A Systematic Comparison of the Intrinsic Properties of Wheat and Oat Bran Fractions and Their Effects on Dough and Bread Properties: Elucidation of Chemical Mechanisms, Water Binding, and Steric Hindrance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Bran Fractions
2.3. Chemical Characterization of Brans
2.3.1. Moisture Content
2.3.2. Proximate Analysis
2.3.3. Total Analysable Carbohydrate Content
2.3.4. β-Glucan Content
2.3.5. Free-, Conjugated-, and Bound Phenolics Content
2.4. Bran Physical Characterization
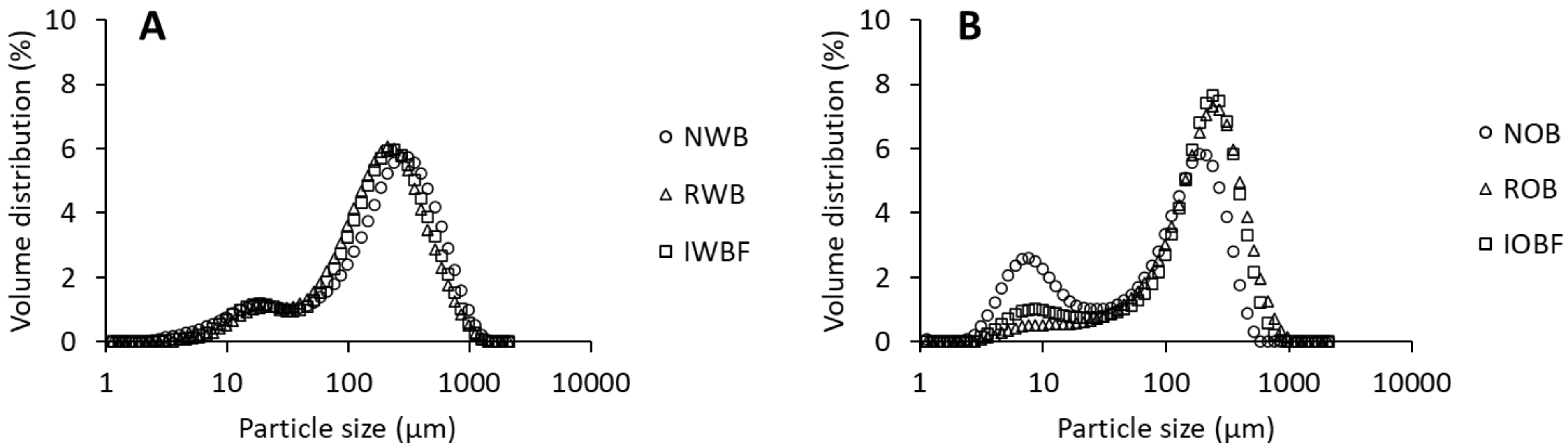
2.4.1. Particle Size Distribution
2.4.2. Water Binding Capacity
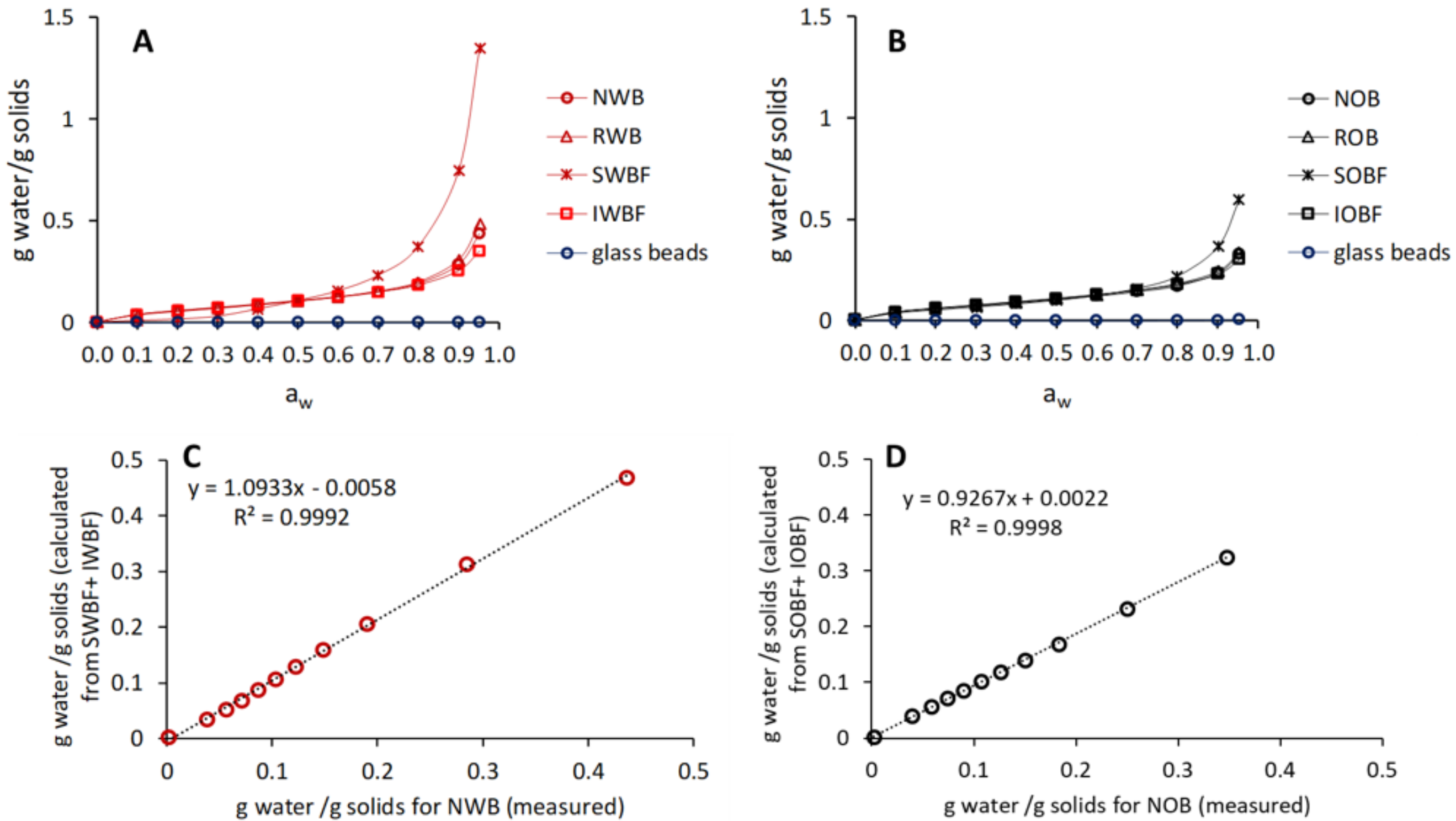
2.4.3. Moisture Sorption Behaviour
2.5. Dough and Bread Preparation and Characterization
2.5.1. Definition of Dough Mixing Conditions
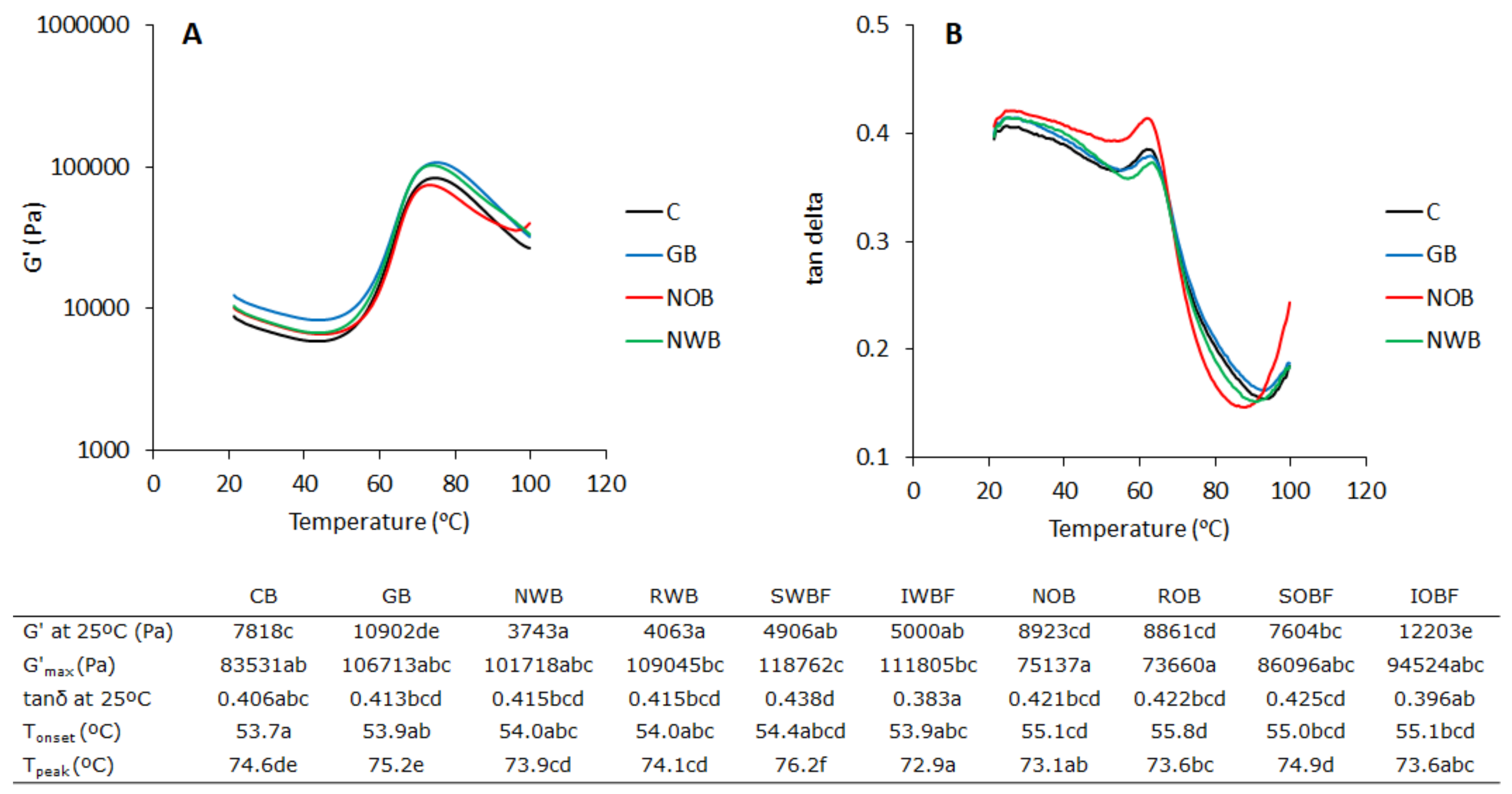
2.5.2. Dynamic Mechanical Thermal Analysis (DMTA) of Dough
2.5.3. Confocal Laser Scanning Microscopy of Dough
2.5.4. Bread Baking and Baking Quality Evaluation
2.5.5. Crumb Texture and Moisture Content
2.6. Statistical Analysis
3. Results
3.1. Chemical Composition and Physical Properties of Brans and Their Fractions
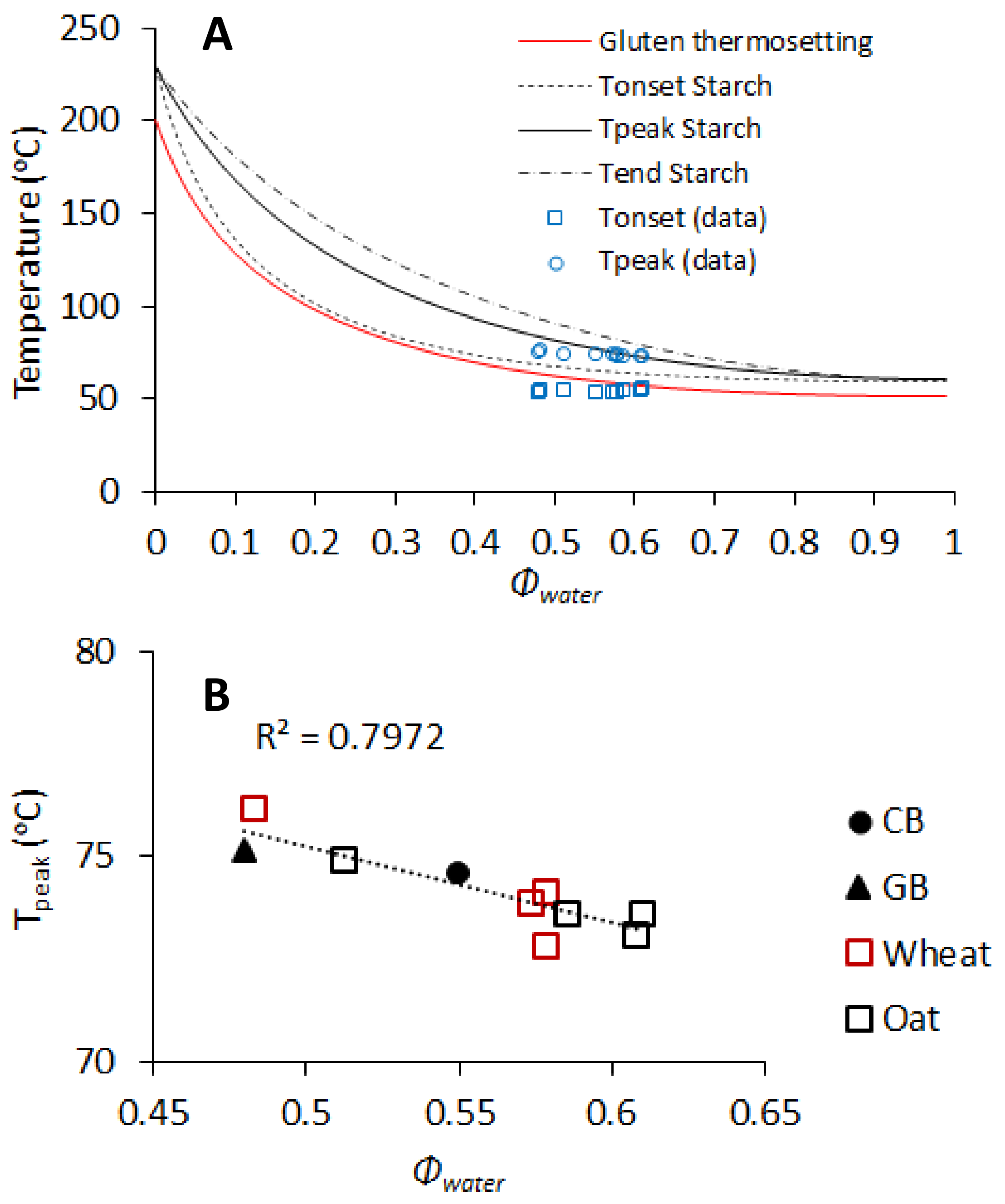
3.2. Dough Thermo-Mechanical Properties during Temperature Sweep
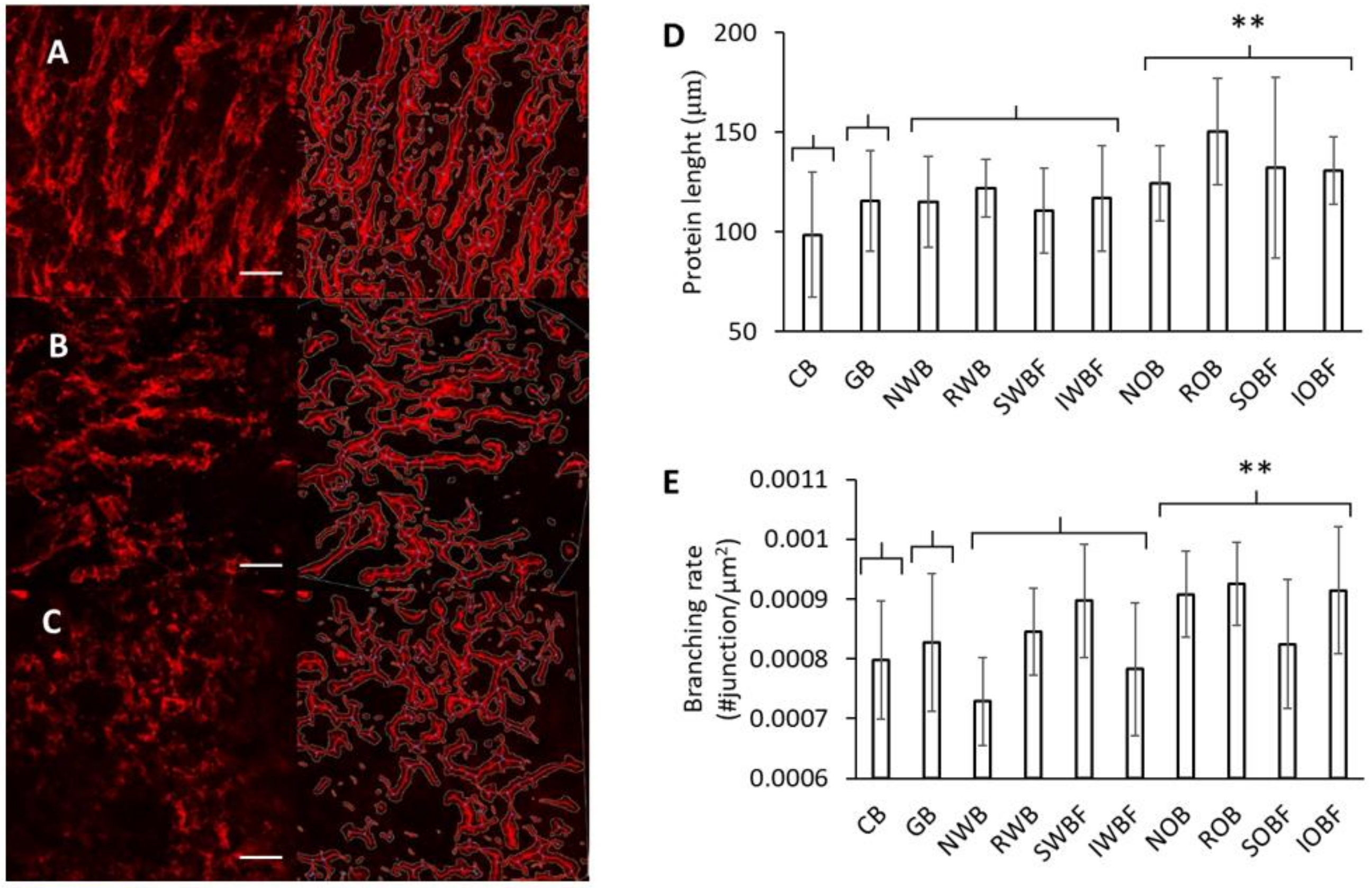
3.3. Protein Network
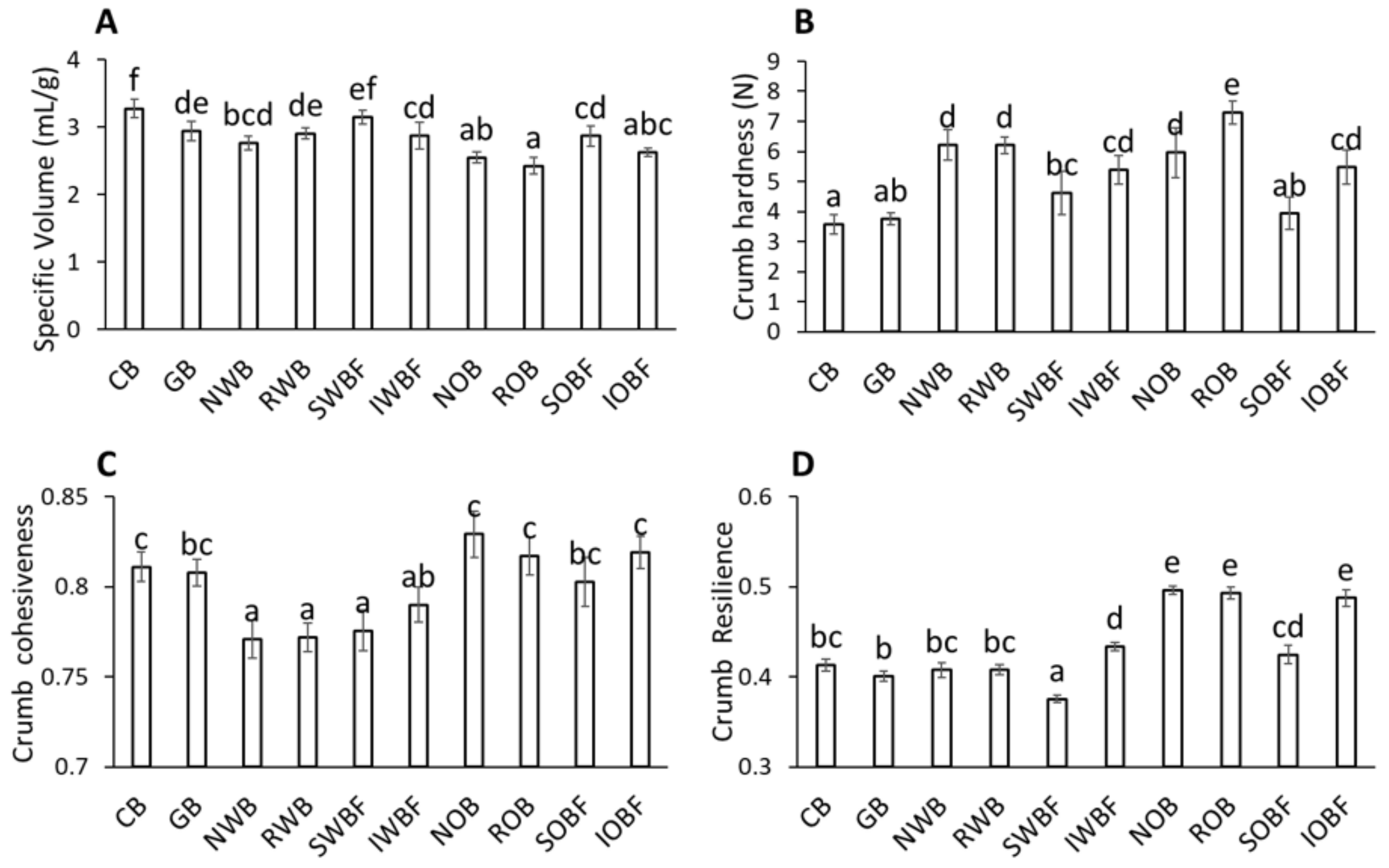
3.4. Bread Quality
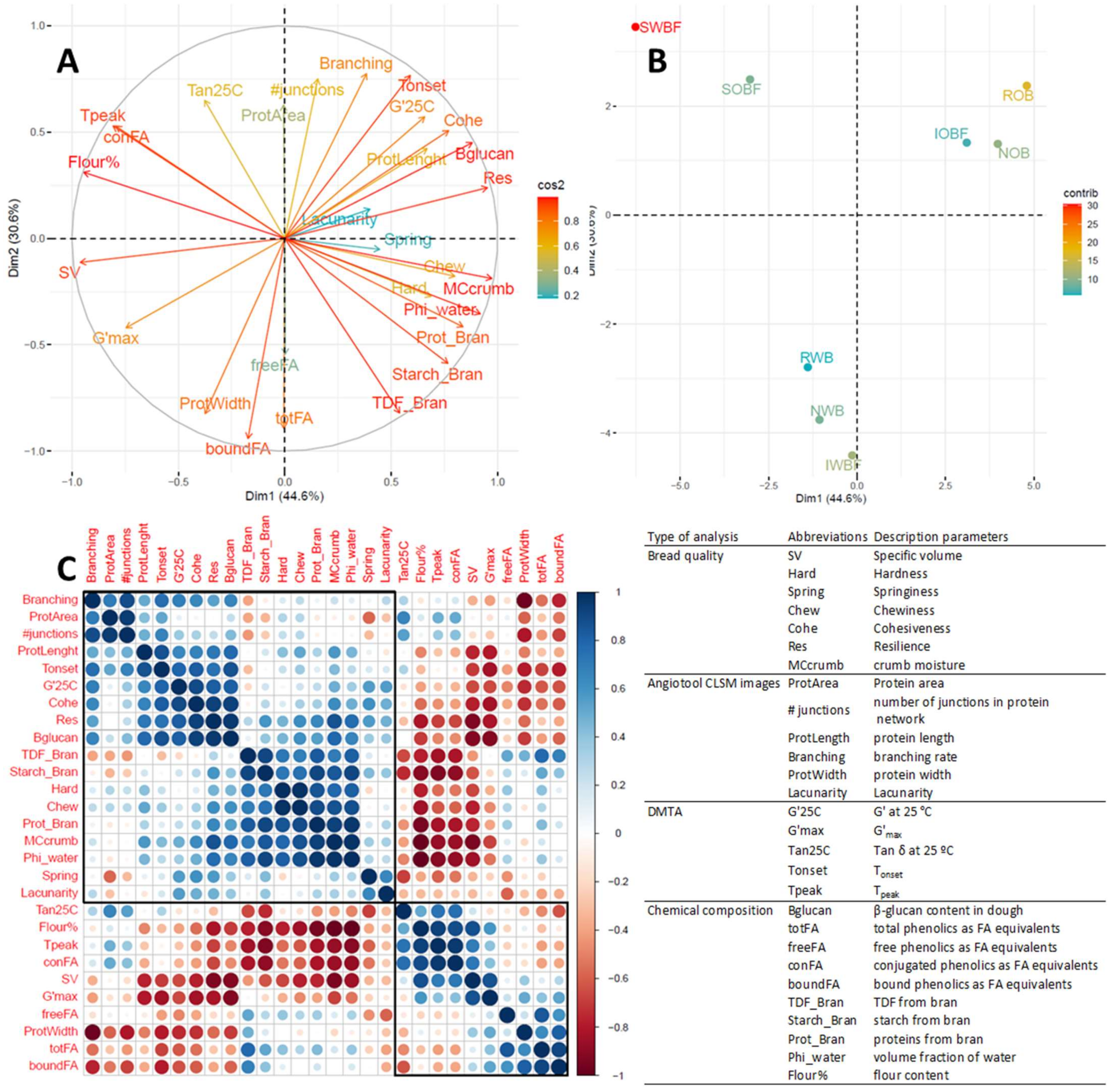
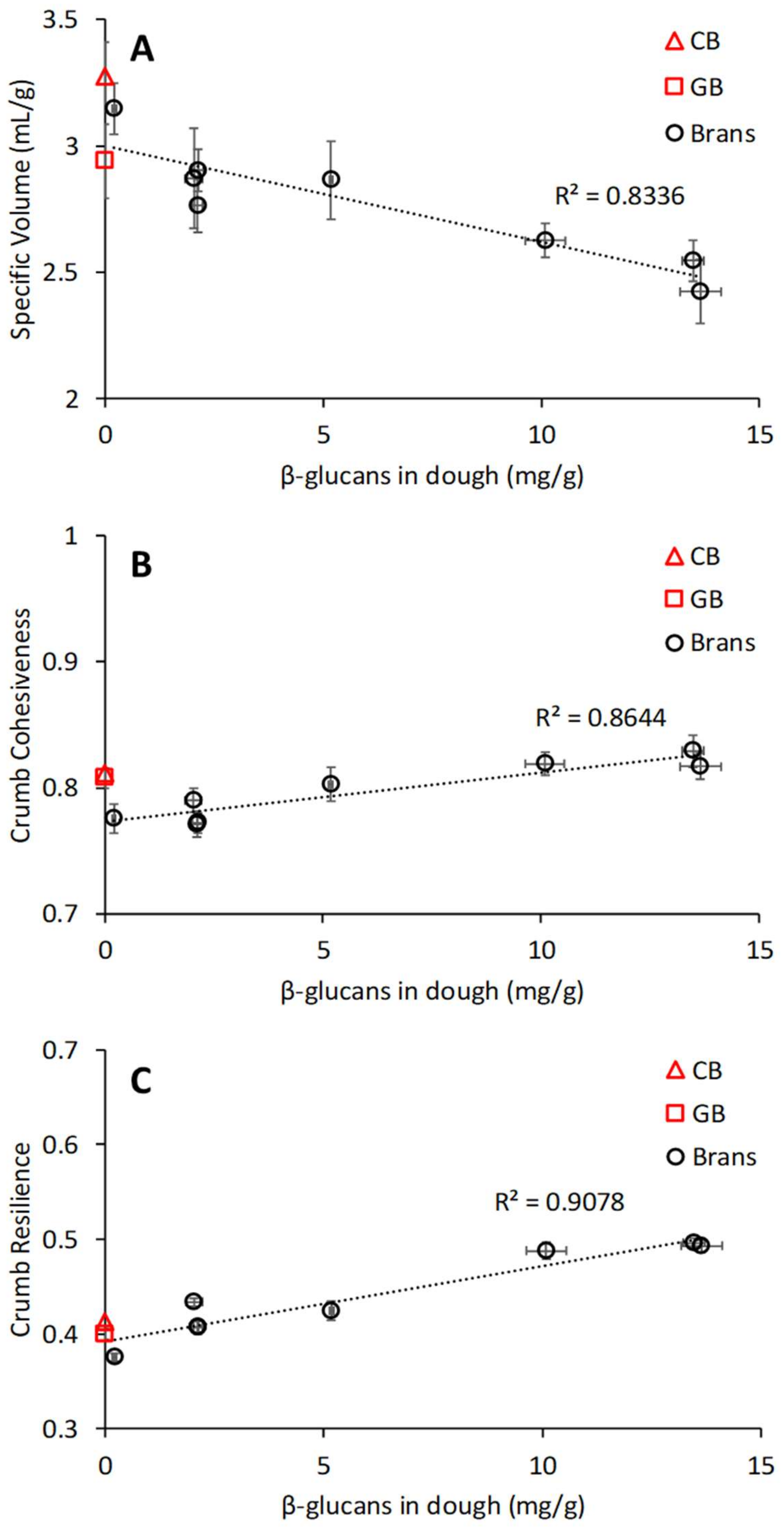
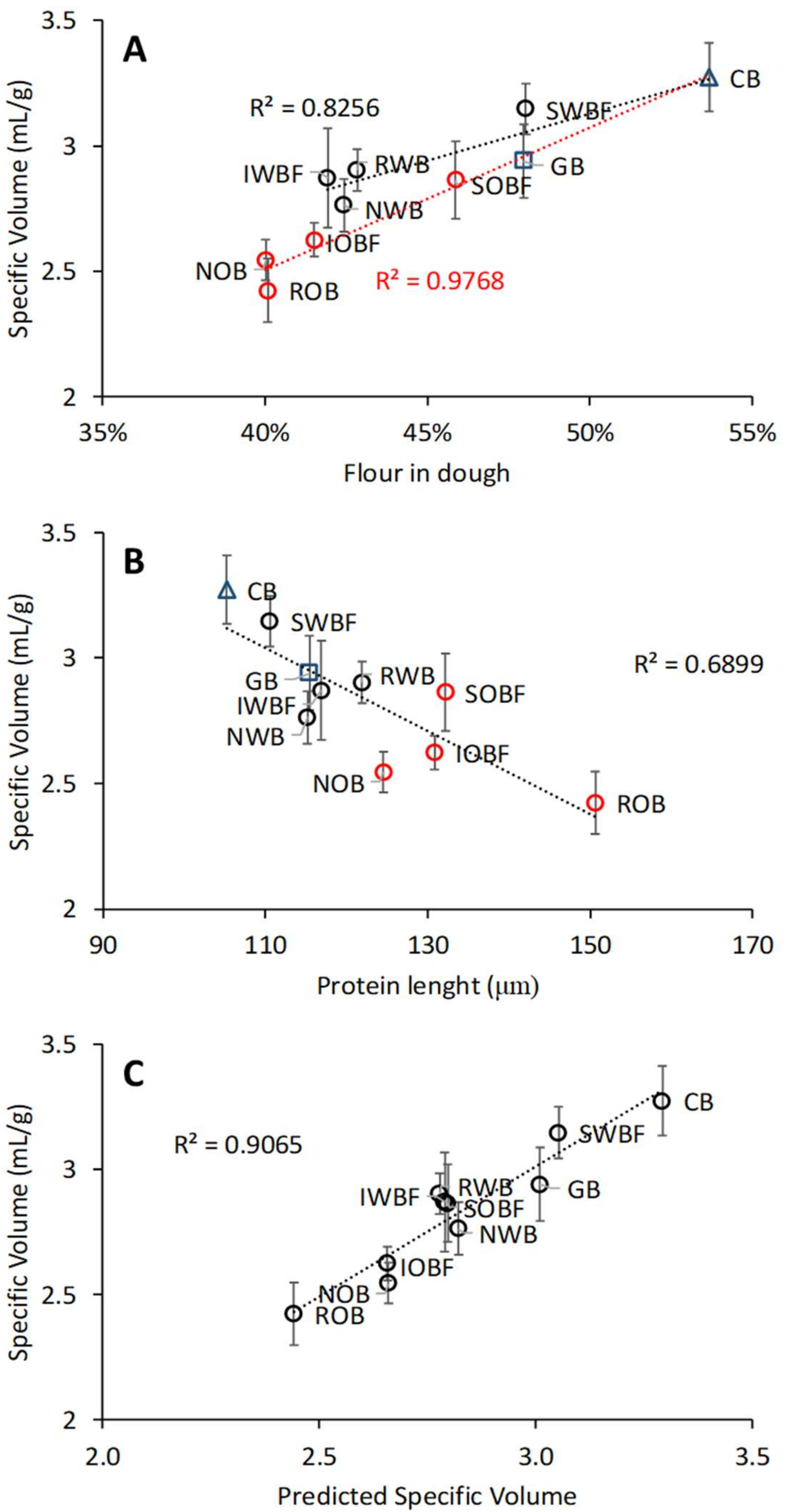
3.5. Relation between Chemical and Physical Properties of Brans, Dough Properties, and Bread Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miller, K.B. Review of whole grain and dietary fiber recommendations and intake levels in different countries. Nutr. Rev. 2020, 78, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Sibakov, J.; Lehtinen, P.; Poutanen, K. Cereal brans as dietary fi bre ingredients. In Fibre-Rich and Wholegrain Foods Improving Quality; Delcour, J.A., Poutanen, K., Eds.; Woodhead Publishing Limited: London, UK, 2013; pp. 170–192. [Google Scholar]
- Robert, W. Nutrient composition and nutritional quality of oats and comparisons with other cereals. In Oats: Chemistry and Technology, 2nd ed.; Webster, H.F., Wood, P.J., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 95–108. [Google Scholar]
- EFSA. Scientific opinion on dietary reference values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1–107. [Google Scholar] [CrossRef] [Green Version]
