Deep Eutectic Solvent-Assisted Extraction, Partially Structural Characterization, and Bioactivities of Acidic Polysaccharides from Lotus Leaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Extraction of Polysaccharides from Lotus Leaves (LLPs)
2.2.1. Preparation of Raw Material
2.2.2. Hot Water Extraction of LLPs
2.2.3. Deep Eutectic Solvent Assisted Extraction (DESE) of LLPs
2.3. Physicochemical and Structural Characterization of Polysaccharides from Lotus Leaves (LLPs)
2.4. Evaluation of Bioactivities of Polysaccharides from Lotus Leaves (LLPs)
2.4.1. Evaluation of In Vitro Antioxidant Activities of LLPs
2.4.2. Evaluation of In Vitro Hypoglycemic Effects of LLPs
2.4.3. Evaluation of Immunomodulatory Activities of LLPs
2.5. Statistical Analysis
3. Results and Discussion
3.1. Optimization of DES Assisted Extraction of LLPs
3.1.1. Single Factor Experimental Analysis
3.1.2. Box-Behnken Design Analysis
3.2. Physicochemical and Structural Properties of LLPs
3.2.1. Chemical Compositions of LLPs
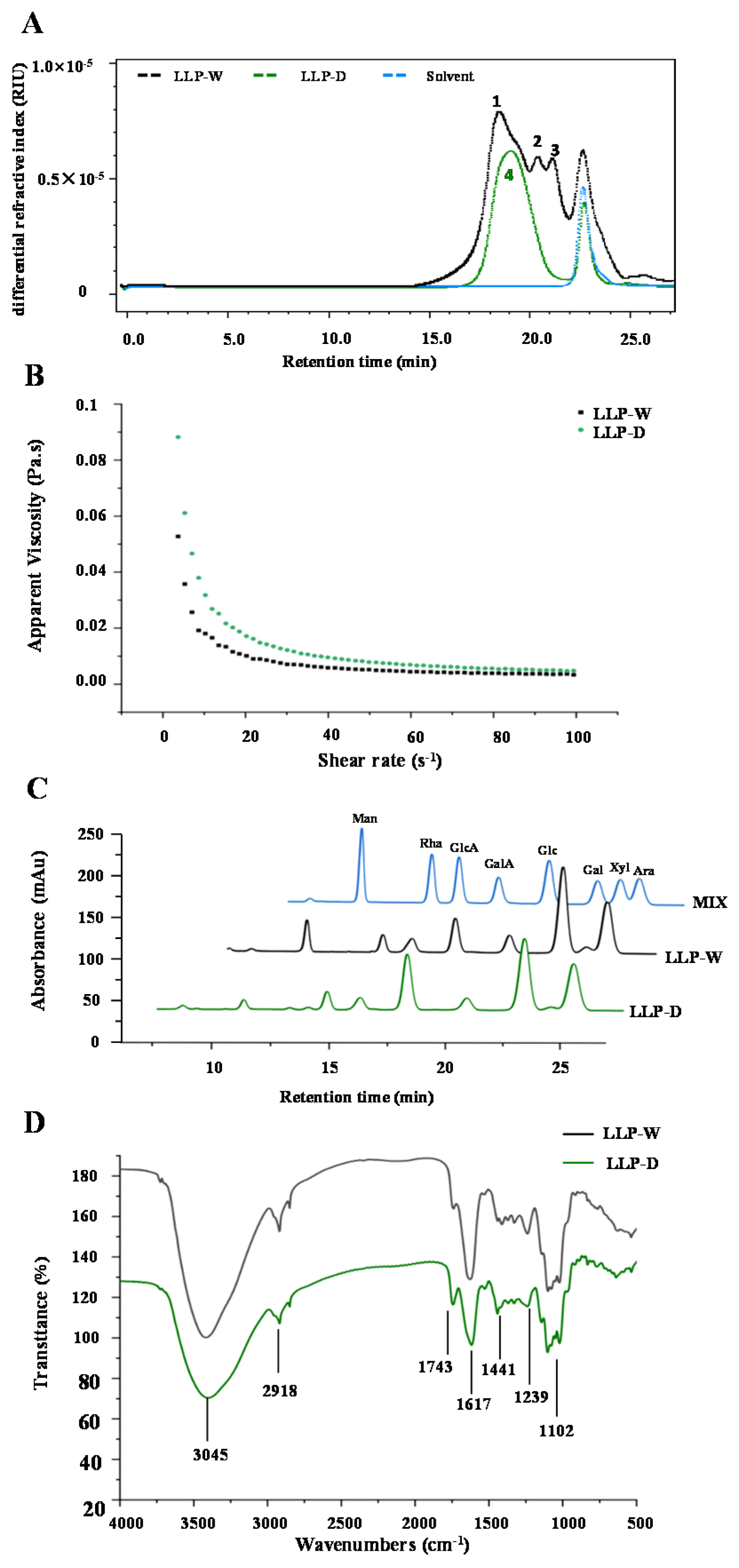
3.2.2. Molecular Weights and Apparent Viscosities of LLPs
3.2.3. Monosaccharide Compositions of LLPs
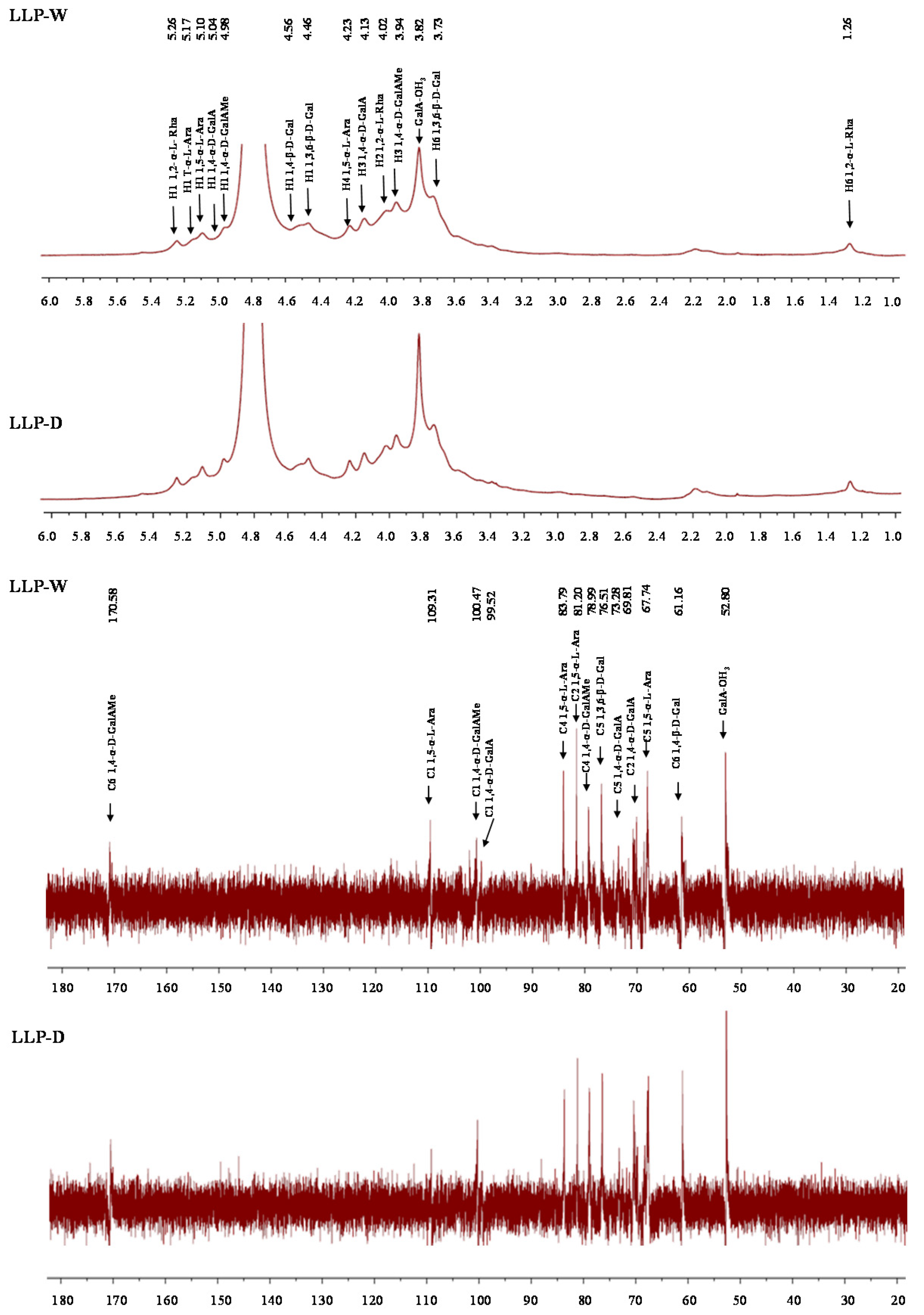
3.2.4. FT-IR and NMR Spectra of LLPs
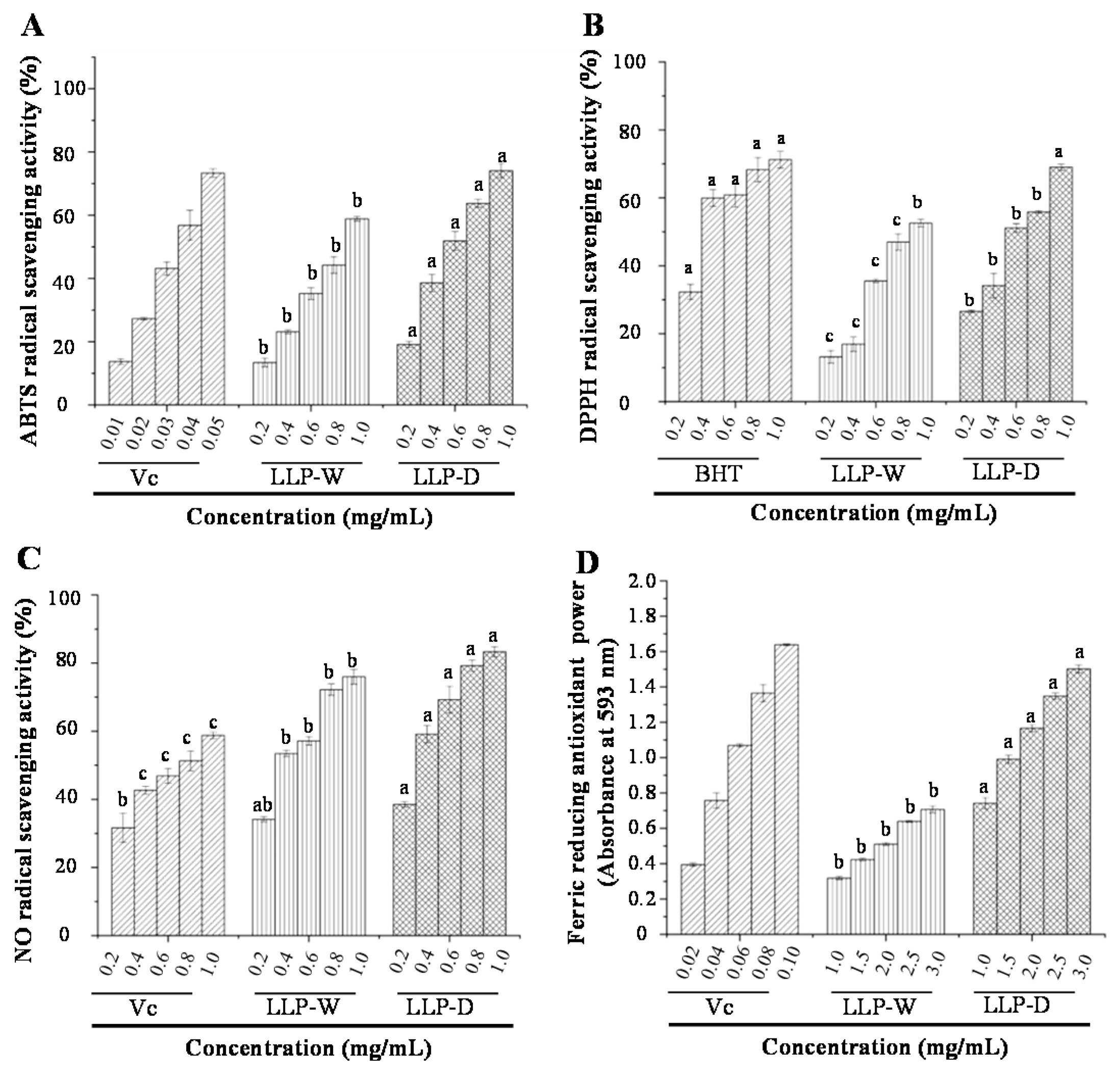
3.3. In Vitro Antioxidant Activities of LLPs
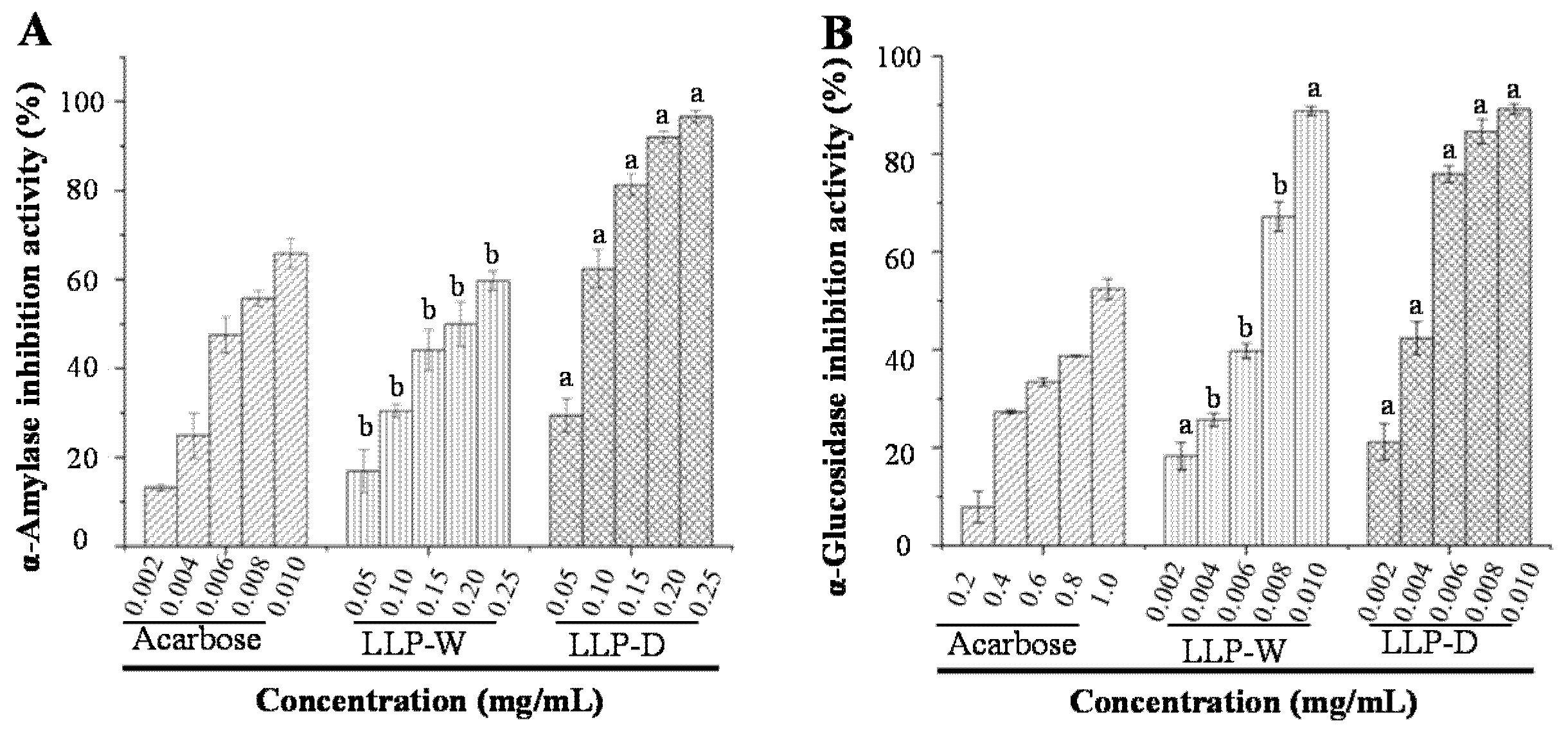
3.4. In Vitro Hypoglycemic Effects of LLPs
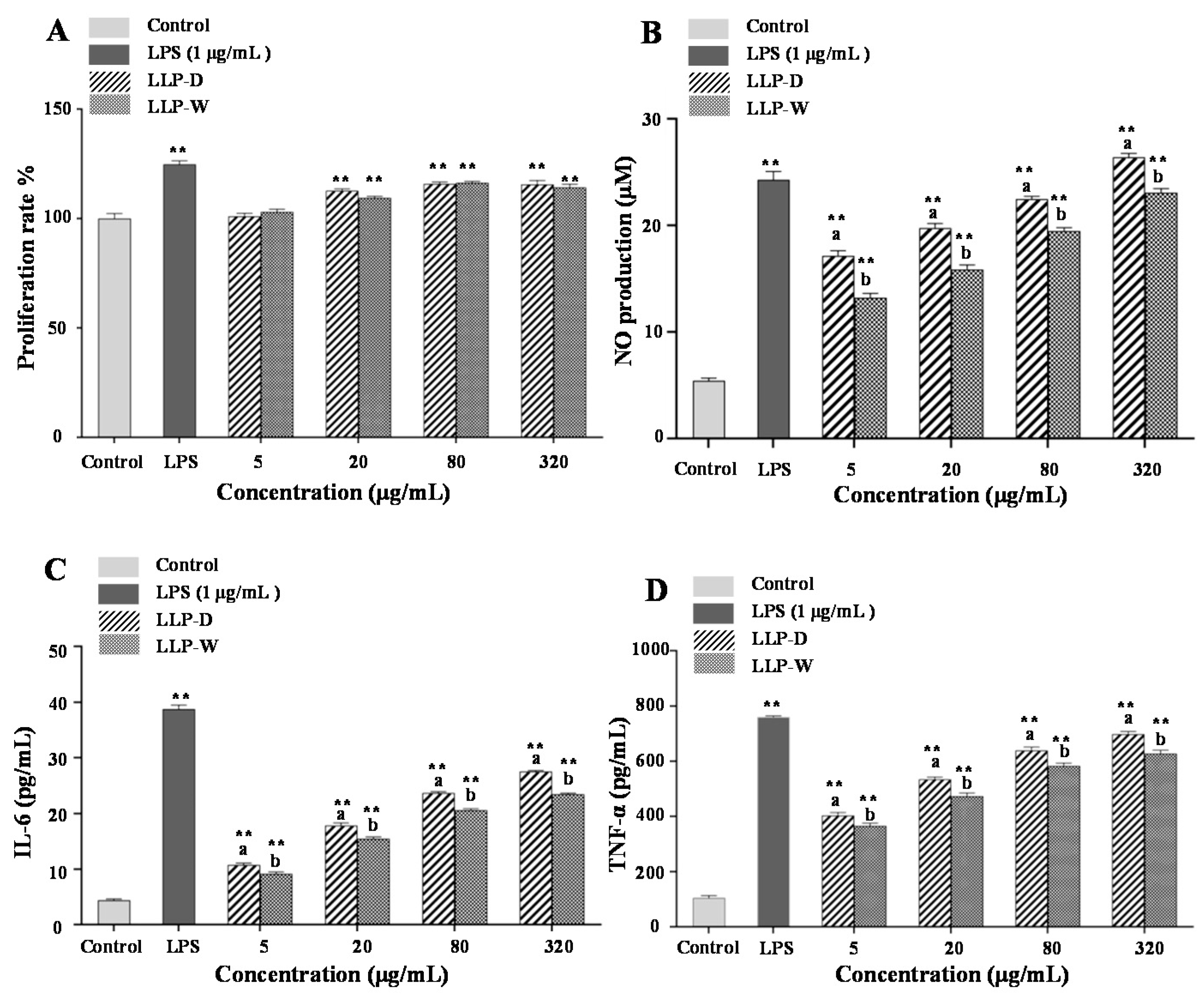
3.5. Immunomodulatory Effects of LLPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, G.; Zhu, M.; Guo, M. Research advances in traditional and modern use of Nelumbo nucifera: Phytochemicals, health promoting activities and beyond. Crit. Rev. Food Sci. Nutr. 2019, 59, S189–S209. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cheng, Y.; Zeng, M.; Wang, Z.; Qin, F.; Wang, Y.; Chen, J.; He, Z. Lotus (Nelumbo nucifera Gaertn.) leaf: A narrative review of its Phytoconstituents, health benefits and food industry applications. Trends Food Sci. Technol. 2021, 112, 631–650. