Structural Characterization of a Neutral Polysaccharide from Cucurbia moschata and Its Uptake Behaviors in Caco-2 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Chemicals
2.2. Extraction and Purification of NPPc
2.3. Homogeneity and Molecular Weight of NPPc
2.4. Monosaccharide Composition of NPPc
2.5. Ultraviolet (UV) and FT-IR Spectrometry of NPPc
2.6. Methylation Analysis of NPPc
2.7. NMR Spectra Analysis of NPPc
2.8. Cell Culture
2.9. Caco-2 Cells Uptake Experiment for NPPc
2.9.1. Fluorescent Labeling of NPPc
2.9.2. Cellular Uptake Quantitative Analysis by the Flow Cytometry Method (FCM)
2.9.3. Cellular Uptake and Localization by a Laser Scanning Confocal Microscopy
2.10. Data Statistical and Analysis
3. Results
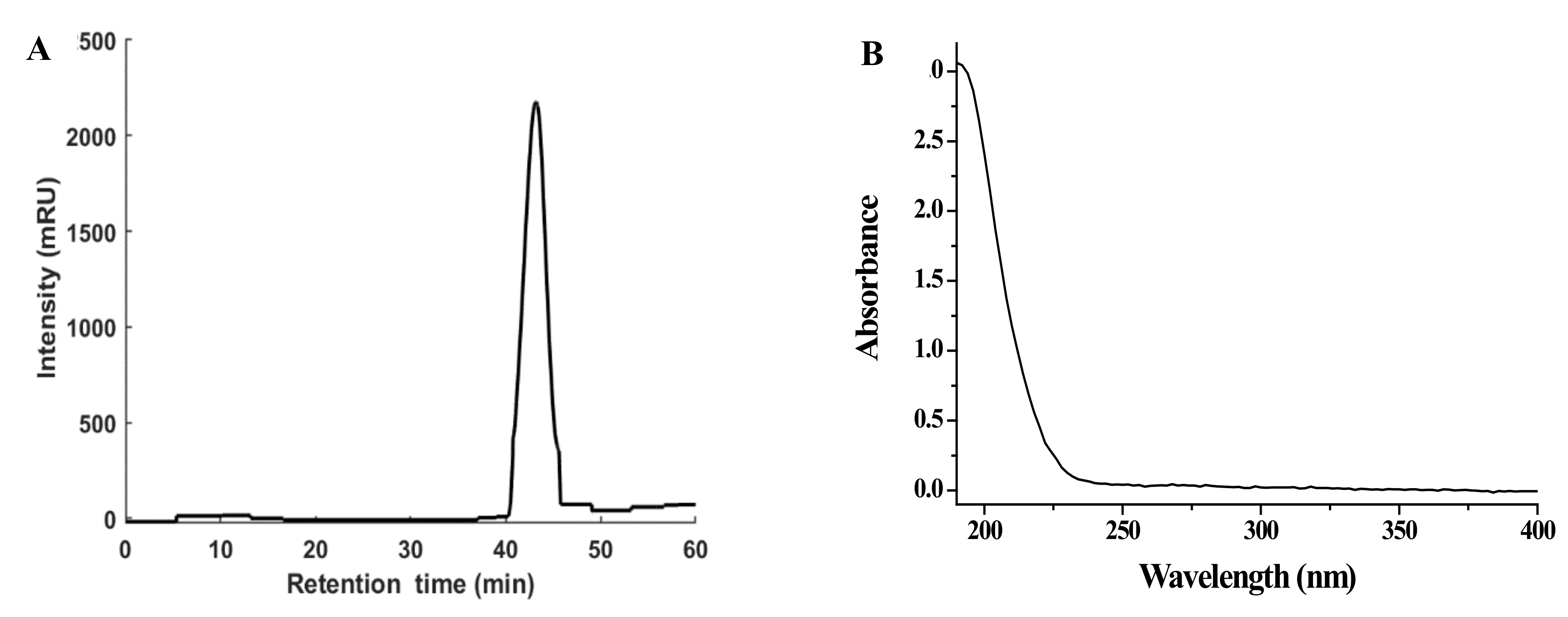
3.1. Preparation of NPPc and Detection of Molecular Weight
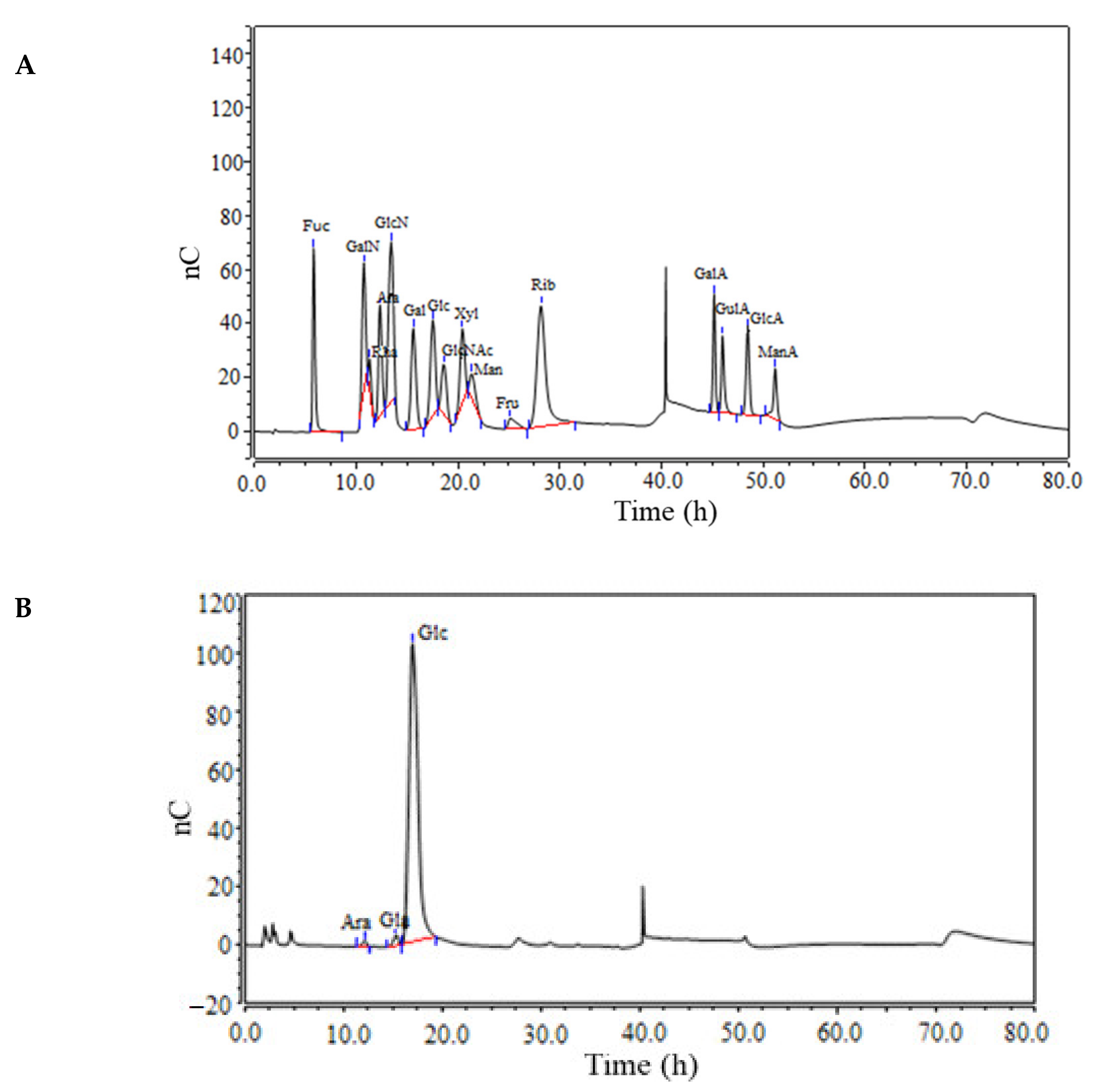
3.2. Monosaccharide Composition
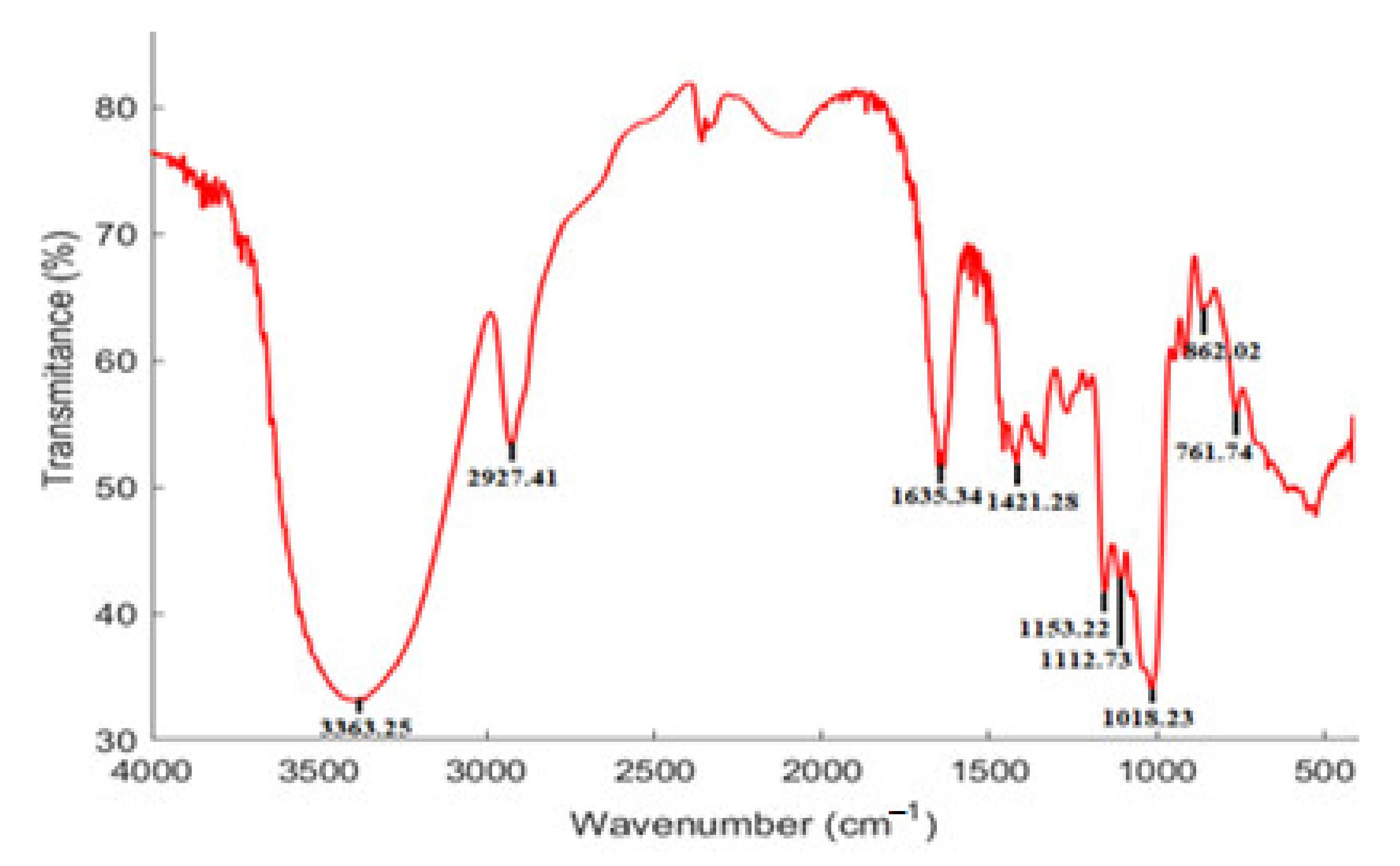
3.3. FT-IR Spectrum Characteristics
3.4. Methylation Analysis
3.5. NMR Spectra Analysis
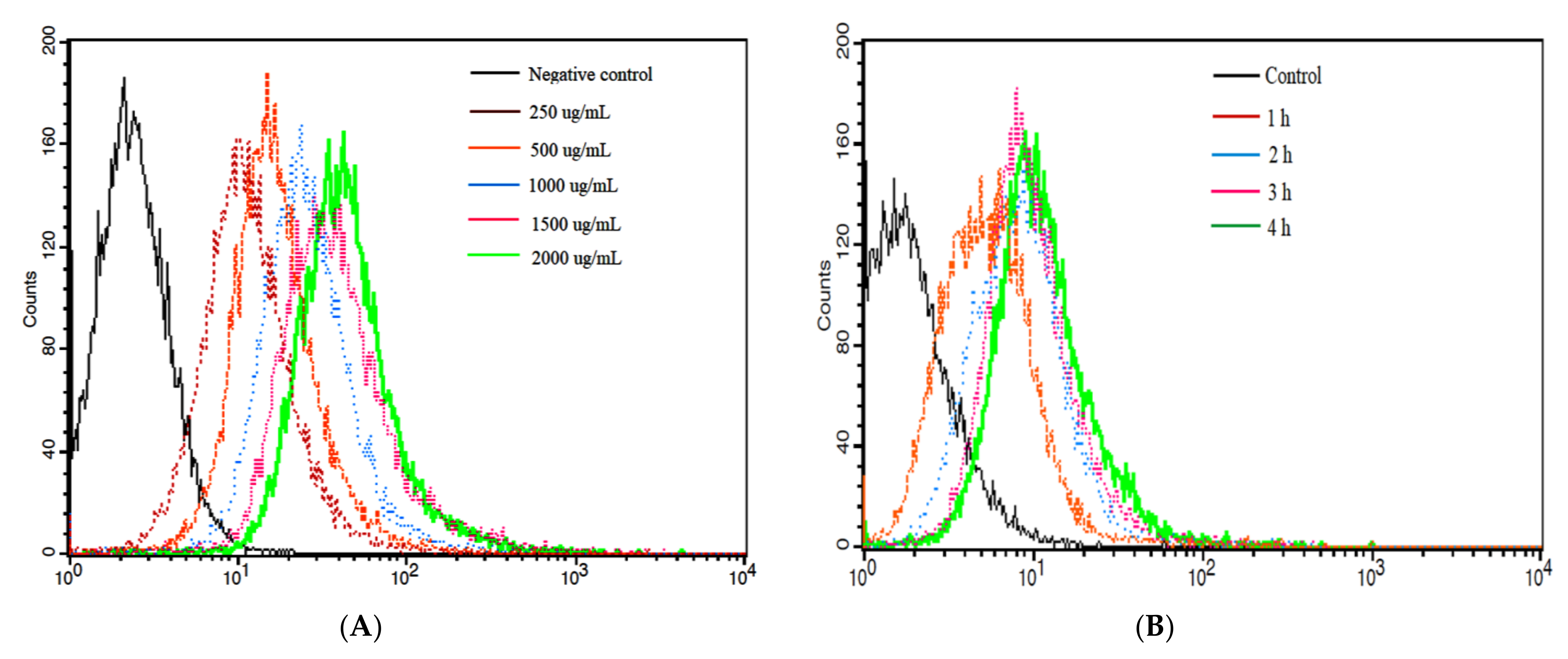
3.6. Cellular Uptake of NPPc by Caco-2 Cell
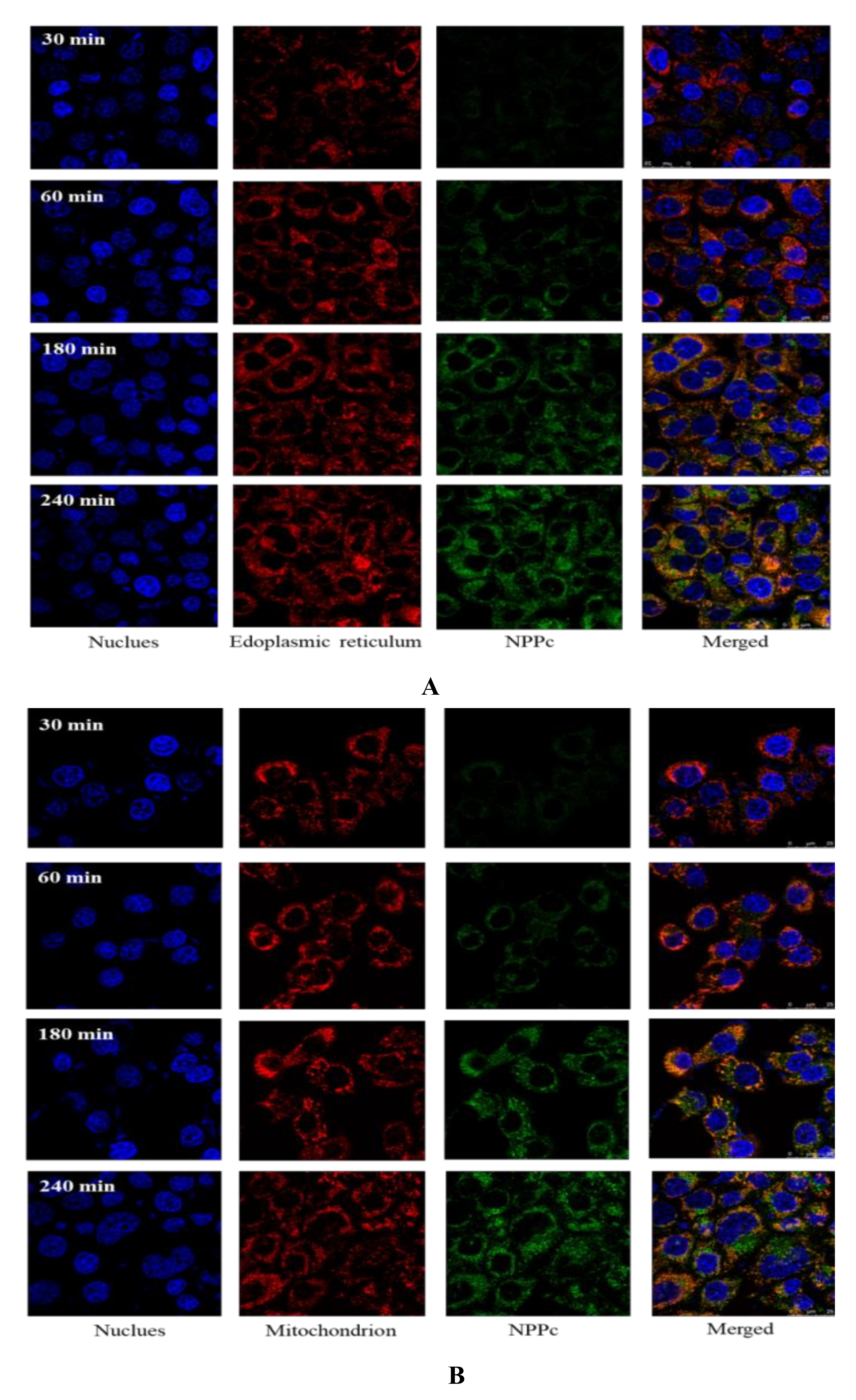
3.7. Subcellular location of NPPc in the Caco-2 Cell
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Q.H.; Fu, C.L.; Rui, Y.K.; Hu, G.H.; Cai, T.Y. Effects of protein-bound polysaccharide isolated from pumpkin on insulin in diabetic rats. Plant Foods Hum. Nutr. 2015, 60, 13–16. [Google Scholar]
- Murkovic, M.; Piironen, V.; Lampi, A.M.; Kraushofer, T.; Sontag, G. Changes in chemical composition of pumpkin seeds during the roasting process for production of pumpkin seed oil. Food Chem. 2004, 84, 359–365. [Google Scholar] [CrossRef]
