In Vitro Study of Cricket Chitosan’s Potential as a Prebiotic and a Promoter of Probiotic Microorganisms to Control Pathogenic Bacteria in the Human Gut
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Chitosan
2.2. Extraction of Chitin and Chitosan
2.2.1. Demineralisation
2.2.2. Deproteinisation
2.2.3. Deacetylation
2.3. Structural Characterisation of Chitin
2.4. Antibacterial Activity and Mechanism of Chitosan
2.4.1. Microorganisms and Cultivation
2.4.2. Antibacterial Assessment
2.4.3. Chitosan Inhibition of S. typhi Growth
2.5. Data Analysis
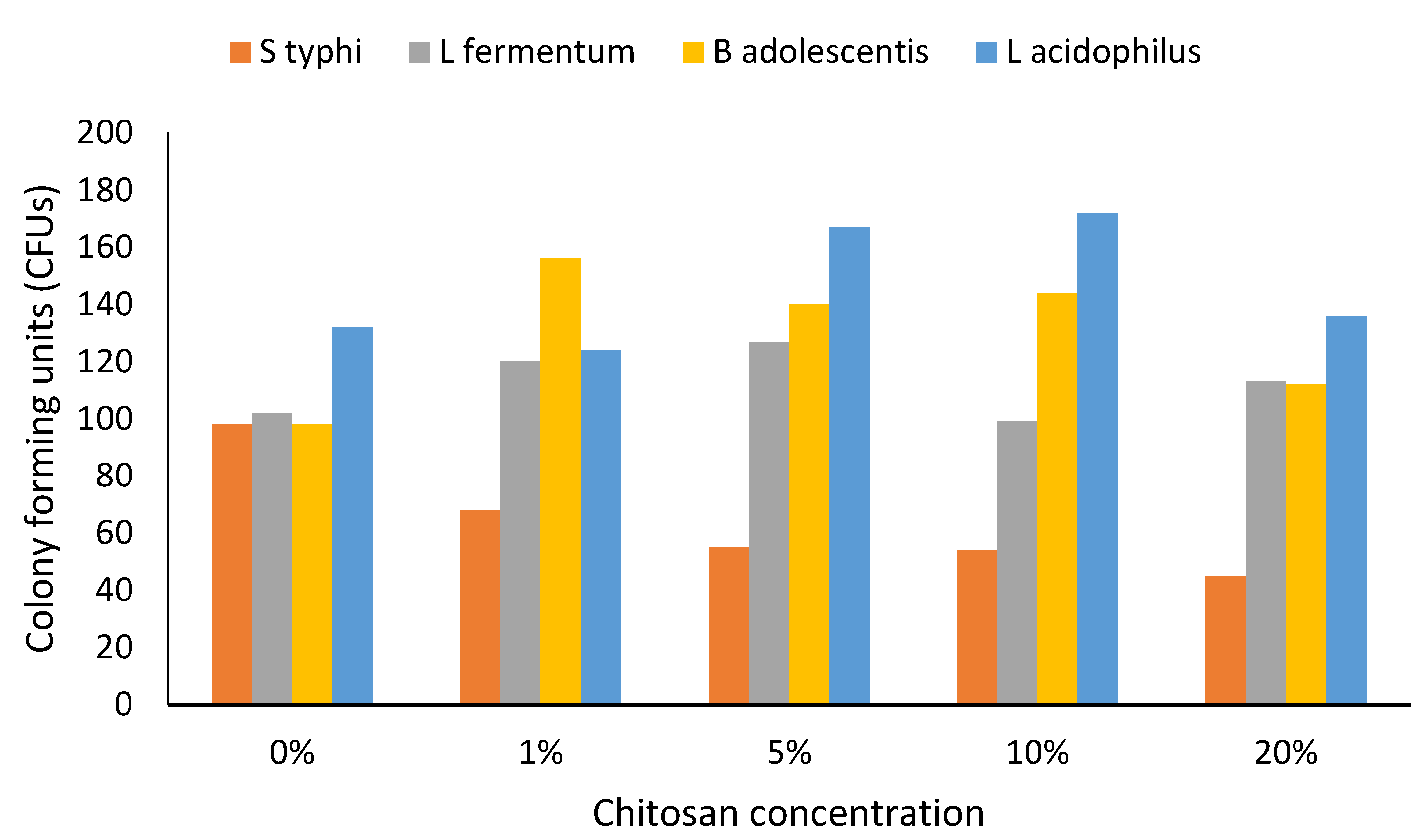
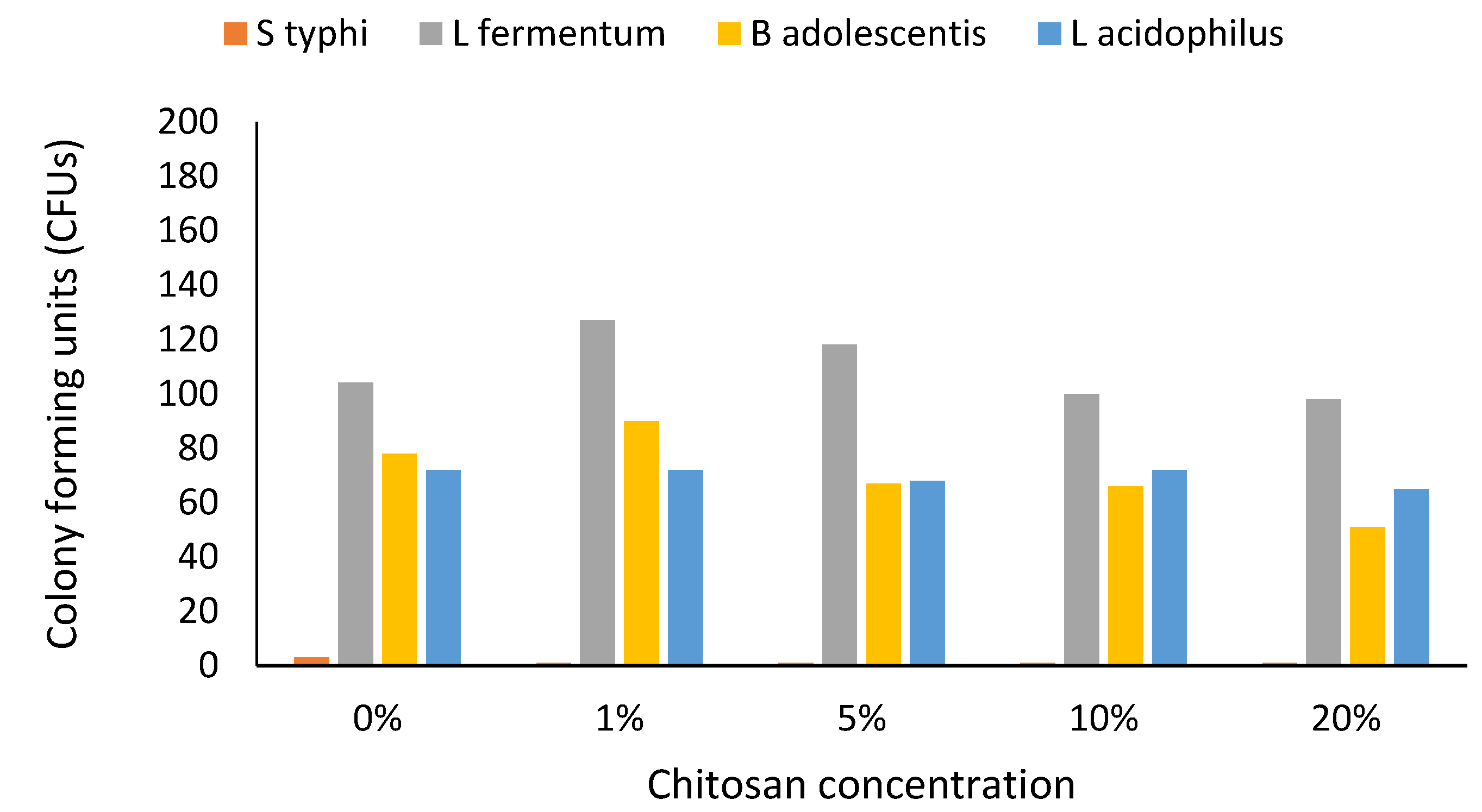
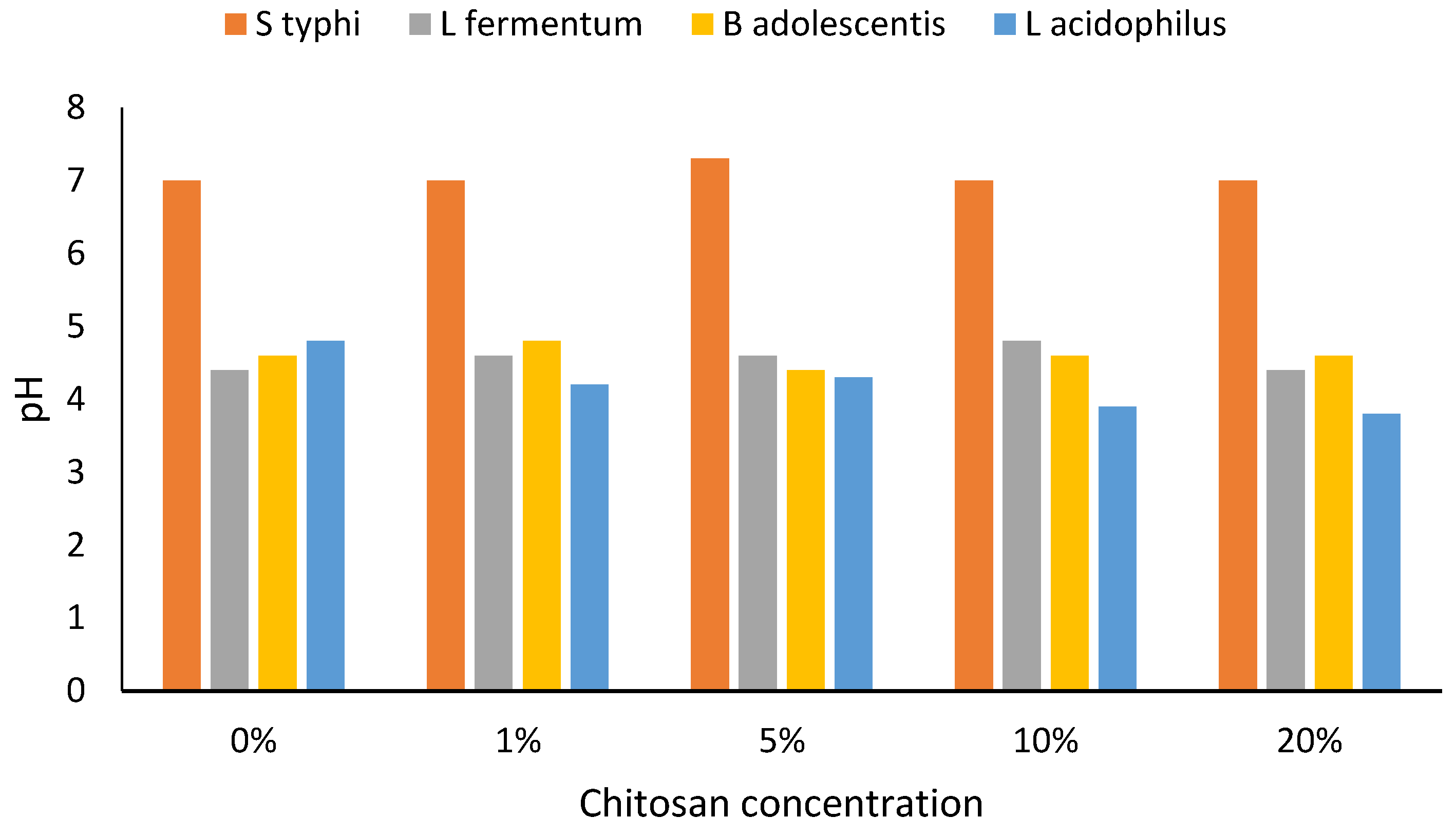
3. Results
4. Discussion
4.1. Chitosan and Chitin Characteristics
4.2. Interactions
4.3. Prebiotics and Gut Health
4.4. Altered Growth and Limitations
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meyer-Rochow, V.B.; Sampat, G.; Jung, C. Farming of insects for food and feed in South Korea: Tradition and innovation. Berl. und Münchener Tierärztliche Wochenschr. 2019, 132, 236–244. [Google Scholar]
- Di Gioia, D.; Biavati, B. Probiotics and Prebiotics in Animal Health and Food Safety: Conclusive Remarks and Future Perspectives, in Probiotics and Prebiotics in Animal Health and Food Safety; Springer: Berlin/Heidelberg, Germany, 2018; pp. 269–273. [Google Scholar]
- Seabrooks, L.; Hu, L. Insects: An underrepresented resource for the discovery of biologically active natural products. Acta Pharm. Sin. B 2017, 7, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, M.G.; Norberto, L.; Damini, R.; Musumeci, S. Human gastric juice contains chitinase that can degrade chitin. Ann. Nutr. Metab. 2007, 51, 244–251. [Google Scholar] [CrossRef]
- Tian, B.; Liu, Y. Chitosan-based biomaterials: From discovery to food application. Polym. Adv. Technol. 2020, 31, 2408–2421. [Google Scholar] [CrossRef]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; Gandía, M.D.L.L.; Caballero, A.H. Cosmetics and cosmeceutical applications of chitin, chitosan and their derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural modification, biological activity and application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef] [PubMed]