- Gan, Z.; Galliard, T.; Ellis, P.R.; Angold, R.E.; Vaughan, J.G. Effect of the Outer Bran Layers on the Loaf Volume of Wheat Bread. J. Cereal Sci. Sci. 1992, 15, 151–163. [Google Scholar] [CrossRef]
- Hemdane, S.; Jacobs, P.J.; Dornez, E.; Verspreet, J.; Delcour, J.A.; Courtin, C.M. Making: A Critical Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 28–42. [Google Scholar] [CrossRef] [Green Version]
- Hemdane, S.; Langenaeken, N.A.; Jacobs, P.J.; Verspreet, J.; Delcour, J.A.; Courtin, C.M. Study of the role of bran water binding and the steric hindrance by bran in straight dough bread making. Food Chem. 2018, 253, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Ma, S.; Li, L.; Zheng, X.; Wang, X. Impact of wheat bran dietary fi ber on gluten and gluten-starch microstructure formation in dough. Food Hydrocoll. 2019, 95, 292–297. [Google Scholar] [CrossRef]
- Lai, C.S.; Hoseney, R.C.; Davis, A.B. Effects of Wheat Bran in Breadmaking. Cereal Chem. 1989, 66, 217–219. [Google Scholar]
- Zanoletti, M.; Marti, A.; Marengo, M.; Iametti, S.; Pagani, M.A.; Renzetti, S. Understanding the influence of buckwheat bran on wheat dough baking performance: Mechanistic insights from molecular and material science approaches. Food Res. Int. 2017, 102, 728–737. [Google Scholar] [CrossRef]
- Noort, M.W.J.; van Haaster, D.; Hemery, Y.; Schols, H.A.; Hamer, R.J. The effect of particle size of wheat bran fractions on bread quality—Evidence for fibre-protein interactions. J. Cereal Sci. 2010, 52, 59–64. [Google Scholar] [CrossRef]
- Zhou, Y.; Dhital, S.; Zhao, C.; Ye, F.; Chen, J.; Zhao, G. Dietary fiber-gluten protein interaction in wheat flour dough: Analysis, consequences and proposed mechanisms. Food Hydrocoll. 2021, 111, 106203. [Google Scholar] [CrossRef]
- Renzetti, S.; van den Hoek, I.A.F.; van der Sman, R.G.M. Mechanisms controlling wheat starch gelatinization and pasting behaviour in presence of sugars and sugar replacers: Role of hydrogen bonding and plasticizer molar volume. Food Hydrocoll. 2021, 119, 106880. [Google Scholar] [CrossRef]
- Van der Sman, R.G.M.; Renzetti, S. Understanding functionality of sucrose in biscuits for reformulation purposes. Crit. Rev. Food Sci. Nutr. 2019, 59, 2225–2239. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Hou, C.; Zhao, X.; Tian, M.; Gu, Z.; Yang, R. Molecular characterization of water-extractable arabinoxylan from wheat bran and its effect on the heat-induced polymerization of gluten and steamed bread quality. Food Hydrocoll. 2019, 87, 570–581. [Google Scholar] [CrossRef]
