Nondestructive Detection of Weight Loss Rate, Surface Color, Vitamin C Content, and Firmness in Mini-Chinese Cabbage with Nanopackaging by Fourier Transform-Near Infrared Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Preparation
2.2. Nanopacking Material
2.3. FT-NIR Spectroscopy
2.4. Quantitative Analysis of Surface Color, Weight Loss Rate, Vitamin C and Firmness
2.5. Freshness Levels Description
2.6. Data Processing
2.7. Evaluation of Models
2.8. Statistical Analysis
3. Results and Discussion
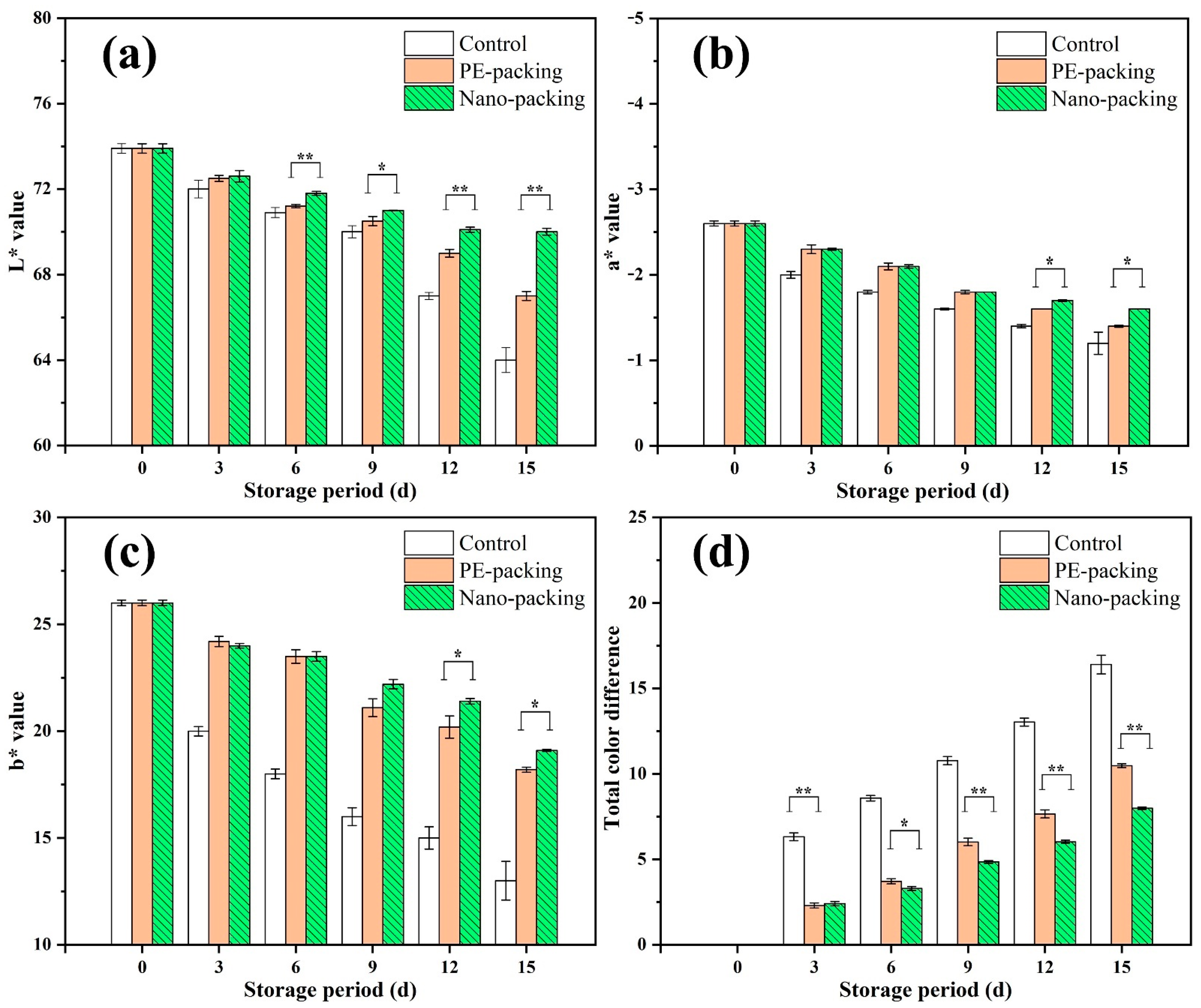
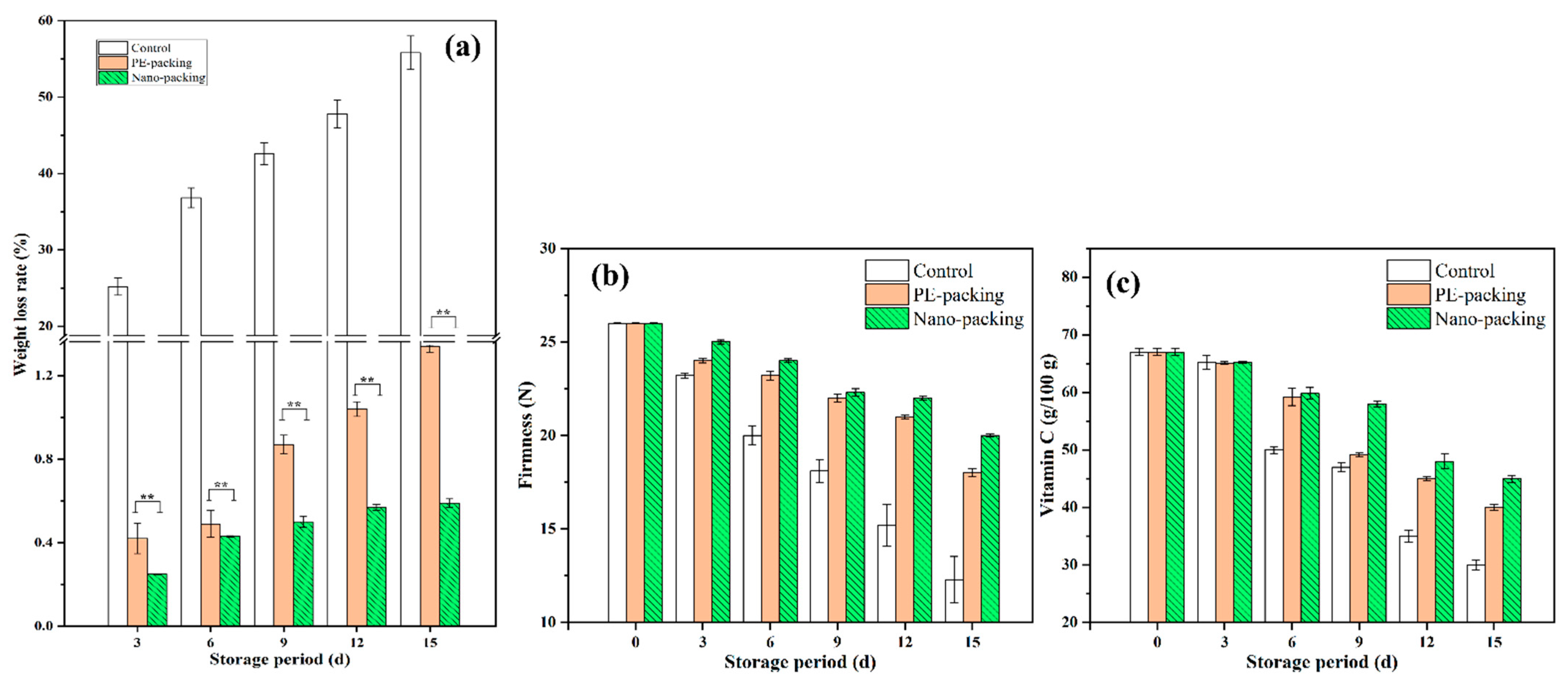
3.1. Analysis of Surface Color, Weight Loss Rate, Vitamin C and Firmness for Mini-Chinese Cabbage during Storage
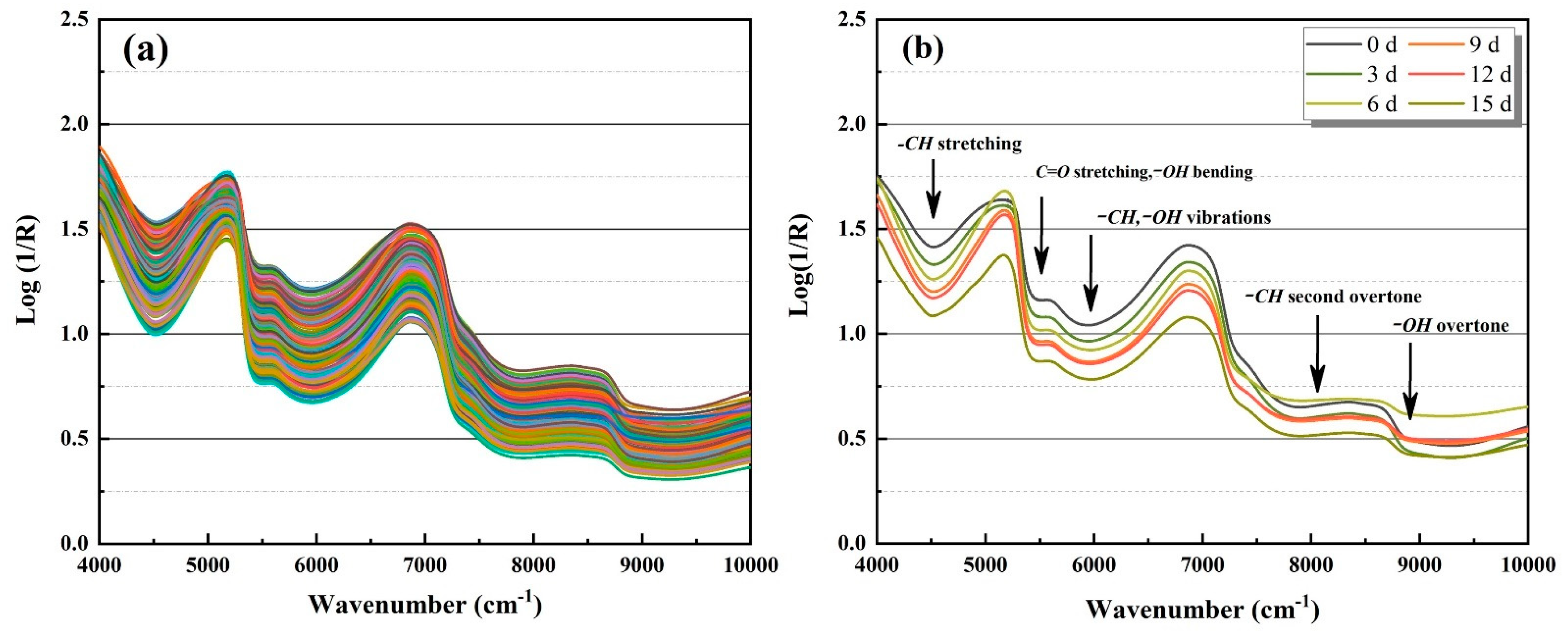
3.2. Spectral Analysis
3.3. Prediction Performance of Surface Color and Quality Attributes Based on FT-NIR Dataset
3.4. Classification Performance of Freshness Levels Based on FT-NIR Dataset
3.5. Independent Test-Set Validation of Freshness Level in Mini-Chinese Cabbage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, L.; Yu, J.; Liao, W.; Zhang, G.; Xie, J.; Lv, J.; Xiao, X.; Yang, B.; Zhou, R.; Bu, R. Moderate ammonium:nitrate alleviates low light intensity stress in mini Chinese cabbage seedling by regulating root architecture and photosynthesis. Sci. Hortic. 2015, 186, 143–153. [Google Scholar] [CrossRef]
- Shawon, R.A.; Kang, B.S.; Lee, S.G.; Kim, S.K.; Lee, H.J.; Katrich, E.; Gorinstein, S.; Ku, Y.G. Influence of drought stress on bioactive compounds, antioxidant enzymes and glucosinolate contents of Chinese cabbage (Brassica rapa). Food Chem. 2020, 308, 125657. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Yeo, H.J.; Park, S.-Y.; Kim, J.K.; Park, S.U. Comparative Phytochemical Analyses and Metabolic Profiling of Different Phenotypes of Chinese Cabbage (Brassica Rapa ssp. Pekinensis). Foods 2019, 8, 587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Mesery, H.S.; Mao, H.; Abomohra, A.E.F. Applications of non-destructive technologies for agricultural and food products quality inspection. Sensors 2019, 19, 846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Zhao, C.; Yang, G. Development of a Non-Destructive Method for Detection of the Juiciness of Pear via VIS/NIR Spectroscopy Combined with Chemometric Methods. Foods 2020, 9, 1778. [Google Scholar] [CrossRef] [PubMed]