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, Z.C.; Wang, H.; Kou, Y.; Wen, Q.H.; Fu, Z.F.; Chang, H.X. Response surface optimization and physicochemical properties of polysaccharides from Nelumbo nucifera leaves. Int. J. Biol. Macromol. 2015, 74, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Xu, Y.; Zhang, B. Antidiabetic activity of a lotus leaf selenium (Se)-polysaccharide in rats with gestational diabetes mellitus. Biol. Trace. Elem. Res. 2017, 176, 321–327. [Google Scholar] [CrossRef]
- Song, Y.R.; Han, A.R.; Park, S.G.; Cho, C.W.; Rhee, Y.K.; Hong, H.D. Effect of enzyme-assisted extraction on the physicochemical properties and bioactive potential of lotus leaf polysaccharides. Int. J. Biol. Macromol. 2020, 153, 169–179. [Google Scholar] [CrossRef]
- Li, W.; Wu, D.T.; Li, F.; Gan, R.Y.; Hu, Y.C.; Zou, L. Structural and biological properties of water soluble polysaccharides from lotus leaves: Effects of drying techniques. Molecules 2021, 26, 4395. [Google Scholar] [CrossRef]
- Song, Y.R.; Han, A.R.; Lim, T.G.; Lee, E.J.; Hong, H.D. Isolation, purification, and characterization of novel polysaccharides from lotus (Nelumbo nucifera) leaves and their immunostimulatory effects. Int. J. Biol. Macromol. 2019, 128, 546–555. [Google Scholar] [CrossRef]
- Fu, Y.; Li, F.; Ding, Y.; Li, H.Y.; Xiang, X.R.; Ye, Q.; Zhang, J.; Zhao, L.; Qin, W.; Gan, R.Y.; et al. Polysaccharides from loquat (Eriobotrya japonica) leaves: Impacts of extraction methods on their physicochemical characteristics and biological activities. Int. J. Biol. Macromol. 2020, 146, 508–517. [Google Scholar] [CrossRef]
- Amamou, S.; Lazreg, H.; Hafsa, J.; Majdoub, H.; Rihouey, C.; Le Cerf, D.; Achour, L. Effect of extraction condition on the antioxidant, antiglycation and α-amylase inhibitory activities of Opuntia macrorhiza fruit peels polysaccharides. LWT Food Sci. Technol. 2020, 127, 109411. [Google Scholar] [CrossRef]