- Ibram, A.; Ionescu, A.-M.; Cadar, E. Comparison of extraction methods of chitin and chitosan from different sources. Eur. J. Med. Nat. Sci. 2021, 5, 44–56. [Google Scholar]
- Mlcek, J.; Borkovcová, M.; Bednářová, M. Biologically active substances of edible insects and their use in agriculture, veterinary and human medicine. J. Cent. Eur. Agric. 2014, 15, 225–237. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Vogt, S.L.; Finlay, B.B. Gut microbiota-mediated protection against diarrheal infections. J. Travel Med. 2017, 24, S39–S43. [Google Scholar] [CrossRef]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Eskandari, M.H.; Djafarian, K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin. Nutr. 2019, 38, 522–528. [Google Scholar] [CrossRef]
- Stull, V.J.; Finer, E.; Bergmans, R.S.; Febvre, H.P.; Longhurst, C.; Manter, D.K.; Patz, J.A.; Weir, T.L. Impact of edible cricket consumption on gut microbiota in healthy adults, a double-blind, randomized crossover trial. Sci. Rep. 2018, 8, 10762. [Google Scholar] [CrossRef] [Green Version]
- Radke, M.; Picaud, J.-C.; Loui, A.; Cambonie, G.; Faas, D.; Lafeber, H.N.; De Groot, N.; Pecquet, S.S.; Steenhout, P.G.; Hascoet, J.-M. Starter formula enriched in prebiotics and probiotics ensures normal growth of infants and promotes gut health: A randomized clinical trial. Pediatr. Res. 2017, 81, 622–631. [Google Scholar] [CrossRef] [Green Version]
- Magara, H.; Tanga, C.; Ayieko, M.; Hugel, S.; Mohamed, S.A.; Khamis, F.; Salifu, D.; Niassy, S.; Subramanian, S.; Fiaboe, K.K.M.; et al. Performance of newly described native edible cricket Scapsipedus icipe (Orthoptera: Gryllidae) on various diets of relevance for farming. J. Econ. Entomol. 2019, 112, 653–664. [Google Scholar] [CrossRef]
- Sørensen, J.; Loeschcke, V. Larval crowding in Drosophila melanogaster induces Hsp70 expression, and leads to increased adult longevity and adult thermal stress resistance. J. Insect Physiol. 2001, 47, 1301–1307. [Google Scholar] [CrossRef]
- Otieno, M.H.J.; Ayieko, M.A.; Niassy, S.; Salifu, D.; Abdelmutalab, A.G.A.; Fathiya, K.M.; Subramanian, S.; Fiaboe, K.K.M.; Roos, N.; Ekesi, S.; et al. Integrating temperature-dependent lifetable data into insect life cycle model for predicting the potential distribution of Scapsipedus icipe Hugel & Tanga. PLoS ONE 2019, 14, e0222941. [Google Scholar] [CrossRef]