- Adams, G.G.; Imran, S.; Wang, S.; Mohammad, A.; Kok, S.; Gray, D.A.; Channell, G.A.; Morris, G.A.; Harding, S.E. The hypoglycaemic effect of pumpkins as anti-diabetic and functional medicines. Food Res. Int. 2011, 44, 862–867. [Google Scholar] [CrossRef]
- Liu, G.M.; Liang, L.; Yu, G.Y.; Li, Q.H. Pumpkin polysaccharide modifies the gut microbiota during alleviation of type 2 diabetes in rats. Int. J. Biol. Macromol. 2018, 115, 711–717. [Google Scholar] [CrossRef]
- Chen, S.; Khan, B.M.; Cheong, K.L.; Liu, Y. Pumpkin polysaccharides: Purification, characterization and hypoglycemic potential. Int. J. Biol. Macromol. 2019, 139, 842–849. [Google Scholar]
- Song, Y.; Yang, Y.; Zhang, Y.; Duan, L.; Zhou, C.; Ni, Y.; Li, Q.; Hu, X. Effect of acetylation on antioxidant and cytoprotective activity of polysaccharides isolated from pumpkin (Cucurbita pepo, lady godiva). Carbohydr. Polym. 2013, 98, 686–691. [Google Scholar] [CrossRef]
- Ker, Y.B.; Chen, K.C.; Peng, C.C.; Hsieh, C.L.; Peng, P.Y. Structural characteristics and antioxidative capability of the soluble polysaccharides present in Dictyophora indusiata (Vent. Ex Pers.) Fish Phallaceae. BMC Complem. Altern. M. 2011, 2011, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bertozzi, C.R.; Kiessling, L.L. Chemical glycobiology. Science 2001, 29, 2357–2364. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Wei, Y.L.; Liang, L.; Yu, G.Y.; Huang, L.L.; Li, Q.H. A novel low-molecular-mass pumpkin polysaccharide: Structuralcharacterization, antioxidant activity, and hypoglycemic potential. Carbohydr. Polym. 2021, 251, 117090. [Google Scholar] [CrossRef]
- Yang, X.B.; Zhao, Y.; Lv, Y. Chemical composition and antioxidant activity of an acidic polysaccharide extracted from Cucurbita moschata Duchesne ex Poiret. J. Agr. Food Chem. 2007, 55, 4684–4690. [Google Scholar] [CrossRef]