- Yuan, Q.; Lin, S.; Fu, Y.; Nie, X.R.; Liu, W.; Su, Y.; Han, Q.H.; Zhao, L.; Zhang, Q.; Lin, D.R.; et al. Effects of extraction methods on the physicochemical characteristics and biological activities of polysaccharides from okra (Abelmoschus esculentus). Int. J. Biol. Macromol. 2019, 127, 178–186. [Google Scholar] [CrossRef]
- Chen, M.; Lahaye, M. Natural deep eutectic solvents pretreatment as an aid for pectin extraction from apple pomace. Food Hydrocoll. 2021, 115, 106601. [Google Scholar] [CrossRef]
- Ruesgas-Ramon, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of deep eutectic solvents (DES) for phenolic compounds extraction: Overview, challenges, and opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Wilpiszewska, K.; Spychaj, T. Deep eutectic solvents for polysaccharides processing. A review. Carbohydr Polym. 2018, 200, 361–380. [Google Scholar] [CrossRef]
- Shang, X.C.; Chu, D.; Zhang, J.X.; Zheng, Y.F.; Li, Y. Microwave-assisted extraction, partial purification and biological activity in vitro of polysaccharides from bladder-wrack (Fucus vesiculosus) by using deep eutectic solvents. Sep. Purif. Technol. 2021, 259, 118169. [Google Scholar] [CrossRef]
- Saravana, P.S.; Cho, Y.N.; Woo, H.C.; Chun, B.S. Green and efficient extraction of polysaccharides from brown seaweed by adding deep eutectic solvent in subcritical water hydrolysis. J. Clean. Prod. 2018, 198, 1474–1484. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, M. Optimization of deep eutectic solvent-based ultrasound-assisted extraction of polysaccharides from Dioscorea opposita Thunb. Int. J. Biol. Macromol. 2017, 95, 675–681. [Google Scholar] [CrossRef]
- Gao, C.; Cai, C.; Liu, J.; Wang, Y.; Chen, Y.; Wang, L.; Tan, Z. Extraction and preliminary purification of polysaccharides from Camellia oleifera Abel. seed cake using a thermoseparating aqueous two-phase system based on EOPO copolymer and deep eutectic solvents. Food Chem. 2020, 313, 126164. [Google Scholar] [CrossRef]