- Ryan, M.P.; Rea, M.C.; Hill, C.; Ross, R. An application in cheddar cheese manufacture for a strain of Lactococcus lactis producing a novel broad-spectrum bacteriocin, lacticin 3147. Appl. Environ. Microbiol. 1996, 62, 612–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goy, R.C.; Morais, S.T.; Assis, O.B. Evaluation of the antimicrobial activity of chitosan and its quaternized derivative on E. coli and S. aureus growth. Rev. Bras. Farm. 2016, 26, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Motulsky, H. The GraphPad Guide to Comparing Dose-Response or Kinetic Curves; GraphPad Software: San Diego, CA, USA, 1998. [Google Scholar]
- Atay, H.Y. Antibacterial activity of chitosan-based systems. Funct. Chitosan 2019, March 6, 457–489. [Google Scholar] [CrossRef]
- Martău, G.A.; Mihai, M.; Vodnar, D.C. The use of chitosan, alginate, and pectin in the biomedical and food sector—biocompatibility, bioadhesiveness, and biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, M.; Vivekanand, V.; Pareek, N. Insect Chitin and Chitosan: Structure, Properties, Production, and Implementation Prospective, in Natural Materials and Products from Insects: Chemistry and Applications; Springer: Cham, Switzerland, 2020; pp. 51–66. [Google Scholar]
- Zainol Abidin, N.A.; Kormin, F.; Zainol Abidin, N.A.; Mohamed Anuar, N.A.F.; Abu Bakar, M.F. The potential of insects as alternative sources of chitin: An overview on the chemical method of extraction from various sources. Int. J. Mol. Sci. 2020, 21, 4978. [Google Scholar] [CrossRef]
- Lee, H.; Zandkarimi, F.; Zhang, Y.; Meena, J.K.; Kim, J.; Zhuang, L.; Tyagi, S.; Ma, L.; Westbrook, T.F.; Steinberg, G.R.; et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat. Cell Biol. 2020, 22, 225–234. [Google Scholar] [CrossRef]
- Anal, A.K.; Singh, H. Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends Food Sci. Technol. 2007, 18, 240–251. [Google Scholar] [CrossRef]
- Sawicki, C.M.; Livingston, K.A.; Obin, M.; Roberts, S.B.; Chung, M.; McKeown, N.M. Dietary fiber and the human gut microbiota: Application of evidence mapping methodology. Nutrients 2017, 9, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarett, J.K.; Carlson, A.; Serao, M.R.; Strickland, J.; Serfilippi, L.; Ganz, H.H. Diets with and without edible cricket support a similar level of diversity in the gut microbiome of dogs. PeerJ 2019, 7, e7661. [Google Scholar] [CrossRef] [PubMed]