- Adedeji, A.A.; Ekramirad, N.; Rady, A.; HamidiSepehr, A.; Donohue, K.D.; Villanueva, R.T.; Parrish, C.A.; Li, M. Non-Destructive Technologies for Detecting Insect Infestation in Fruits and Vegetables under Postharvest Conditions: A Critical Review. Foods 2020, 9, 927. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Fang, Y.; Yang, Y.; Ma, N.; Zhao, L. Effect of nanocomposite-based packaging on postharvest quality of ethylene-treated kiwifruit (Actinidia deliciosa) during cold storage. Food Res. Int. 2011, 44, 1589–1596. [Google Scholar] [CrossRef]
- Wang, L.; Shao, S.; Madebo, M.P.; Hou, Y.; Zheng, Y.; Jin, P. Effect of nano-SiO2 packing on postharvest quality and antioxidant capacity of loquat fruit under ambient temperature storage. Food Chem. 2020, 315, 126295. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, Y.; Wang, H.; Feng, S. Impact of nano-CaCO3 -LDPE packaging on quality of fresh-cut sugarcane. J. Sci. Food Agric. 2014, 94, 3273–3280. [Google Scholar] [CrossRef]
- An, J.; Zhang, M.; Wang, S.; Tang, J. Physical, chemical and microbiological changes in stored green asparagus spears as affected by coating of silver nanoparticles-PVP. LWT Food Sci. Technol. 2008, 41, 1100–1107. [Google Scholar] [CrossRef]
- Erkan, M.; Wang, S.Y.; Wang, C.Y. Effect of UV treatment on antioxidant capacity, antioxidant enzyme activity and decay in strawberry fruit. Postharvest Biol. Technol. 2008, 48, 163–171. [Google Scholar] [CrossRef]
- Moscetti, R.; Haff, R.P.; Ferri, S.; Raponi, F.; Liang, P.; Massantini, R.; Monarca, D. Real-Time Monitoring of Organic Carrot (var. Romance) During Hot-Air Drying Using Near-Infrared Spectroscopy. Food Bioprocess Technol. 2017, 10, 2046–2059. [Google Scholar] [CrossRef]
- Botros, L.L.; Jablonski, J.; Chang, C.; Bergana, M.M.; Wehling, P.; Harnly, J.M.; Downey, G.; Harrington, P.; Potts, A.R.; Moore, J.C. Exploring Authentic Skim and Nonfat Dry Milk Powder Variance for the Development of Nontargeted Adulterant Detection Methods Using Near-Infrared Spectroscopy and Chemometrics. J. Agric. Food Chem. 2013, 61, 9810–9818. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, G.A.; Castilhos, F.; Renard, C.M.; Bureau, S. Comparison of NIR and MIR spectroscopic methods for determination of individual sugars, organic acids and carotenoids in passion fruit. Food Res. Int. 2014, 60, 154–162. [Google Scholar] [CrossRef]
