |
| 9 | 7.18 | 17.41 | 74.13 | 86.82 | 49.24 | 41.74 |
| 10 | 10.19 | 24.16 | 74.68 | 71.34 | 52.94 | 42.00 |
| 11 | 4.84 | 10.88 | 75.88 | 99.58 | 51.12 | 44.55 |
| 12 | 7.98 | 18.97 | 73.97 | 91.34 | 59.57 | 42.55 |
| 13 | 7.26 | 17.85 | 73.55 | 93.06 | 49.65 | 40.66 |
| 14 | 9.74 | 23.24 | 72.91 | 76.37 | 51.88 | 42.04 |
| 15 | 7.61 | 17.35 | 73.08 | 95.55 | * na | 44.60 |
| 16 | 8.79 | 22.02 | 73.22 | 84.52 | 52.13 | 40.23 |
| 17 | 9.69 | 22.45 | 72.99 | 80.28 | 53.17 | 43.25 |
| 18 | 6.83 | 17.86 | 69.72 | 97.52 | * na | 38.40 |
| 19 | 10.49 | 26.62 | 72.55 | 75.76 | 51.76 | 39.66 |
| 20 | 9.94 | 22.94 | 72.73 | 83.17 | 50.99 | 43.62 |
| 21 | 8.20 | 20.62 | 74.36 | 97.23 | 42.64 | 39.47 |
| 22 | 9.43 | 26.84 | 70.56 | 83.90 | 49.14 | 35.52 |
| s.e. | 0.381 | 0.950 | 0.448 | 1.544 | 0.787 | 0.765 |
| Prob | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Variety | Yield (t ha−1) | Grain Number (1000 m−2) | Groat Content (%) | Hullability (%) | Hectoliter Weight (kg hL−1) | TGW (g) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (Vi) | bi | Mean (Vi) | bi | Mean (Vi) | bi | Mean (Vi) | bi | Mean (Vi) | bi | Mean (Vi) | bi | |
| Balado | 7.98 | 1.151 | 19.31 | 1.218 | 70.82 | 1.744 | 83.64 | 1.522 | 48.09 | 1.085 | 41.81 | 1.381 |
| Gerald | 8.24 | 0.982 | 22.16 | 1.078 | 72.81 | 1.096 | 87.75 | 1.052 | 52.16 | 0.998 | 37.44 | 0.988 |
| Mascani | 8.18 | 1.020 | 18.05 | 0.912 | 76.78 | 0.525 | 98.54 | 0.129 | 52.22 | 0.926 | 45.39 | 0.799 |
| Tardis | 8.25 | 0.844 | 19.74 | 0.785 | 72.23 | 0.590 | 81.38 | 1.290 | 49.16 | 0.990 | 41.90 | 0.808 |
| s.e. | 0.167 | 0.1046 | 0.420 | 0.1022 | 0.227 | 0.1108 | 0.761 | 0.0957 | 0.358 | 0.0907 | 0.341 | 0.1184 |
| Prob. | 0.625 | 0.270 | <0.001 | 0.028 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.691 | <0.001 | 0.005 |
| Environment | Width (mm) | Length (mm) | Roundness | Oil (%) | Protein (%) | β-Glucan (%) |
|---|---|---|---|---|---|---|
| 1 | 3.10 | 10.83 | 0.287 | 7.82 | 8.42 | 3.72 |
| 2 | 3.13 | 12.79 | 0.245 | 7.03 | 10.40 | 4.61 |
| 3 | 3.06 | 10.74 | 0.286 | 7.79 | 7.77 | 4.70 |
| 4 | 3.30 | 11.04 | 0.300 | 7.33 | 10.18 | 3.82 |
| 5 | 3.34 | 10.69 | 0.312 | 7.21 | 9.54 | 4.44 |
| 6 | 3.25 | 11.79 | 0.276 | 7.37 | 9.59 | 4.35 |
| 7 | 3.19 | 10.42 | 0.306 | 7.21 | 9.65 | 3.61 |
| 8 | 3.08 | 12.58 | 0.244 | 6.48 | 12.11 | 4.88 |
| 9 | 3.11 | 11.09 | 0.281 | 7.24 | 8.36 | 4.60 |
| 10 | 3.16 | 12.31 | 0.258 | 7.66 | 11.85 | 4.11 |
| 11 | 3.27 | 11.10 | 0.295 | 6.59 | 11.10 | 3.98 |
| 12 | 3.19 | 10.67 | 0.300 | 7.27 | 10.83 | 4.19 |
| 13 | 3.18 | 12.24 | 0.262 | 7.00 | 10.88 | 3.97 |
| 14 | 3.13 | 12.18 | 0.258 | 7.34 | 9.70 | 4.29 |
| 15 | 3.31 | 10.86 | 0.306 | 7.83 | 9.06 | 3.35 |
| 16 | 3.08 | 11.92 | 0.259 | 6.99 | 9.97 | 4.63 |
| 17 | 3.20 | 10.79 | 0.297 | 7.69 | 8.42 | 4.27 |
| 18 | 3.14 | 10.26 | 0.307 | 7.74 | 8.95 | 3.16 |
| 19 | 3.08 | 12.55 | 0.246 | 7.59 | 8.79 | 4.21 |
| 20 | 3.21 | 10.98 | 0.293 | 7.19 | 7.88 | 4.43 |
| 21 | 3.11 | 10.52 | 0.297 | 7.23 | 10.88 | 3.74 |
| 22 | 2.94 | 10.67 | 0.276 | 7.70 | 12.33 | 4.61 |
| s.e. | 0.024 | 0.113 | 0.0033 | 0.104 | 0.19 | 0.098 |
| Prob. | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Variety | Width (mm) | Length (mm) | Roundness | Oil, % | Protein, % | β-Glucan, % | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (Vi) | bi | Mean (Vi) | bi | Mean (Vi) | bi | Mean (Vi) | bi | Mean (Vi) | bi | Mean (Vi) | bi | |