- Marzorati, M.; Maquet, V.; Possemiers, S. Fate of chitin-glucan in the human gastrointestinal tract as studied in a dynamic gut simulator (SHIME®). J. Funct. Foods 2017, 30, 313–320. [Google Scholar] [CrossRef]
- Buruiana, C.-T.; Gómez, B.; Vizireanu, C.; Garrote, G. Manufacture and evaluation of xylo-oligosaccharides from corn stover as emerging prebiotic candidates for human health. LWT 2017, 77, 449–459. [Google Scholar] [CrossRef]
- Álvarez-Ordóñez, A.; Begley, M.; Prieto, M.; Messens, W.; López, M.; Bernardo, A.; Hill, C. Salmonella spp. survival strategies within the host gastrointestinal tract. Microbiology 2011, 157, 3268–3281. [Google Scholar] [CrossRef] [Green Version]
- Bäumler, A.J.; Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 2016, 535, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Satokari, R. Modulation of gut microbiota for health by current and next-generation probiotics. Nutrients 2019, 11, 1921. [Google Scholar] [CrossRef] [Green Version]
- Díaz, L.D.; Fernández-Ruiz, V.; Cámara, M. An international regulatory review of food health-related claims in functional food products labeling. J. Funct. Foods 2020, 68, 103896. [Google Scholar] [CrossRef]
- Dong, L.; Ariëns, R.M.C.; Tomassen, M.M.; Wichers, H.J.; Govers, C. In vitro studies toward the use of chitin as nutraceutical: Impact on the intestinal epithelium, macrophages, and microbiota. Mol. Nutr. Food Res. 2020, 64, e2000324. [Google Scholar] [CrossRef] [PubMed]
- Prodeus, A.; Niborski, V.; Schrezenmeir, J.; Gorelov, A.; Shcherbina, A.; Rumyantsev, A. Fermented milk consumption and common infections in children attending day-care centers: A randomized trial. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 534–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slomka, V.; Herrero, E.R.; Boon, N.; Bernaerts, K.; Trivedi, H.M.; Daep, C. Oral prebiotics and the influence of environmental conditions in vitro. J. Peridontol. 