- Li, J.H.; Li, W.; Luo, S.; Ma, C.H.; Liu, S.X. Alternate ultrasound/microwave digestion for deep eutectic hydro-distillation extraction of essential oil and polysaccharide from Schisandra chinensis (Turcz.) Baill. Molecules 2019, 24, 1288. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.T.; Liu, W.; Han, Q.H.; Du, G.; Li, H.Y.; Yuan, Q.; Fu, Y.; Zhao, L.; Zhang, Q.; Li, S.Q.; et al. Physicochemical characteristics and antioxidant activities of non-starch polysaccharides from different kiwifruits. Int. J. Biol. Macromol. 2019, 136, 891–900. [Google Scholar] [CrossRef]
- Wu, D.T.; He, Y.; Fu, M.X.; Gan, R.Y.; Hu, Y.C.; Peng, L.X.; Zhao, G.; Zou, L. Structural characteristics and biological activities of a pectic-polysaccharide from okra affected by ultrasound assisted metal-free Fenton reaction. Food Hydrocoll. 2022, 122, 107085. [Google Scholar] [CrossRef]
- Wu, H.; Li, M.; Yang, X.; Wei, Q.; Sun, L.; Zhao, J.; Shang, H. Extraction optimization, physicochemical properties and antioxidant and hypoglycemic activities of polysaccharides from roxburgh rose (Rosa roxburghii Tratt.) leaves. Int. J. Biol. Macromol. 2020, 165, 517–529. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Li, C.; Huang, Q.; You, L.J.; Chen, C.; Fu, X.; Liu, R.H. Comparative study on the physicochemical properties and bioactivities of polysaccharide fractions extracted from Fructus Mori at different temperatures. Food Funct. 2019, 10, 410–421. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.; Wang, Z.; Kan, J. A comparison of a polysaccharide extracted from ginger (Zingiber officinale) stems and leaves using different methods: Preparation, structure characteristics, and biological activities. Int. J. Biol. Macromol. 2020, 151, 635–649. [Google Scholar] [CrossRef]
- Sun, Y.; Hou, S.; Song, S.; Zhang, B.; Ai, C.; Chen, X.; Liu, N. Impact of acidic, water and alkaline extraction on structural features, antioxidant activities of Laminaria japonica polysaccharides. Int. J. Biol. Macromol. 2018, 112, 985–995. [Google Scholar] [CrossRef]
- Yao, H.Y.; Wang, J.Q.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. A review of NMR analysis in polysaccharide structure and conformation: Progress, challenge and perspective. Food Res. Int. 2021, 143, 110290. [Google Scholar] [CrossRef]