- Renzetti, S.; van den Hoek, I.A.F.; van der Sman, R.G.M. Amino acids, polyols and soluble fibres as sugar replacers in bakery applications: Egg white proteins denaturation controlled by hydrogen bond density of solutions. Food Hydrocoll. 2020, 108, 106034. [Google Scholar] [CrossRef]
- Wang, M.; Oudgenoeg, G.; van Vliet, T.; Hamer, R.J. Interaction of water unextractable solids with gluten protein: Effect on dough properties and gluten quality. J. Cereal Sci. 2003, 38, 95–104. [Google Scholar] [CrossRef]
- Wang, M.; van Vliet, T.; Hamer, R.J. Evidence that pentosans and xylanase affect the re-agglomeration of the gluten network. J. Cereal Sci. 2004, 39, 341–349. [Google Scholar] [CrossRef]
- Wang, M.; van Vliet, T.; Hamer, R.J. How gluten properties are affected by pentosans. J. Cereal Sci. 2004, 39, 395–402. [Google Scholar] [CrossRef]
- Wang, M.; Hamer, R.J.; Van Vliet, T.; Gruppen, H.; Marseille, H.; Weegels, P.L. Effect of Water Unextractable Solids on Gluten Formation and Properties: Mechanistic Considerations. J. Cereal Sci. 2003, 37, 55–64. [Google Scholar] [CrossRef]
- Thebaudin, J.Y.; Lefebvre, A.C.; Harrington, M.; Bourgeois, C.M. Dietary fibres: Nutritional and technological interest. Trends Food Sci. Technol. 1997, 8, 41–48. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, X.; Yang, R.; Zhou, Y.; Zhou, Q.; Gu, Z.; Jiang, D. Water-Extractable Arabinoxylan-Induced Changes in the Conformation and Polymerization Behavior of Gluten upon Thermal Treatment. J. Agric. Food Chem. 2020, 68, 4005–4016. [Google Scholar] [CrossRef]
- Sosulski, F.W.; Wu, K.K. High-Fiber Breads Containing Field Pea Hulls, Wheat, Corn, and Wild Oat Brans. Cereal Chem. 1983, 65, 186–191. [Google Scholar]
- Campbell, G.M.; Martin, P.J.; Centre, S. Bread aeration and dough rheology: An introduction. In Breadmaking: Improving Quality, 2nd ed.; Woodhead Publishing Limited: Cambridge, UK, 2012; pp. 299–336. [Google Scholar]
- Pauly, A.; Delcour, J.A. Impact of water-extractable components from different cereals on the quality of oat bread. J. Cereal Sci. 2018, 79, 134–140. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Saeman, J.F.; Moore, W.E.; Mitchell, R.L.; Millett, M.A. Techniques for the determination of pulp constituents by quantitiative paper chromatography. Tappi J. 1954, 37, 336–343. [Google Scholar]