- Boughattas, F.; Le Fur, B.; Karoui, R. Mid infrared spectroscopy coupled with chemometric tools for qualitative analysis of canned tuna with sunflower medium. J. Food Compos. Anal. 2020, 91, 103519. [Google Scholar] [CrossRef]
- Giovenzana, V.; Beghi, R.; Civelli, R.; Guidetti, R. Optical techniques for rapid quality monitoring along minimally processed fruit and vegetable chain. Trends Food Sci. Technol. 2015, 46, 331–338. [Google Scholar] [CrossRef]
- Liu, Q.; Wei, K.; Xiao, H.; Tu, S.; Sun, K.; Sun, Y.; Pan, L.; Tu, K. Near-Infrared Hyperspectral Imaging Rapidly Detects the Decay of Postharvest Strawberry Based on Water-Soluble Sugar Analysis. Food Anal. Methods 2019, 12, 936–946. [Google Scholar] [CrossRef]
- Li, H.; Li, F.; Wang, L.; Sheng, J.; Xin, Z.; Zhao, L.; Xiao, H.; Zheng, Y.; Hu, Q. Effect of nano-packing on preservation quality of Chinese jujube (Ziziphus jujuba Mill. var. inermis (Bunge) Rehd). Food Chem. 2009, 114, 547–552. [Google Scholar] [CrossRef]
- Ibáñez, G.; Cebolla-Cornejo, J.; Martí, R.; Roselló, S.; Valcárcel, M. Non-destructive determination of taste-related compounds in tomato using NIR spectra. J. Food Eng. 2019, 263, 237–242. [Google Scholar] [CrossRef]
- Szigedi, T.; Lénárt, J.; Dernovics, M.; Turza, S.; Fodor, M. Protein content determination in Brassica oleracea species using FT-NIR technique and PLS regression. Int. J. Food Sci. Technol. 2011, 47, 436–440. [Google Scholar] [CrossRef]
- Caramês, E.T.S.; Alamar, P.D.; Pallone, J.A.L. Bioactive Compounds and Antioxidant Capacity in Freeze-Dried Red Cabbage by FT-NIR and MIR Spectroscopy and Chemometric Tools. Food Anal. Methods 2019, 13, 78–85. [Google Scholar] [CrossRef]
- Brasil, I.; Gomes, C.; Puerta-Gomez, A.; Castell-Perez, M.; Moreira, R.G. Polysaccharide-based multilayered antimicrobial edible coating enhances quality of fresh-cut papaya. LWT Food Sci. Technol. 2012, 47, 39–45. [Google Scholar] [CrossRef]