- Xu, Y.; Niu, X.; Liu, N.; Gao, Y.; Wang, L.; Xu, G.; Li, X.; Yang, Y. Characterization, antioxidant and hypoglycemic activities of degraded polysaccharides from blackcurrant (Ribes nigrum L.) fruits. Food Chem. 2018, 243, 26–35. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, L.; Yao, J.; Shi, Y.; Li, P.; Ding, K. An arabinogalactan from flowers of Panax notoginseng inhibits angiogenesis by BMP2/Smad/Id1 signaling. Carbohydr. Polym. 2015, 121, 328–335. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Zheng, Y.; Zhang, H.; Chen, J.; Yan, L.; Ding, T.; Linhardt, R.J.; Orfila, C.; Liu, D.; et al. Fast preparation of rhamnogalacturonan I enriched low molecular weight pectic polysaccharide by ultrasonically accelerated metal-free Fenton reaction. Food Hydrocoll. 2019, 95, 551–561. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, X.; Xie, H.; Li, X.; Shi, L. Structural characterization and antitumor activity of a polysaccharide from ramulus mori. Carbohydr. Polym. 2018, 190, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Li, J.E.; Cui, S.W.; Nie, S.P.; Xie, M.Y. Structure and biological activities of a pectic polysaccharide from Mosla chinensis Maxim. cv. Jiangxiangru. Carbohydr. Polym. 2014, 105, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Bai, J.; Bu, X.; Yin, Y.; Wang, L.; Yang, Y.; Xu, Y. Characterization of selenized polysaccharides from Ribes nigrum L. and its inhibitory effects on alpha-amylase and alpha-glucosidase. Carbohydr. Polym. 2021, 259, 117729. [Google Scholar] [CrossRef]
- Lv, Q.Q.; Cao, J.J.; Liu, R.; Chen, H.Q. Structural characterization, alpha-amylase and alpha-glucosidase inhibitory activities of polysaccharides from wheat bran. Food Chem. 2021, 341, 128218. [Google Scholar] [CrossRef]
- Dou, Z.; Chen, C.; Fu, X. The effect of ultrasound irradiation on the physicochemical properties and α-glucosidase inhibitory effect of blackberry fruit polysaccharide. Food Hydrocoll. 2019, 96, 568–576. [Google Scholar] [CrossRef]