- Gilbert-lópez, B.; Mendiola, J.A.; Fontecha, J. Downstream processing of Isochrysis galbana: A step towards microalgal biorefi nery. Green Chem. 2015, 17, 4599–4609. [Google Scholar] [CrossRef] [Green Version]
- Erickson, D.P.; Renzetti, S.; Jurgens, A.; Campanella, O.H.; Hamaker, B.R. Modulating state transition and mechanical properties of viscoelastic resins from maize zein through interactions with plasticizers and co-proteins. J. Cereal Sci. 2014, 60, 576–583. [Google Scholar] [CrossRef]
- Cai, L.M.; Choi, I.; Lee, C.K.; Park, K.K.; Baik, B.K. Bran characteristics and bread-baking quality of whole grain wheat flour. Cereal Chem. 2016, 91, 398–405. [Google Scholar] [CrossRef]
- Bernklau, I.; Lucas, L.; Jekle, M.; Becker, T. Protein network analysis—A new approach for quantifying wheat dough microstructure. Food Res. Int. 2016, 89, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Van der Sman, R.G.M.; Renzetti, S. Understanding functionality of sucrose in cake for reformulation purposes. Crit. Rev. Food Sci. Nutr. 2020, 61, 2756–2772. [Google Scholar] [CrossRef]
- Jekle, M.; Mühlberger, K.; Becker, T. Starch-gluten interactions during gelatinization and its functionality in dough like model systems. Food Hydrocoll. 2016, 54, 196–201. [Google Scholar] [CrossRef]
- Xie, F.; Yu, L.; Chen, L.; Li, L. A new study of starch gelatinization under shear stress using dynamic mechanical analysis. Carbohydr. Polym. 2008, 72, 229–234. [Google Scholar] [CrossRef]
- Verbauwhede, A.E.; Lambrecht, M.A.; Jekle, M.; Lucas, I.; Fierens, E.; Shegay, O.; Brijs, K.; Delcour, J.A. Original article Microscopic investigation of the formation of a thermoset wheat gluten network in a model system relevant for bread making. Int. J. Food Sci. Technol. 2020, 55, 891–898. [Google Scholar] [CrossRef]
- Si, X.; Li, T.; Zhang, Y.; Zhang, W.; Qian, H.; Li, Y.; Zhang, H.; Qi, X.; Wang, L. Interactions between gluten and water-unextractable arabinoxylan during the thermal treatment. Food Chem. 2021, 345, 128785. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ye, F.; Li, S.; Wei, F.; Chen, J.; Zhao, G. Wheat flour enriched with oat b-glucan: A study of hydration, rheological and fermentation properties of dough. J. Cereal Sci. 2017, 75, 143–150. [Google Scholar] [CrossRef]
- De Bondt, Y.; Hermans, W.; Moldenaers, P.; Courtin, C.M. Selective modification of wheat bran affects its impact on gluten-starch dough rheology, microstructure and bread volume. Food Hydrocoll. 2021, 113, 106348. [Google Scholar] [CrossRef]