2018, 89, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Platts-Mills, J.A.; Liu, J.; Rogawski, E.T.; Kabir, F.; Lertsethtakarn, P.; Siguas, M.; Khan, S.S.; Praharaj, I.; Murei, A.; Nshama, R.; et al. Use of quantitative molecular diagnostic methods to assess the aetiology, burden, and clinical characteristics of diarrhoea in children in low-resource settings: A reanalysis of the MAL-ED cohort study. Lancet Glob. Health 2018, 6, e1309–e1318. [Google Scholar] [CrossRef] [Green Version]
- Goy, R.C.; de Britto, D.; Assis, O.B.G. A review of the antimicrobial activity of chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Vasiljevic, T.; Shah, N.P. Fermented milk: Health benefits beyond probiotic effect. In Handbook of Food Products Manufacturing; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; Volume 2, pp. 99–115. [Google Scholar]
- Su, Z.; Han, Q.; Zhang, F.; Meng, X.; Liu, B. Preparation, characterization and antibacterial properties of 6-deoxy-6-arginine modified chitosan. Carbohydr. Polym. 2020, 230, 115635. [Google Scholar] [CrossRef]
- Yang, T.-C.; Chou, C.-C.; Li, C.-F. Antibacterial activity of N-alkylated disaccharide chitosan derivatives. Int. J. Food Microbiol. 2005, 97, 237–245. [Google Scholar] [CrossRef]
- Singh, R.; Mishra, N.K.; Kumar, V.; Vinayak, V.; Joshi, K.B. Transition metal ion-mediated tyrosine-based short-peptide amphiphile nanostructures inhibit bacterial growth. ChemBioChem 2018, 19, 1630–1637. [Google Scholar] [CrossRef]
- Hojsak, I. Probiotics in functional gastrointestinal disorders. Adv. Exp. Med. Biol. 2019, 1125, 121–137. [Google Scholar]
- Vandenplas, Y.; Savino, F. Probiotics and prebiotics in pediatrics: What is new? Nutrients 2019, 11, 431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galdeano, C.M.; Cazorla, S.I.; Dumit, J.M.L.; Vélez, E.; Perdigón, G. Beneficial effects of probiotic consumption on the immune system. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar]
- Jeong, C.-W.; Choi, H.-J.; Yoo, G.-Y.; Lee, S.-H.; Kim, Y.-C.; Okorie, O.E.; Lee, J.-H.; Jun, K.-D.; Choi, S.-M.; Kim, K.-W.; et al. Effects of dietary probiotics supplementation on juvenile Olive Flounder Paralichthys olivaceus. Korean J. Fish. Aquat. Sci. 2006, 39, 460–465. [Google Scholar]