- Nicolaï, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, K.; Zhao, N.; Yang, J.; Zhang, Y.; Ma, C.; Pan, L.; Tu, K. Information fusion of hyperspectral imaging and electronic nose for evaluation of fungal contamination in strawberries during decay. Postharvest Biol. Technol. 2019, 153, 152–160. [Google Scholar] [CrossRef]
- Iqbal, A.; Sun, D.-W.; Allen, P. Prediction of moisture, color and pH in cooked, pre-sliced turkey hams by NIR hyperspectral imaging system. J. Food Eng. 2013, 117, 42–51. [Google Scholar] [CrossRef]
- Ma, C.; Feng, L.; Pan, L.; Wei, K.; Liu, Q.; Tu, K.; Zhao, L.; Peng, J. Relationships between optical properties of peach flesh with firmness and tissue structure during storage. Postharvest Biol. Technol. 2020, 163, 111134. [Google Scholar] [CrossRef]
- Wang, F.; Hu, Q.; Mariga, A.M.; Cao, C.; Yang, W. Effect of nano packaging on preservation quality of Nanjing 9108 rice variety at high temperature and humidity. Food Chem. 2018, 239, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Yang, Z.; Cai, Y.; Zheng, Y. Fatty acid composition and antioxidant system in relation to susceptibility of loquat fruit to chilling injury. Food Chem. 2011, 127, 1777–1783. [Google Scholar] [CrossRef]
- Saeys, W.; Trong, N.N.D.; Van Beers, R.; Nicolaï, B.M. Multivariate calibration of spectroscopic sensors for postharvest quality evaluation: A review. Postharvest Biol. Technol. 2019, 158, 110981. [Google Scholar] [CrossRef]
- Beć, K.; Huck, C.W. Breakthrough Potential in Near-Infrared Spectroscopy: Spectra Simulation. A Review of Recent Developments. Front. Chem. 2019, 7, 48. [Google Scholar] [CrossRef] [Green Version]
- Cevoli, C.; Gori, A.; Nocetti, M.; Cuibus, L.; Caboni, M.F.; Fabbri, A. FT-NIR and FT-MIR spectroscopy to discriminate competitors, non compliance and compliance grated Parmigiano Reggiano cheese. Food Res. Int. 2013, 52, 214–220. [Google Scholar] [CrossRef]
- Ma, T.; Li, X.; Inagaki, T.; Yang, H.; Tsuchikawa, S. Noncontact evaluation of soluble solids content in apples by near-infrared hyperspectral imaging. J. Food Eng. 2018, 224, 53–61. [Google Scholar] [CrossRef]
- Czarnecki, M.A.; Morisawa, Y.; Futami, Y.; Ozaki, Y. Advances in Molecular Structure and Interaction Studies Using Near-Infrared Spectroscopy. Chem. Rev. 2015, 115, 9707–9744. [Google Scholar] [CrossRef]