- Krishnan, P.G.; Chang, K.C.; Brown, G. Effect of commercial oat bran on the characteristics and composition of bread. Cereal Chem. 1987, 64, 55–58. [Google Scholar]
- Westerlund, E.; Andersson, R.; Aman, P. Isolation and chemical characterization of water-soluble mixed-linked beta-glucans and arabinoxylans in oat milling fractions. Carbohydr. Polym. 1993, 20, 115–123. [Google Scholar] [CrossRef]
- Wang, J.; Bai, J.; Fan, M.; Li, T.; Li, Y.; Qian, H.; Wang, L.I.; Zhang, H.; Qi, X.; Rao, Z. Cereal-derived arabinoxylans: Structural features and structure—activity correlations. Trends Food Sci. Technol. 2020, 96, 157–165. [Google Scholar] [CrossRef]
- Roye, C.; van de Walle, D.; Courtin, C.M. Side-by-side comparison of composition and structural properties of wheat, rye, oat, and maize bran and their impact on in vitro fermentability. Cereal Chem. 2019, 97, 20–33. [Google Scholar] [CrossRef] [Green Version]
- Hemdane, S.; Langenaeken, N.A.; Jacobs, P.J.; Verspreet, J.; Delcour, J.A.; Courtin, C.M. Study of the intrinsic properties of wheat bran and pearlings obtained by sequential debranning and their role in bran-enriched bread making. J. Cereal Sci. 2016, 71, 78–85. [Google Scholar] [CrossRef]
- Westenbrink, S.; Brunt, K.; van der Kamp, J.W. Dietary fibre: Challenges in production and use of food composition data. Food Chem. 2013, 140, 562–567. [Google Scholar] [CrossRef]
- Aydogdu, A.; Sumnu, G.; Sahin, S. Effects of addition of different fibers on rheological characteristics of cake batter and quality of cakes. J. Food Sci. Technol. 2018, 55, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Muhammad, F.; Zahoor, T.; Nawaz, H.; Ahmed, Z. Extraction and characterization of beta-d-glucan from oat for industrial utilization. Int. J. Biol. Macromol. 2010, 46, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Rieder, A.; Ballance, S.; Knutsen, S.H. Viscosity based quantification of endogenous beta-glucanase activity in flour. Carbohydr. Polym. 2015, 115, 104–111. [Google Scholar] [CrossRef]
- Chen, H.; Rubenthaler, G.L.; Leung, H.K.; Baranowski, J.D. Chemical, Physical, and Baking Properties of Apple Fiber Compared with Wheat and Oat Bran. Cereal Chem. 1988, 65, 244–247. [Google Scholar]
- Netto, F.M.; Desobry, S.A.; Labuza, T.P. Effect of water content on the glass transition, caking and stickiness of protein hydrolysates. Int. J. Food Prop. 1998, 1, 141–161. [Google Scholar] [CrossRef]
- Van der Sman, R.G.M.; Meinders, M.B.J. Prediction of the state diagram of starch water mixtures using the Flory-Huggins free volume theory. Soft Matter 2011, 7, 429–442. [Google Scholar] [CrossRef]