- Fissore, E.N.; Ponce, N.M.A.; Pla, M.d.E.; Stortz, C.A.; Rojas, A.M.; Gerschenson, L.N. Characterization of acid-extracted pectin-enriched products obtained from Red Beet (Beta vulgaris L. var. conditiva) and Butternut (Cucurbita moschata Duch ex Poiret). J. Agr. Food. Chem. 2010, 58, 3793–3800. [Google Scholar] [CrossRef]
- Huang, L.L.; Zhao, J.; Wei, Y.L.; Yu, G.Y.; Li, F.; Li, Q.H. Structural characterization and mechanisms of macrophage immunomodulatory activity of a pectic polysaccharide from Cucurbita moschata Duch. Carbohyd. Polym. 2021, 269, 118288. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, F.M.; Liu, X.Y.; Ange, K.S.; Zhang, A.Q.; Li, Q.H.; Linhardt, R.J. Isolation of a lectin binding rhamnogalacturonan-I containing pectic polysaccharide from pumpkin. Carbohyd. Polym. 2017, 163, 330–336. [Google Scholar] [CrossRef]
- Chen, L.; Huang, G. Extraction, characterization and antioxidant activities of pumpkin polysaccharide. Int. J. Biol. Macromol. 2018, 118, 770–774. [Google Scholar] [CrossRef]
- Song, Y.; Juan, L.; Hu, X.S.; Ni, Y.Y.; Li, Q.H. Structural characterization of a polysaccharide isolated from Lady Godiva pumpkins (Cucurbita pepo lady godiva). Macromol. Res. 2011, 19, 1172–1178. [Google Scholar] [CrossRef]
- Zhou, C.L.; Liu, W.; Kong, Q.; Song, Y.; Ni, Y.Y.; Li, Q.H.; O’Riordan, D. Isolation, characterisation and sulphation of soluble polysaccharides isolated from Cucurbita maxima. Int. J. Food Sci. Technol. 2014, 49, 508–514. [Google Scholar] [CrossRef]
- Xiang, Q.; Zhang, W.; Li, Q.; Zhao, J.; Feng, W.; Zhao, T.; Mao, G.; Chen, Y.; Wu, X.; Yang, L.; et al. Investigation of the uptake and transport of polysaccharide from Se-enriched Grifola frondosa in Caco-2 cells model. Int. J. Biol. Macromol. 2020, 158, 1330–1341. [Google Scholar] [CrossRef]