- Beć, K.B.; Grabska, J.; Huck, C.W.; Czarnecki, M.A. Effect of conformational isomerism on NIR spectra of ethanol isotopologues. Spectroscopic and anharmonic DFT study. J. Mol. Liq. 2020, 310, 113271. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Kim, S.-H.; Chung, I.-M. Exogenous phytohormones increase the accumulation of health-promoting metabolites, and influence the expression patterns of biosynthesis related genes and biological activity in Chinese cabbage (Brassica rapa spp. pekinensis). Sci. Hortic. 2015, 193, 136–146. [Google Scholar] [CrossRef]
- Li, F.; Huang, H.; Ding, X.; Liu, J.; He, M.; Shan, Y.; Qu, H.; Jiang, Y. Effect of CPPU on postharvest attributes of Chinese flowering cabbage during storage. Postharvest Biol. Technol. 2021, 174, 111438. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, D.; Tu, S.; Xiao, H.; Zhang, B.; Sun, Y.; Pan, L.; Tu, K. Quantitative Visualization of Fungal Contamination in Peach Fruit Using Hyperspectral Imaging. Food Anal. Methods 2020, 13, 1262–1270. [Google Scholar] [CrossRef]
- Shen, F.; Zhang, B.; Cao, C.; Jiang, X. On-line discrimination of storage shelf-life and prediction of post-harvest quality for strawberry fruit by visible and near infrared spectroscopy. J. Food Process Eng. 2018, 41, 12866. [Google Scholar] [CrossRef]
- Liu, C.; Yang, S.X.; Deng, L. Determination of internal qualities of Newhall navel oranges based on NIR spectroscopy using machine learning. J. Food Eng. 2015, 161, 16–23. [Google Scholar] [CrossRef]
- Balage, J.M.; da Luz e Silva, S.; Gomide, C.A.; de Nadai Bonin, M.; Figueira, A.C. Predicting pork quality using Vis/NIR spectroscopy. Meat Sci. 2015, 108, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lv, W.; Zhao, R.; Guo, H.; Liu, J.; Han, D. Non-destructive assessment of quality parameters in ‘Friar’ plums during low temperature storage using visible/near infrared spectroscopy. Food Control. 2017, 73, 1334–1341. [Google Scholar] [CrossRef]

| Freshness Levels | Description |
|---|---|
| Level 1 | Surface without visible defects, smells fresh. Quality attributes including weight loss rate < 30%, L* > 71 and Vc content > 59 mg/100 g. |
| Level 2 | Surface with visible defect points or peculiar smells. Quality attributes including 30% ≤ weight loss rate < 50%, 68 < L* ≤ 71 and 47 < Vc content ≤ 59 mg/100 g. |