- Chen, C.; Wang, P.P.; Huang, Q.; You, L.J.; Liu, R.H.; Zhao, M.M.; Fu, X.; Luo, Z.G. A comparison study on polysaccharides extracted from Fructus Mori using different methods: Structural characterization and glucose entrapment. Food Funct. 2019, 10, 3684–3695. [Google Scholar] [CrossRef]
- Wang, N.; Jia, G.; Wang, X.; Liu, Y.; Li, Z.; Bao, H.; Guo, Q.; Wang, C.; Xiao, D. Fractionation, structural characteristics and immunomodulatory activity of polysaccharide fractions from asparagus (Asparagus officinalis L.) skin. Carbohydr. Polym. 2021, 256, 117514. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, M.; Wen, C.; Zhou, J.; Gu, J.; Duan, Y.; Zhang, H.; Ren, X.; Ma, H. Structural characterization and immunostimulatory activity of a novel polysaccharide isolated with subcritical water from Sagittaria sagittifolia L. Int. J. Biol. Macromol. 2019, 133, 11–20. [Google Scholar] [CrossRef]
- Yuan, P.; Aipire, A.; Yang, Y.; Wei, X.; Fu, C.; Zhou, F.; Mahabati, M.; Li, J.; Li, J. Comparison of the structural characteristics and immunostimulatory activities of polysaccharides from wild and cultivated Pleurotus feruleus. J. Funct. Food 2020, 72, 104050. [Google Scholar] [CrossRef]
- Ketha, K.; Gudipati, M. Immunomodulatory activity of non starch polysaccharides isolated from green gram (Vigna radiata). Food Res. Int. 2018, 113, 269–276. [Google Scholar] [CrossRef]
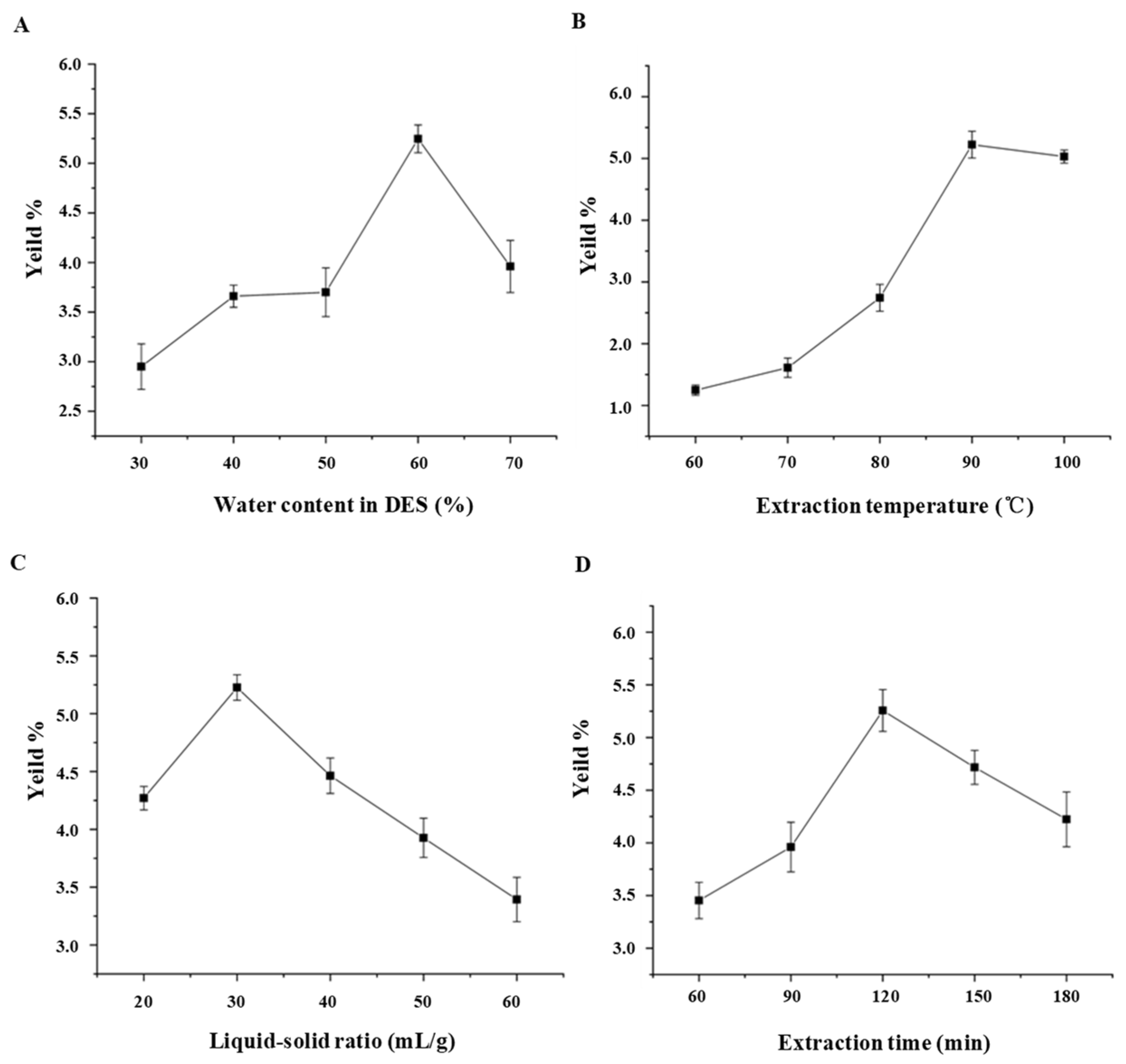
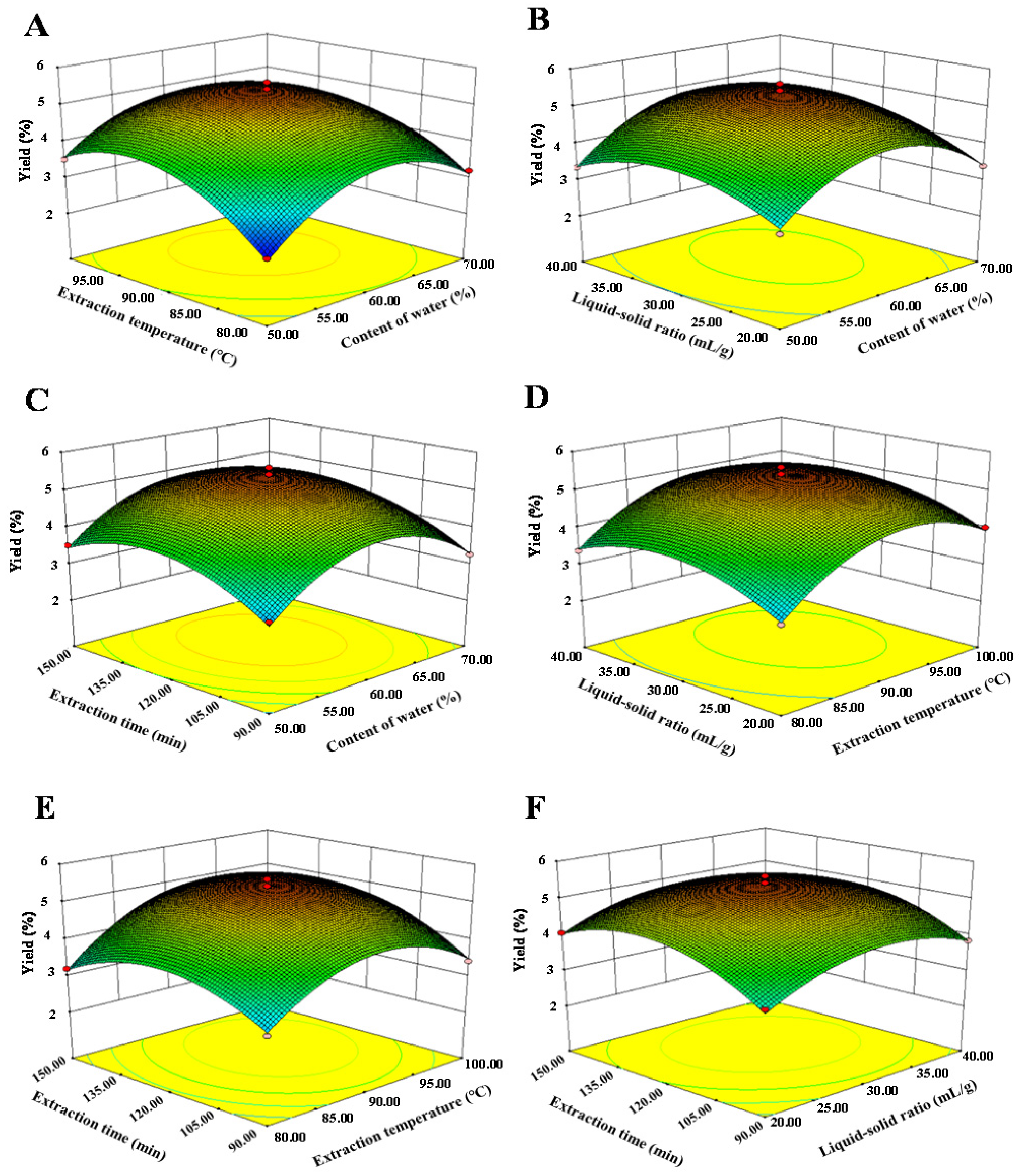
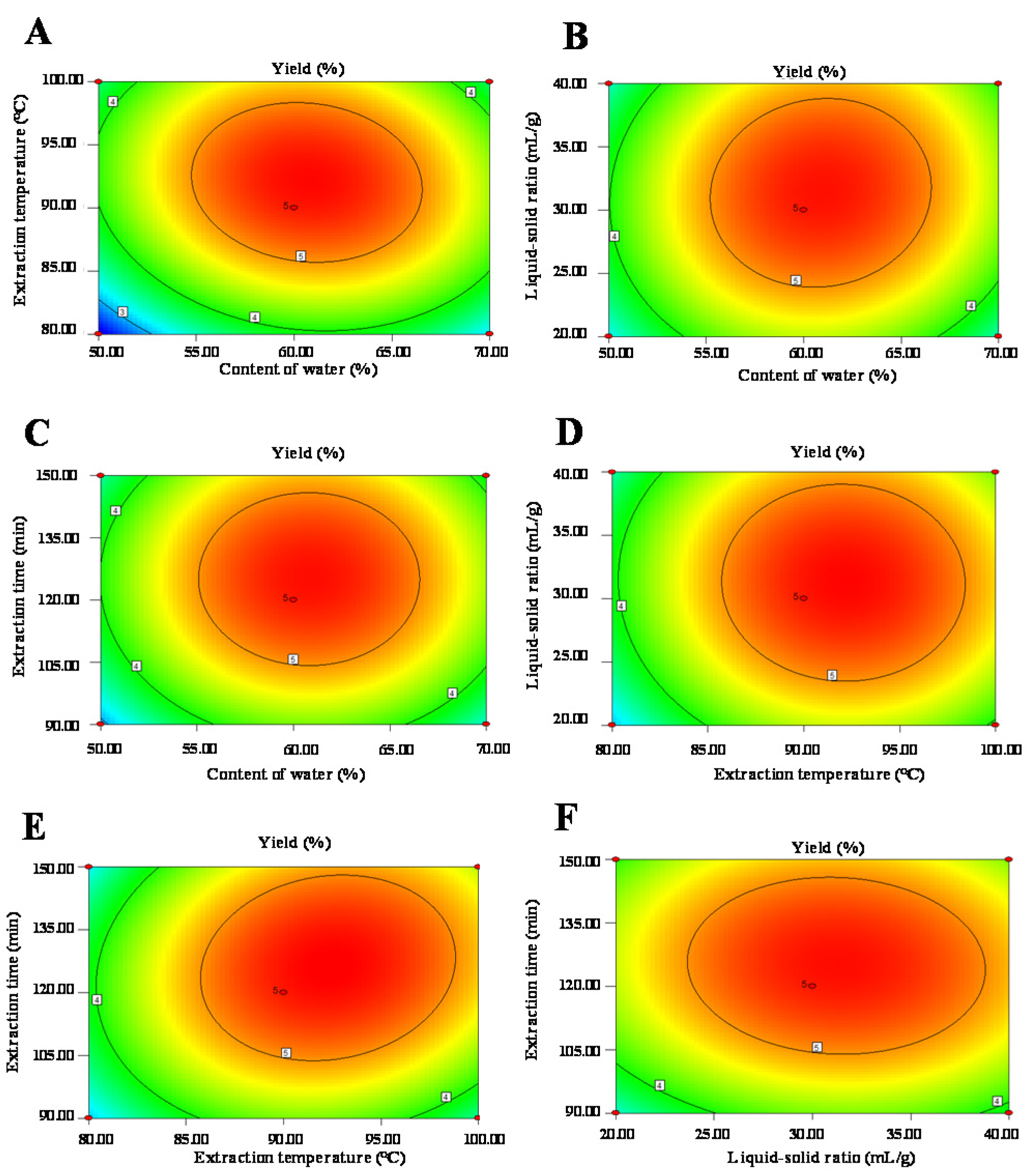
| Runs | Levels of Independent Parameters | Yields (%) | |||
|---|---|---|---|---|---|
| X1 (%) | X2 (°C) | X3 (mL/g) | X4 (min) | ||
| 1 | 0 (60) | −1 (80) | 0 (30) | 1 (150) | 3.20 |
| 2 | 0 (60) | 1 (100) | −1 (20) | 0 (120) | 4.01 |
| 3 | 1 (50) | −1 (80) | 0 (30) | 0 (120) | 2.41 |
| 4 | 1 (70) | 0 (90) | 1 (40) | 0 (120) | 4.06 |
| 5 | 1 (50) | 1 (100) | 0 (30) | 0 (120) | 3.51 |
| 6 | −1 (50) | 0 (90) | −1 (20) | 0 (120) | 3.10 |
| 7 | 0 (60) | 0 (90) | 0 (30) | 0 (120) | 5.40 |
| 8 | 0 (60) | 1 (100) | 0 (30) | 1 (150) | 4.47 |