- Sanchez, A.B.; Calpena, A.C.; Mallandrich, M.; Clares, B. Validation of an ex vivo permeation method for the intestinal permeability of different BCS drugs and its correlation with Caco-2 in vitro experiments. Pharmaceutics 2019, 11, 638. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Wei, Y.L.; Zhao, J.; Yu, G.Y.; Huang, L.L.; Li, Q.H. Transport mechanism and subcellular localization of a polysaccharide from Cucurbia Moschata across Caco-2 cells model. Int. J. Biol. Macromol. 2021, 182, 1002–1014. [Google Scholar] [CrossRef]
- Wang, K.; Cheng, F.; Pan, X.; Zhou, T.; Liu, X.; Zheng, Z.; Zhang, Y. Investigation of the transport and absorption of Angelica sinensis polysaccharide through gastrointestinal tract both in vitro and in vivo. Drug Deliv. 2017, 24, 1360–1371. [Google Scholar] [CrossRef] [Green Version]
- Zhang, E.; Chu, E.; Xu, L.; Liang, H.; Song, S.; Ji, A. Use of fluorescein isothiocyanate isomer I to study the mechanism of intestinal absorption of fucoidan sulfate in vivo and in vitro. Biopharm. Drug Dispos. 2018, 39, 298–307. [Google Scholar] [CrossRef]
- Sims, I.M.; Carnachan, S.M.; Bell, T.J.; Hinkley, S.F.R. Methylation analysis of polysaccharides: Technical advice. Carbohydr. Polym. 2018, 188, 1–7. [Google Scholar] [CrossRef]
- Zhao, L.; Wu, M.; Xiao, C.; Yang, L.; Zhou, L.; Gao, N.; Li, Z.; Chen, J.; Chen, J.C.; Liu, J.K.; et al. Discovery of an intrinsic tenase complex inhibitor: Pure nonasaccharide from fucosylated glycosaminoglycan. Proc. Natl. Acad. Sci. USA 2015, 112, 8284–8289. [Google Scholar] [CrossRef] [Green Version]