| Level 3 | Surface with visible defect areas and unpleasant smell. Quality attributes including weight loss rate ≥ 51%, L* ≤ 68 and Vc content ≤ 47 mg/100 g. |
| Quality Attributes | Pretreatment | Model | Calibration | Cross-Validation | Prediction | ||||
|---|---|---|---|---|---|---|---|---|---|
| RC2 | RMSEC | RCV2 | RMSECV | Rp2 | RMSEP | RPD | |||
| Weight loss rate | SNV | PLSR | 0.95 | 1.334 | 0.92 | 1.339 | 0.96 | 1.332 | 3.612 |
| 1-st | 0.90 | 1.340 | 0.86 | 1.573 | 0.87 | 1.360 | 2.191 | ||
| 2-nd | 0.82 | 1.654 | 0.80 | 1.732 | 0.80 | 1.564 | 3.212 | ||
| MSC | 0.88 | 1.365 | 0.85 | 1.537 | 0.88 | 1.354 | 2.435 | ||
| Autoscale | 0.86 | 1.573 | 0.81 | 1.691 | 0.84 | 1.476 | 2.830 | ||
| SNV | SVR | 0.83 | 1.643 | 0.80 | 1.733 | 0.79 | 1.590 | 2.700 | |
| 1-st | 0.89 | 1.378 | 0.87 | 1.520 | 0.87 | 1.359 | 2.191 | ||
| 2-nd | 0.85 | 1.587 | 0.81 | 1.720 | 0.83 | 1.489 | 2.795 | ||
| MSC | 0.87 | 1.520 | 0.82 | 1.647 | 0.85 | 1.435 | 2.546 | ||
| Autoscale | 0.87 | 1.489 | 0.82 | 1.649 | 0.85 | 1.461 | 2.544 | ||
| Firmness | SNV | PLSR | 0.58 | 2.542 | 0.50 | 2.714 | 0.57 | 2.606 | 1.437 |
| 1-st | 0.60 | 2.403 | 0.51 | 2.704 | 0.58 | 2.578 | 2.066 | ||
| 2-nd | 0.57 | 2.604 | 0.49 | 2.821 | 0.44 | 2.706 | 1.128 | ||
| MSC | 0.51 | 2.704 | 0.44 | 2.781 | 0.51 | 2.695 | 2.042 | ||
| Autoscale | 0.45 | 2.775 | 0.40 | 3.305 | 0.40 | 2.789 | 1.195 | ||
| SNV | SVR | 0.60 | 2.463 | 0.51 | 2.812 | 0.57 | 2.598 | 1.608 | |
| 1-st | 0.55 | 2.671 | 0.48 | 2.901 | 0.50 | 2.701 | 2.159 | ||
| 2-nd | 0.58 | 2.534 | 0.47 | 2.953 | 0.49 | 2.735 | 2.124 | ||
| MSC | 0.56 | 2.638 | 0.41 | 3.217 | 0.48 | 2.780 | 2.091 | ||
| Autoscale | 0.60 | 2.479 | 0.55 | 2.671 | 0.60 | 2.453 | 2.205 | ||
| Vitamin C | SNV | PLSR | 0.90 | 3.213 | 0.85 | 3.474 | 0.86 | 3.43 | 2.727 |
| 1-st | 0.91 | 3.231 | 0.84 | 3.481 | 0.89 | 3.25 | 2.883 | ||
| 2-nd | 0.81 | 3.500 | 0.75 | 4.002 | 0.80 | 3.46 | 2.015 | ||
| MSC | 0.90 | 3.113 | 0.85 | 3.474 | 0.95 | 3.19 | 2.681 | ||
| Autoscale | 0.87 | 3.453 | 0.74 | 4.110 | 0.78 | 3.48 | 2.113 | ||
| SNV | SVR | 0.82 | 3.496 | 0.74 | 4.024 | 0.79 | 3.47 | 2.238 | |
| 1-st | 0.86 | 3.467 | 0.81 | 3.500 | 0.87 | 3.24 | 2.513 | ||