| 9 | 0 (60) | 0 (90) | −1 (20) | 1 (150) | 4.06 |
| 10 | 0 (60) | −1 (80) | 0 (30) | −1 (90) | 2.96 |
| 11 | 1 (70) | 0 (90) | 0 (30) | −1 (90) | 3.27 |
| 12 | 0 (60) | 0 (90) | 0 (30) | 0 (120) | 5.27 |
| 13 | −1 (50) | 0 (90) | 1 (40) | 0 (120) | 3.35 |
| 14 | 0 (60) | 0 (90) | 0 (30) | 0 (120) | 5.32 |
| 15 | 0 (60) | 1 (100) | 1 (40) | 0 (120) | 4.31 |
| 16 | 0 (60) | −1 (80) | −1 (20) | 0 (120) | 2.97 |
| 17 | −1 (50) | 0 (90) | 0 (30) | −1 (90) | 3.01 |
| 18 | 1 (70) | −1 (80) | 0 (30) | 0 (120) | 3.20 |
| 19 | 0 (60) | 0 (90) | 1 (40) | −1 (90) | 3.84 |
| 20 | 0 (60) | 0 (90) | 0 (30) | 0 (120) | 5.38 |
| 21 | 0 (60) | 0 (90) | 0 (30) | 0 (120) | 5.42 |
| 22 | −1 (50) | 0 (90) | 0 (30) | 1 (150) | 3.52 |
| 23 | 0 (60) | 0 (90) | −1 (20) | −1 (90) | 3.46 |
| 24 | 0 (60) | −1 (80) | 1 (40) | 0 (120) | 3.38 |
| 25 | 1 (70) | 0 (90) | 0 (30) | 1 (150) | 3.76 |
| 26 | 0 (60) | 1 (100) | 0 (30) | −1 (90) | 3.41 |
| 27 | 1 (70) | 0 (90) | −1 (20) | 0 (120) | 3.39 |
| 28 | 0 (60) | 0 (90) | 1 (40) | 1 (150) | 4.17 |
| 29 | 1 (70) | 1 (100) | 0 (30) | 0 (120) | 3.35 |
| Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|
| Model | 19.51 | 14 | 1.39 | 147.7 | <0.0001 ** |
| X1 | 0.44 | 1 | 0.44 | 47.13 | <0.0001 ** |
| X2 | 2.18 | 1 | 2.18 | 231.54 | <0.0001 ** |
| X3 | 0.37 | 1 | 0.37 | 39.70 | <0.0001 ** |
| X4 | 0.87 | 1 | 0.87 | 92.15 | <0.0001 ** |
| X1X2 | 0.15 | 1 | 0.15 | 15.71 | 0.0014 ** |
| X1X3 | 0.044 | 1 | 0.044 | 4.67 | 0.0484 * |
| X1X4 | 1.000 × 10−4 | 1 | 1.000 × 10−4 | 0.011 | 0.9195 |
| X2 X3 | 3.025 × 10−3 | 1 | 3.025 × 10−3 | 0.32 | 0.5802 |
| X2X4 | 0.17 | 1 | 0.17 | 17.82 | 0.0009 ** |
| X3X4 | 0.018 | 1 | 0.018 | 1.93 | 0.1863 |
| X12 | 9.03 | 1 | 9.03 | 957.01 | <0.0001 ** |
| X22 | 6.80 | 1 | 6.80 | 720.31 | <0.0001 ** |
| X32 | 3.01 | 1 | 3.01 | 318.91 | <0.0001 ** |
| X42 | 4.18 | 1 | 4.18 | 442.57 | <0.0001 ** |
| Residual | 0.13 | 14 | 9.435 × 10−3 | ||
| Lack of fit | 0.12 | 10 | 0.012 | 3.06 | 0.1464 |
| Pure error | 0.1464 | 4 | 3.820 × 10−3 | ||
| Correlation total | 19.64 | 28 |