- Renzetti, S.; Voogt, J.A.; Oliver, L.; Meinders, M.B.J. Water migration mechanisms in amorphous powder material and related agglomeration propensity. J. Food Eng. 2012, 110, 160–168. [Google Scholar] [CrossRef]
- Van der Sman, R.G.M. Moisture sorption in mixtures of biopolymer, Disaccharides and water. Food Hydrocoll. 2013, 32, 186–194. [Google Scholar] [CrossRef]
- Jacobs, P.J.; Hemdane, S.; Delcour, J.A.; Courtin, C.M. Dry heat treatment affects wheat bran surface properties and hydration kinetics. Food Chem. 2016, 203, 513–520. [Google Scholar] [CrossRef]
- Weng, L.; Stott, S.L.; Toner, M. Exploring Dynamics and Structure of Biomolecules, Cryoprotectants, and Water Using Molecular Dynamics Simulations: Implications for Biostabilization and Biopreservation. Annu. Rev. Biomed. Eng. 2019, 21, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Van der Sman, R.G.M.; van den Hoek, I.A.F.; Renzetti, S. Sugar replacement with zwitterionic plasticizers like amino acids. Food Hydrocoll. 2020, 109, 106113. [Google Scholar] [CrossRef]
- Verbauwhede, A.E.; Lambrecht, M.A.; Fierens, E.; Hermans, S.; Shegay, O.; Brijs, K.; Delcour, J.A. Thermo-reversible inhibition makes aqualysin 1 from Thermus aquaticus a potent tool for studying the contribution of the wheat gluten network to the crumb texture of fresh bread. Food Chem. 2018, 264, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Heiniö, R.L.; Noort, M.W.; Katina, K.; Alam, S.A.; Sozer, N.; De Kock, H.L.; Hersleth, M.; Poutanen, K. Sensory characteristics of wholegrain and bran-rich cereal foods—A review. Trends Food Sci. Technol. 2016, 47, 25–38. [Google Scholar] [CrossRef] [Green Version]
- Frederix, S.A.; van Hoeymissen, K.E.; Courtin, C.M.; Delcour, J.A. Water-extractable and water-unextractable arabinoxylans affect gluten agglomeration behavior during wheat flour gluten-starch separation. J. Agric. Food Chem. 2004, 52, 7950–7956. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, F.; Wang, Y.; Li, J.; Teng, C. Effects of different molecular weight water-extractable arabinoxylans on the physicochemical properties and structure of wheat gluten. J. Food Sci. Technol. 2019, 56, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Londono, D.M.; Gilissen, L.J.W.J.; Visser, R.G.F.; Smulders, M.J.M.; Hamer, R.J. Understanding the role of oat b-glucan in oat-based dough systems. J. Cereal Sci. 2015, 62, 1–7. [Google Scholar] [CrossRef]