| 2-nd | 0.88 | 3.405 | 0.80 | 3.557 | 0.87 | 3.25 | 2.512 | ||
| MSC | 0.89 | 3.436 | 0.81 | 3.501 | 0.85 | 3.46 | 2.442 | ||
| Autoscale | 0.90 | 3.298 | 0.80 | 3.557 | 0.82 | 3.31 | 2.379 | ||
| Quality Attributes | Pretreatment | Model | Calibration | Cross-Validation | Prediction | ||||
|---|---|---|---|---|---|---|---|---|---|
| RC2 | RMSEC | RCV2 | RMSECV | Rp2 | RMSEP | RPD | |||
| L* | SNV | PLSR | 0.72 | 2.571 | 0.68 | 2.823 | 0.70 | 2.324 | 2.458 |
| 1-st | 0.69 | 2.803 | 0.62 | 3.074 | 0.67 | 2.415 | 2.258 | ||
| 2-nd | 0.74 | 2.472 | 0.67 | 2.854 | 0.71 | 2.183 | 2.015 | ||
| MSC | 0.77 | 2.372 | 0.68 | 2.823 | 0.72 | 2.051 | 2.453 | ||
| Autoscale | 0.65 | 2.762 | 0.60 | 3.227 | 0.60 | 2.903 | 2.469 | ||
| SNV | SVR | 0.74 | 2.586 | 0.64 | 2.974 | 0.65 | 2.372 | 1.541 | |
| 1-st | 0.73 | 2.594 | 0.65 | 2.914 | 0.74 | 2.134 | 2.445 | ||
| 2-nd | 0.68 | 2.908 | 0.61 | 3.146 | 0.60 | 2.961 | 2.098 | ||
| MSC | 0.84 | 2.051 | 0.74 | 2.586 | 0.82 | 2.013 | 3.069 | ||
| Autoscale | 0.80 | 2.162 | 0.71 | 2.584 | 0.75 | 2.122 | 2.483 | ||
| a* | SNV | PLSR | 0.75 | 1.241 | 0.68 | 1.586 | 0.72 | 1.309 | 2.088 |
| 1-st | 0.70 | 1.443 | 0.62 | 1.733 | 0.68 | 1.528 | 1.781 | ||
| 2-nd | 0.68 | 1.587 | 0.61 | 1.748 | 0.64 | 1.691 | 1.332 | ||
| MSC | 0.71 | 1.401 | 0.62 | 1.733 | 0.68 | 1.528 | 1.781 | ||
| Autoscale | 0.74 | 1.287 | 0.67 | 1.593 | 0.69 | 1.501 | 1.855 | ||
| SNV | SVR | 0.77 | 1.032 | 0.71 | 1.403 | 0.73 | 1.288 | 2.145 | |
| 1-st | 0.72 | 1.317 | 0.68 | 1.557 | 0.70 | 1.402 | 1.987 | ||
| 2-nd | 0.67 | 1.594 | 0.60 | 1.756 | 0.65 | 1.625 | 1.501 | ||
| MSC | 0.72 | 1.317 | 0.67 | 1.594 | 0.70 | 1.402 | 1.987 | ||
| Autoscale | 0.75 | 1.243 | 0.68 | 1.557 | 0.71 | 1.388 | 2.051 | ||
| b* | SNV | PLSR | 0.80 | 1.211 | 0.71 | 1.302 | 0.78 | 1.278 | 1.943 |
| 1-st | 0.85 | 1.204 | 0.72 | 1.283 | 0.85 | 1.264 | 2.432 | ||
| 2-nd | 0.79 | 1.236 | 0.72 | 1.289 | 0.72 | 1.323 | 1.893 | ||
| MSC | 0.81 | 1.218 | 0.73 | 1.301 | 0.73 | 1.312 | 1.897 | ||
| Autoscale | 0.68 | 1.324 | 0.61 | 1.374 | 0.67 | 1.421 | 1.457 | ||
| SNV | SVR | 0.73 | 1.278 | 0.61 | 1.374 | 0.70 | 1.376 | 1.541 | |
| 1-st | 0.78 | 1.245 | 0.62 | 1.370 | 0.73 | 1.356 | 1.896 | ||
| 2-nd | 0.78 | 1.234 | 0.62 | 1.370 | 0.75 | 1.321 | 1.913 | ||