| LLP-W | LLP-D | |
|---|---|---|
| Yields and chemical compositions | ||
| Extraction yield (%) | 3.22 ± 0.16 b | 5.38 ± 0.11 a |
| Total polysaccharides (%) | 77.94 ± 0.48 b | 82.10 ± 1.29 a |
| Total uronic acids (%) | 22.98 ± 1.15 b | 39.96 ± 0.32 a |
| Total proteins (%) | 3.39 ± 0.39 b | 5.97 ± 0.52 a |
| Degree of esterification (%) | 16.46 ± 0.08 b | 28.92 ± 0.77 a |
| Mw× 104 (Da) | ||
| Peak 1 (15 min to 20 min) | 12.90 ± 0.09 | - |
| Peak 2 (20 min to 21 min) | 4.70 ± 0.07 | - |
| Peak 3 (21 min to 22 min) | 3.43 ± 0.09 | - |
| Peak 4 (17 min to 21 min) | - | 4.03 ± 0.07 |
| Monosaccharides and molar ratios | ||
| Galactose | 1.00 | 1.00 |
| Galacturonic acid | 0.70 | 1.36 |
| Arabinose | 0.64 | 0.70 |
| Rhamnose | 0.21 | 0.27 |
| Mannose | 0.26 | 0.10 |
| Glucose | 0.22 | 0.19 |
| Glucuronic acid | 0.21 | 0.18 |
| Xylose | trace | trace |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.-T.; Feng, K.-L.; Huang, L.; Gan, R.-Y.; Hu, Y.-C.; Zou, L. Deep Eutectic Solvent-Assisted Extraction, Partially Structural Characterization, and Bioactivities of Acidic Polysaccharides from Lotus Leaves. Foods 2021, 10, 2330. https://doi.org/10.3390/foods10102330
Wu D-T, Feng K-L, Huang L, Gan R-Y, Hu Y-C, Zou L. Deep Eutectic Solvent-Assisted Extraction, Partially Structural Characterization, and Bioactivities of Acidic Polysaccharides from Lotus Leaves. Foods. 2021; 10(10):2330. https://doi.org/10.3390/foods10102330
Chicago/Turabian StyleWu, Ding-Tao, Kang-Lin Feng, Ling Huang, Ren-You Gan, Yi-Chen Hu, and Liang Zou. 2021. "Deep Eutectic Solvent-Assisted Extraction, Partially Structural Characterization, and Bioactivities of Acidic Polysaccharides from Lotus Leaves" Foods 10, no. 10: 2330. https://doi.org/10.3390/foods10102330
APA StyleWu, D.-T., Feng, K.-L., Huang, L., Gan, R.-Y., Hu, Y.-C., & Zou, L. (2021). Deep Eutectic Solvent-Assisted Extraction, Partially Structural Characterization, and Bioactivities of Acidic Polysaccharides from Lotus Leaves. Foods, 10(10), 2330. https://doi.org/10.3390/foods10102330