- Ahmed, J. Effect of barley b-glucan concentrate on oscillatory and creep behavior of composite wheat flour dough. J. Food Eng. 2015, 152, 85–94. [Google Scholar] [CrossRef]
- Van der Sman, R.G.M.; Mauer, L.J. Starch gelatinization temperature in sugar and polyol solutions explained by hydrogen bond density. Food Hydrocoll. 2019, 94, 371–380. [Google Scholar] [CrossRef]
- Huang, Z. Effect of barley b-glucan on water redistribution and thermal properties of dough. Int. J. Food Sci. Technol. 2019, 54, 2329–2337. [Google Scholar] [CrossRef]
- De Erive, M.O.; He, F.; Wang, T.; Chen, G. Development of β-glucan enriched wheat bread using soluble oat fiber. J. Cereal Sci. 2020, 95, 103051. [Google Scholar] [CrossRef]
- Zhuang, H.; Chen, Z.; Feng, T.; Yang, Y.; Zhang, J.; Liu, G.; Li, Z.; Ye, R. Characterization of Lentinus edodes β-glucan influencing the in vitro starch digestibility of wheat starch gel. Food Chem. 2017, 224, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Brennan, M.; Brennan, C. An insight into the mechanism of interactions between mushroom polysaccharides and starch. Curr. Opin. Food Sci. 2021, 37, 17–25. [Google Scholar] [CrossRef]
| Water Absorption Properties | ||
|---|---|---|
| Samples | Water Absorption a (Baker’s %) | Dough Water Content b (%wb) |
| Control (CB) | 55.9 | 44.9 |
| Glass beads (GB) | 46.6 | 39.8 |
| Native wheat bran (NWB) | 63.0 | 45.7 |
| Reconstituted wheat bran (RWB) | 64.6 | 46.4 |
| Soluble wheat bran fraction (SWBF) | 46.3 | 39.7 |
| Insoluble wheat bran fraction (IWBF) | 65.9 | 46.7 |
| Native oat bran (NOB) | 75.3 | 49.6 |
| Reconstituted oat bran (ROB) | 75.9 | 49.8 |
| Soluble oat bran fraction (SOBF) | 53.4 | 42.5 |
| Insoluble oat bran fraction (IOBF) | 68.8 | 47.7 |
| NWB | RWB | SWBF | IWBF | NOB | ROB | SOBF | IOBF | |
|---|---|---|---|---|---|---|---|---|
| Chemical compositions | ||||||||
| Starch | 31.6 ± 0.6d | 25.6 ± 0.5c | 2.4 ± 0.0a | 42.9 ± 1.6f | 35.9 ± 0.8e | 35.7 ± 0.5e | 6.4 ± 0.1b | 41.2 ± 0.6f |
| Protein | 19.7 ± 0.3a | 19.4 ± 0.4a | 30.4 ± 2.0c | 16.7 ± 0.7a | 23.8 ± 0.4b | 23.4 ± 0.1b | 24.7 ± 0.2b | 23.8 ± 0.6b |
| TDF | 35.0 ± 0.4ef | 32.4 ± 0.2de | 20.6 ± 0.9a | 38.4 ± 1.8g | 28.2 ± 1.4bc | 30.0 ± 1.2cd | 36.9 ± 0.1fg | 26.3 ± 0.3b |
| Total AX | 14.7 ± 0.3c | 15.0 ± 0.1c | 3.25 ± 0.2ab | 16.3 ± 0.0c | 5.0 ± 0.9b | 5.2 ± 0.9b | 1.4 ± 0.0a | 5.4 ± 1.2b |
| WEAX | 0.65 ± 0.00c | 0.89 ± 0.00d | 3.25 ± 0.2e | 0.12 ± 0.0a | <0.1 | <0.1 | 0.4 ± 0.0b | <0.1 |