- Chylinska, M.; Szymanska-Chargot, M.; Zdunek, A. FT-IR and FT-Raman characterization of non-cellulosic polysaccharides fractions isolated from plant cell wall. Carbohydr. Polym. 2016, 154, 48–54. [Google Scholar]
- Jeff, I.B.; Li, S.; Peng, X.; Kassim, R.M.R.; Liu, B.; Zhou, Y. Purification, structural elucidation and antitumor activity of a novel mannogalactoglucan from the fruiting bodies of Lentinus Edodes. Fitotherapy 2013, 84, 338–346. [Google Scholar] [CrossRef]
- Choma, A.; Nowak, K.; Komaniecka, I.; Wasko, A.; Pleszczynska, M.; Siwulski, M.; Wiater, A. Chemical characterization of alkali-soluble polysaccharides isolated from a Boletus edulis (Bull.) fruiting body and their potential for heavy metal biosorption. Food Chem. 2018, 266, 329–334. [Google Scholar] [CrossRef]
- Xiao, X.; Tan, C.; Sun, X.; Zhao, Y.; Zhang, J.; Zhu, Y.; Bai, J.; Dong, Y.; Zhou, X. Effects of fermentation on structural characteristics and in vitro physiological activities of barley beta-glucan. Carbohydr. Polym. 2020, 231, 115685. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, M.; Wang, C.; Yang, Y.; Chen, J.; Ding, J.; Guo, W. Characterization and in vitro antitumor activity of polysaccharides from the mycelium of Sarcodon aspratus. Int. J. Biol. Macromol. 2013, 52, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, L.; Li, Q.; Liu, C.; Han, J.; Zhu, L.; Zhu, D.; He, Y.; Liu, H. Rheological properties and chain conformation of soy hull water-soluble polysaccharide fractions obtained by gradient alcohol precipitation. Food Hydrocoll. 2019, 9, 34–39. [Google Scholar] [CrossRef]