- Moreno-Vásquez, M.J.; Valenzuela-Buitimea, E.L.; Plascencia-Jatomea, M.; Encinas, J.C.; Rodríguez-Félix, F.; Sánchez-Valdes, S.; Rosas-Burgos, E.; Ocaño-Higuera, V.M.; Graciano-Verdugo, A.Z. Functionalization of chitosan by a free radical reaction: Characterization, antioxidant and antibacterial potential. Carbohydr. Polym. 2017, 155, 117–127. [Google Scholar] [CrossRef]
- Cremar, L.; Gutierrez, J.; Martinez, J.; Materon, L.; Gilkerson, R.; Xu, F.; Lozano, K. Development of antimicrobial chitosan based nanofiber dressings for wound healing applications. Nanomed. J. 2018, 5, 6–14. [Google Scholar]
- Abdelmalek, B.E.; Sila, A.; Haddar, A.; Bougatef, A.; Ayadi, M.A. β-Chitin and chitosan from squid gladius: Biological activities of chitosan and its application as clarifying agent for apple juice. Int. J. Biol. Macromol. 2017, 104, 953–962. [Google Scholar] [CrossRef]
- Shao, W.; Wu, J.; Ye, S.; Wang, S.; Jiang, L.; Wei, S.; Jimin, W.; Shan, Y.; Shuxia, W.; Lei, J. A facile and green method to prepare chitin based composites with antibacterial activity. J. Bionanosci. 2017, 11, 75–79. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B. Can insects help to ease the probem of world food shrtage? Search 1975, 6, 261–262. [Google Scholar]
- Chien, P.-J.; Sheu, F.; Lin, H.-R. Coating citrus (Murcott tangor) fruit with low molecular weight chitosan increases postharvest quality and shelf life. Food Chem. 2007, 100, 1160–1164. [Google Scholar] [CrossRef]
- Butnaru, E.; Stoleru, E.; Brebu, M.A.; Darie-Nita, R.N.; Bargan, A.; Vasile, C. Chitosan-Based bionanocomposite films prepared by emulsion technique for food preservation. Materials 2019, 12, 373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noorbakhsh-Soltani, S.; Zerafat, M.; Sabbaghi, S. A comparative study of gelatin and starch-based nano-composite films modified by nano-cellulose and chitosan for food packaging applications. Carbohydr. Polym. 2018, 189, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Freepons, D. Enhancing food production with chitosan seed-coating technology. In Applications of Chitin and Chitosan; CRC Press: Boca Raton, FL, USA, 2020; pp. 128–139. [Google Scholar]
- da Costa, J.C.M.; Miki, K.S.L.; da Silva Ramos, A.; Teixeira-Costa, B.E. Development of biodegradable films based on purple yam starch/chitosan for food application. Heliyon 2020, 6, e03718. [Google Scholar] [CrossRef]