| β-glucan | 2.3 ± 0.1a | 2.3 ± 0.1a | 1.9 ± 0.2a | 2.3 ± 0.2a | 15.7 ± 0.2c | 16.0 ± 0.3c | 33.7 ± 0.2d | 13.0 ± 0.6b |
| Total phenolics * | 0.47 ± 0.12b | 0.67 ± 0.02bc | 0.83 ± 0.06d | 0.53 ± 0.01bc | 0.22 ± 0.04a | 0.25 ± 0.03a | 0.68 ± 0.02cd | 0.18 ± 0.01a |
| Free phenolics | 0.11 ± 0.01ab | 0.26 ± 0.00bc | 0.49 ± 0.03d | 0.12 ± 0.00ab | 0.12 ± 0.00ab | 0.11 ± 0.00ab | 0.41 ± 0.01cd | 0.05 ± 0.00a |
| Bound phenolics | 0.32 ±0.03c | 0.34 ±0.03c | 0.06 ±0.00ab | 0.39 ±0.00c | 0.06 ± 0.05a | 0.09 ± 0.01ab | 0.14 ± 0.01b | 0.11 ± 0.00ab |
| Conjugated free phenolics | 0.05 ± 0.01ab | 0.08 ± 0.01bc | 0.27 ± 0.03d | 0.02 ± 0.00a | 0.05 ± 0.00ab | 0.05 ± 0.01ab | 0.13 ± 0.00c | 0.02 ± 0.01a |
| Physical properties | ||||||||
| D[4,3] ** (μm) | 288.5 ± 6.0d | 243.0 ± 1.4c | - | 254.5 ± 5.0c | 134.0 ±1.4a | 241.0 ±1.4b | - | 217.0 ± 4.9c |
| WBC (g/g dm) | 2.27 ± 0.16a | 2.30 ± 0.01a | - | 2.74 ± 0.01a | 4.79 ± 0.54b | 2.39±0.42a | 4.72 ± 1.14b | 2.69 ± 0.18a |
| gwater/gsolids at aw = 0.95 | 0.44 ± 0.00c | 0.49 ± 0.00d | 1.35 ± 0.00f | 0.35 ± 0.00b | 0.33 ± 0.00ab | 0.34 ± 0.00ab | 0.60 ± 0.00e | 0.30 ± 0.00a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renzetti, S.; Theunissen, M.; Horrevorts, K. A Systematic Comparison of the Intrinsic Properties of Wheat and Oat Bran Fractions and Their Effects on Dough and Bread Properties: Elucidation of Chemical Mechanisms, Water Binding, and Steric Hindrance. Foods 2021, 10, 2311. https://doi.org/10.3390/foods10102311
Renzetti S, Theunissen M, Horrevorts K. A Systematic Comparison of the Intrinsic Properties of Wheat and Oat Bran Fractions and Their Effects on Dough and Bread Properties: Elucidation of Chemical Mechanisms, Water Binding, and Steric Hindrance. Foods. 2021; 10(10):2311. https://doi.org/10.3390/foods10102311
Chicago/Turabian StyleRenzetti, Stefano, Mira Theunissen, and Karlijn Horrevorts. 2021. "A Systematic Comparison of the Intrinsic Properties of Wheat and Oat Bran Fractions and Their Effects on Dough and Bread Properties: Elucidation of Chemical Mechanisms, Water Binding, and Steric Hindrance" Foods 10, no. 10: 2311. https://doi.org/10.3390/foods10102311
APA StyleRenzetti, S., Theunissen, M., & Horrevorts, K. (2021). A Systematic Comparison of the Intrinsic Properties of Wheat and Oat Bran Fractions and Their Effects on Dough and Bread Properties: Elucidation of Chemical Mechanisms, Water Binding, and Steric Hindrance. Foods, 10(10), 2311. https://doi.org/10.3390/foods10102311