- Gao, J.; Lin, L.; Sun, B.; Zhao, M. Comparison study on polysaccharide fractions from Laminaria japonica: Structural characterization and bile acid binding aapacity. J. Agric. Food Chem. 2017, 65, 9790–9798. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dobruchowska, J.M.; Gerwig, G.J.; Dijkhuizen, L.; Kamerling, J.P. Structural investigation of water-soluble polysaccharides extracted from the fruit bodies of Coprinus comatus. Carbohydr. Polym. 2013, 91, 314–321. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Shang, F.N.; Yang, Z.M.; Wu, M.Y.; Zhao, J.H. Structural analysis of a homogeneous polysaccharide from Achatina fulica. Int. J. Biol. Macromol. 2017, 98, 786–792. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, L.; Yan, A.P.; Feng, L.; Wan, Y.Q. Fractionation, structure and conformation characterization of polysaccharides from Anoectochilus roxburghii. Carbohydr. Polym. 2020, 231, 115688. [Google Scholar] [CrossRef]
- Meng, F.; Li, Q.; Qi, Y.; He, C.; Wang, C.; Zhang, Q. Characterization and immunoregulatory activity of two polysaccharides from the root of Ilex asprella. Cabohydr. Polym. 2018, 197, 9–16. [Google Scholar] [CrossRef]
- Shi, X.D.; Li, O.Y.; Yin, J.Y.; Nie, S.P. Structure identification of α-glucans from Dictyophora echinovolvata by methylation and 1D/2D NMR spectroscopy. Food Chem. 2019, 271, 338–344. [Google Scholar] [CrossRef]
- Wang, J.Q.; Nie, S.P.; Cui, S.W.; Wang, Z.J.; Phillips, G.O.; Li, Y.J.; Xie, M.Y. Structural characterization and immunostimulatory activity of a glucan from natural Cordyceps sinensis. Food Hydrocoll. 2017, 67, 139–147. [Google Scholar] [CrossRef]
- Zdorovenko, E.L.; Shashkov, A.S.; Kadykova, A.A.; Kiseleva, E.P.; Savich, V.V.; Novik, G.I.; Knirel, Y.A. Structures of O-specific polysaccharides of Pseudomonas psyhrotolerans BIM B-1158G. Carbohydr. Res. 2018, 465, 35–39. [Google Scholar] [CrossRef]