| MSC | 0.80 | 1.216 | 0.72 | 1.289 | 0.74 | 1.310 | 1.906 | ||
| Autoscale | 0.77 | 1.270 | 0.70 | 1.311 | 0.71 | 1.368 | 1.632 | ||
| Models | Freshness Levels | Accuracy/% | |||
|---|---|---|---|---|---|
| Calibration | Level 1 | Level 2 | Level 3 | ||
| Level 1 | 40 | 1 | 4 | 88.8 | |
| Level 2 | 8 | 32 | 5 | 71.1 | |
| Level 3 | 2 | 3 | 39 | 86.6 | |
| Total accuracy/% | 82.1 | ||||
| Prediction | Level 1 | 13 | 1 | 1 | 86.6 |
| Level 2 | 1 | 11 | 3 | 73.3 | |
| Level 3 | 0 | 3 | 12 | 80.0 | |
| Total accuracy/% | 79.9 | ||||
| Models | Freshness Levels | Accuracy/% | |||
|---|---|---|---|---|---|
| Calibration | Level 1 | Level 2 | Level 3 | ||
| Level 1 | 43 | 1 | 1 | 95.5 | |
| Level 2 | 4 | 38 | 3 | 84.4 | |
| Level 3 | 1 | 4 | 40 | 88.8 | |
| Total accuracy/% | 89.6 | ||||
| Prediction | Level 1 | 14 | 1 | 0 | 93.3 |
| Level 2 | 0 | 13 | 2 | 86.6 | |
| Level 3 | 0 | 2 | 13 | 86.6 | |
| Total accuracy/% | 88.8 | ||||
| Models | Freshness Levels | Accuracy/% | |||
|---|---|---|---|---|---|
| SVC | Level 1 | Level 2 | Level 3 | ||
| Level 1 | 38 | 6 | 1 | 84.4 | |
| Level 2 | 7 | 34 | 4 | 75.6 | |
| Level 3 | 0 | 6 | 39 | 86.7 | |
| Total accuracy/% | 82.2% | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Chen, S.; Zhou, D.; Ding, C.; Wang, J.; Zhou, H.; Tu, K.; Pan, L.; Li, P. Nondestructive Detection of Weight Loss Rate, Surface Color, Vitamin C Content, and Firmness in Mini-Chinese Cabbage with Nanopackaging by Fourier Transform-Near Infrared Spectroscopy. Foods 2021, 10, 2309. https://doi.org/10.3390/foods10102309
Liu Q, Chen S, Zhou D, Ding C, Wang J, Zhou H, Tu K, Pan L, Li P. Nondestructive Detection of Weight Loss Rate, Surface Color, Vitamin C Content, and Firmness in Mini-Chinese Cabbage with Nanopackaging by Fourier Transform-Near Infrared Spectroscopy. Foods. 2021; 10(10):2309. https://doi.org/10.3390/foods10102309
Chicago/Turabian StyleLiu, Qiang, Shaoxia Chen, Dandan Zhou, Chao Ding, Jiahong Wang, Hongsheng Zhou, Kang Tu, Leiqing Pan, and Pengxia Li. 2021. "Nondestructive Detection of Weight Loss Rate, Surface Color, Vitamin C Content, and Firmness in Mini-Chinese Cabbage with Nanopackaging by Fourier Transform-Near Infrared Spectroscopy" Foods 10, no. 10: 2309. https://doi.org/10.3390/foods10102309