| Balado | 3.16 | 1.316 | 11.62 | 1.030 | 0.273 | 0.986 | 7.53 | 1.450 | 9.92 | 1.056 | 4.76 | 1.101 |
| Gerald | 3.09 | 0.952 | 10.53 | 0.866 | 0.294 | 0.890 | 7.36 | 0.786 | 9.54 | 0.956 | 3.57 | 0.986 |
| Mascani | 3.23 | 0.817 | 11.23 | 1.066 | 0.290 | 1.142 | 6.70 | 0.906 | 9.76 | 1.011 | 4.26 | 1.068 |
| Tardis | 3.17 | 0.901 | 11.89 | 1.038 | 0.268 | 0.982 | 7.72 | 0.817 | 10.13 | 0.977 | 4.07 | 0.843 |
| s.e. | 0.011 | 0.113 | 0.050 | 0.063 | 0.0014 | 0.0666 | 0.047 | 0.127 | 0.09 | 0.065 | 0.043 | 0.097 |
| Prob. | <0.001 | 0.025 | <0.001 | 0.119 | <0.001 | 0.074 | <0.001 | 0.004 | <0.001 | 0.729 | <0.001 | 0.263 |
| Variable | December to April Cumulative Rainfall | March to July Cumulative Rainfall | June Cumulative Rainfall | June and July Cumulative Rainfall | November Mean Monthly Temperature | March mean Monthly Temperature | July Mean Monthly Temperature | June Cumulative Radiation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yield (t ha−1) | −0.042 | −0.772 | *** | −0.788 | *** | −0.768 | *** | −0.448 | * | −0.422 | 0.404 | 0.467 | * | |||
| Groat content (%) | −0.441 | * | −0.179 | −0.217 | −0.137 | −0.385 | −0.121 | −0.062 | −0.361 | |||||||
| Hullability (%) | −0.169 | 0.758 | *** | 0.739 | *** | 0.803 | *** | 0.454 | * | 0.612 | ** | −0.481 | * | −0.485 | * | |
| Hectoliter weight $ (kg hL−1) | −0.234 | −0.715 | *** | −0.545 | * | −0.611 | ** | −0.625 | ** | −0.414 | 0.110 | 0.170 | ||||
| Grain number m−2 | 0.039 | −0.680 | *** | −0.718 | *** | −0.698 | *** | −0.296 | −0.339 | 0.492 | * | 0.551 | ** | |||
| TGW (g) | −0.222 | −0.099 | −0.072 | −0.065 | −0.357 | −0.116 | −0.266 | −0.312 | ||||||||
| Grain width (mm) | −0.268 | 0.249 | 0.278 | 0.313 | −0.050 | 0.164 | −0.531 | * | −0.516 | ** | ||||||
| Grain length (mm) | −0.059 | −0.477 | * | −0.560 | ** | −0.539 | * | −0.202 | −0.634 | ** | 0.297 | 0.103 | ||||
| Grain roundness | −0.047 | 0.526 | * | 0.612 | ** | 0.607 | ** | 0.175 | 0.634 | ** | 0.472 | * | 0.287 | |||
| Grain protein (%) | −0.727 | *** | −0.174 | −0.122 | −0.002 | −0.171 | 0.015 | 0.080 | −0.254 | |||||||
| Grain β-glucan (%) | 0.260 | −0.730 | *** | −0.800 | *** | −0.846 | *** | −0.633 | ** | −0.553 | ** | 0.605 | ** | 0.594 | ** | |
| Grain oil (%) | 0.109 | −0.114 | 0.006 | −0.031 | −0.004 | 0.187 | −0.139 | 0.290 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Howarth, C.J.; Martinez-Martin, P.M.J.; Cowan, A.A.; Griffiths, I.M.; Sanderson, R.; Lister, S.J.; Langdon, T.; Clarke, S.; Fradgley, N.; Marshall, A.H. Genotype and Environment Affect the Grain Quality and Yield of Winter Oats (Avena sativa L.). Foods 2021, 10, 2356. https://doi.org/10.3390/foods10102356
Howarth CJ, Martinez-Martin PMJ, Cowan AA, Griffiths IM, Sanderson R, Lister SJ, Langdon T, Clarke S, Fradgley N, Marshall AH. Genotype and Environment Affect the Grain Quality and Yield of Winter Oats (Avena sativa L.). Foods. 2021; 10(10):2356. https://doi.org/10.3390/foods10102356
Chicago/Turabian StyleHowarth, Catherine J., Pilar M. J. Martinez-Martin, Alexander A. Cowan, Irene M. Griffiths, Ruth Sanderson, Susan J. Lister, Tim Langdon, Sarah Clarke, Nick Fradgley, and Athole H. Marshall. 2021. "Genotype and Environment Affect the Grain Quality and Yield of Winter Oats (Avena sativa L.)" Foods 10, no. 10: 2356. https://doi.org/10.3390/foods10102356