- Dou, T.; Wang, J.; Han, C.; Shao, X.; Zhang, J.; Lu, W. Cellular uptake and transport characteristics of chitosan modified nanoparticles in Caco-2 cell monolayers. Int. J. Biol. Macromol. 2019, 138, 791–799. [Google Scholar] [CrossRef]
- Chen, P.; Zhao, M.; Chen, Q.; Fan, L.; Gao, F.; Zhao, L. Absorption characteristics of chitobiose and chitopentaose in the human intestinal cell line Caco-2 and everted gut sacs. J. Agric. Food Chem. 2019, 67, 4513–4523. [Google Scholar] [CrossRef]
- Wang, L.; Li, X. Radioprotective effect of Hohenbuehelia serotina polysaccharides through mediation of ER apoptosis pathway in vivo. Int. J. Biol. Macromol. 2019, 127, 18–26. [Google Scholar] [CrossRef]
- Shen, W.; Guan, Y.; Wang, J.; Hu, Y.; Tan, Q.; Song, X.; Jin, Y.H.; Liu, Y.; Zhang, Y. A polysaccharide from pumpkin induces apoptosis of HepG2 cells by activation of mitochondrial pathway. Tumor Biolog. 2016, 37, 5239–5245. [Google Scholar] [CrossRef]
- Yuan, Q.X.; Zhao, L.Y.; Cha, Q.Q.; Sun, Y.; Ye, H.; Zeng, X.X. Structural characterization and immunostimulatory activity of a homogeneous polysaccharide from Sinonovacula constricta. J. Agr. Food Chem. 2015, 63, 7986–7994. [Google Scholar] [CrossRef]


| Retention Time | Methylated Sugar | Mass Fragments (m/z) | Molar Ratio | Types of Linkage |
|---|---|---|---|---|
| 16.182 | 2,3,4,6-Me4-Glcp | 43,71,87,101,117,129,145,161,205 | 9.7 | Glcp-(1→ |
| 21.755 | 2,3,6-Me3-Glcp | 43,87,99,101,113,117,129,131,161,173,233 | 70.1 | →4)-Glcp-(1→ |
| 22.425 | 2,3,4-Me3-Glcp | 43,87,99,101,117,129,161,189,233 | 13 | →6-Glcp-(1→ |
| 27.255 | 2,3-Me2-Glcp | 43,71,85,87,99,101,117,127,159,161,201 | 7.2 | →4,6)-Glcp-(1→ |
| Glycosyl Residues | Chemical Shift δ H/C (ppm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6a | 6b | |||
| A | →4-α-D-Glcp-(1→ | H | 5.29 | 3.46 | 3.86 | 3.54 | 3.30 | 3.62 | 3.73 |