- Piotrowska, B. Toxic Components of Food Packaging Materials; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Kritchenkov, A.S.; Egorov, A.; Yagafarov, N.Z.; Volkova, O.V.; Zabodalova, L.A.; Suchkova, E.P.; Kurliuk, A.V.; Khrustalev, V. Efficient reinforcement of chitosan-based coatings for Ricotta cheese with non-toxic, active, and smart nanoparticles. Prog. Org. Coat. 2020, 145, 105707. [Google Scholar] [CrossRef]
- Călinoiu, L.F.; Vodnar, D.C. Thermal processing for the release of phenolic compounds from wheat and oat bran. Biomolecules 2020, 10, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Bacterial Species | |||
|---|---|---|---|
| Chitosan Concentration | L. fermentum | B. adolescentis | L. acidophilus |
| 0% | 10 ± 0.3 a | 10 ± 0.2 a | 10 ± 0.3 a |
| 1% | 11 ± 0.3 a | 10 ± 0.3 a | 11 ± 0.2 a |
| 5% | 11 ± 0.3 a | 10 ± 0.1 a | 12 ± 0.2 b |
| 10% | 16 ± 0.3 b | 12 ± 0.2 b | 12 ± 0.3 b |
| 20% | 13 ± 0.3 c | 14 ± 0.3 c | 10 ± 0.1 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kipkoech, C.; Kinyuru, J.N.; Imathiu, S.; Meyer-Rochow, V.B.; Roos, N. In Vitro Study of Cricket Chitosan’s Potential as a Prebiotic and a Promoter of Probiotic Microorganisms to Control Pathogenic Bacteria in the Human Gut. Foods 2021, 10, 2310. https://doi.org/10.3390/foods10102310
Kipkoech C, Kinyuru JN, Imathiu S, Meyer-Rochow VB, Roos N. In Vitro Study of Cricket Chitosan’s Potential as a Prebiotic and a Promoter of Probiotic Microorganisms to Control Pathogenic Bacteria in the Human Gut. Foods. 2021; 10(10):2310. https://doi.org/10.3390/foods10102310
Chicago/Turabian StyleKipkoech, Carolyne, John N. Kinyuru, Samuel Imathiu, Victor Benno Meyer-Rochow, and Nanna Roos. 2021. "In Vitro Study of Cricket Chitosan’s Potential as a Prebiotic and a Promoter of Probiotic Microorganisms to Control Pathogenic Bacteria in the Human Gut" Foods 10, no. 10: 2310. https://doi.org/10.3390/foods10102310
APA StyleKipkoech, C., Kinyuru, J. N., Imathiu, S., Meyer-Rochow, V. B., & Roos, N. (2021). In Vitro Study of Cricket Chitosan’s Potential as a Prebiotic and a Promoter of Probiotic Microorganisms to Control Pathogenic Bacteria in the Human Gut. Foods, 10(10), 2310. https://doi.org/10.3390/foods10102310