| C | 99.61 | 71.50 | 73.30 | 76.81 | 69.47 | 60.42 | |||
| B | →6)-α-D-Glcp-(1→ | H | 4.87 | 3.61 | 3.87 | 3.51 | 3.71 | 3.87 | |
| C | 97.68 | 72.90 | 73.44 | 71.40 | 70.80 | 65.36 | |||
| C | →4,6)-α-D-Glcp-(1→ | H | 5.25 | 3.44 | 3.82 | 3.51 | 3.48 | 3.63 | |
| C | 99.71 | 71.64 | 70.07 | 78.01 | 73.07 | 65.59 | |||
| D | α-D-Glcp-(1→ | H | 4.86 | 3.45 | 3.62 | 3.58 | 3.31 | 3.63 | 3.73 |
| C | 97.67 | 71.27 | 73.07 | 73.07 | 69.23 | 60.44 | |||
| Rα | →4)-α-D-Glcp | H | 5.11 | 3.43 | 3.78 | 3.64 | 3.72 | 3.62 | 3.71 |
| C | 91.76 | 69.33 | 70.20 | 75.97 | 71.14 | 60.56 | |||
| Rβ | →4)-β-D-Glcp | H | 4.53 | 3.16 | 3.63 | 3.73 | 3.57 | 3.62 | 3.73 |
| C | 95.63 | 73.81 | 71.67 | 76.58 | 71.74 | 60.61 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Pak, S.; Zhao, J.; Wei, Y.; Zhang, Y.; Li, Q. Structural Characterization of a Neutral Polysaccharide from Cucurbia moschata and Its Uptake Behaviors in Caco-2 Cells. Foods 2021, 10, 2357. https://doi.org/10.3390/foods10102357
Li F, Pak S, Zhao J, Wei Y, Zhang Y, Li Q. Structural Characterization of a Neutral Polysaccharide from Cucurbia moschata and Its Uptake Behaviors in Caco-2 Cells. Foods. 2021; 10(10):2357. https://doi.org/10.3390/foods10102357
Chicago/Turabian StyleLi, Fei, Solju Pak, Jing Zhao, Yunlu Wei, Yuyu Zhang, and Quanhong Li. 2021. "Structural Characterization of a Neutral Polysaccharide from Cucurbia moschata and Its Uptake Behaviors in Caco-2 Cells" Foods 10, no. 10: 2357. https://doi.org/10.3390/foods10102357
APA StyleLi, F., Pak, S., Zhao, J., Wei, Y., Zhang, Y., & Li, Q. (2021). Structural Characterization of a Neutral Polysaccharide from Cucurbia moschata and Its Uptake Behaviors in Caco-2 Cells. Foods, 10(10), 2357. https://doi.org/10.3390/foods10102357





