In-Bag Dry- vs. Wet-Aged Lamb: Quality, Consumer Acceptability, Oxidative Stability and In Vitro Digestibility
Abstract
:1. Introduction
2. Materials and Methods
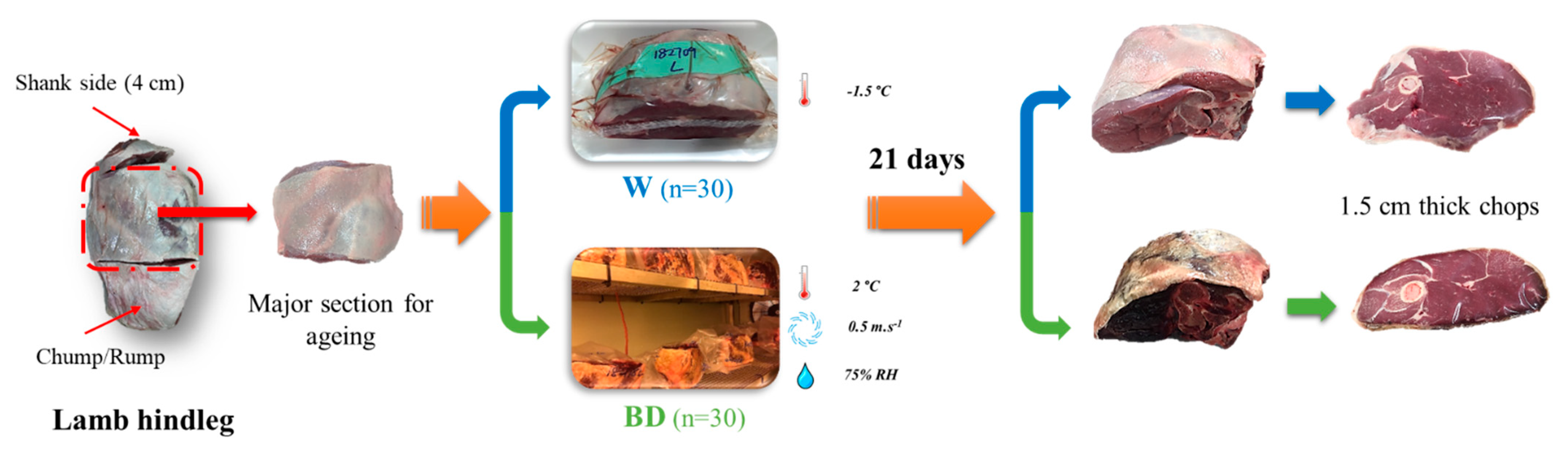
2.1. Sample Collection and Ageing Regimes
2.2. Weight Loss, pH and Proximate Content
2.2.1. Ageing Weight Loss (%)
2.2.2. Cook Loss and Percentage Total Loss (%)
2.2.3. pH
2.2.4. Proximate Content
2.3. Surface Microorganism Enumeration
2.4. Instrumental Colour
2.5. Instrumental Texture Profile Analysis
2.6. Consumer Sensory Evaluation
2.7. Protein Carbonyl Content
2.8. Lipid Oxidation and Fatty Acid (FA) Profile
2.9. In Vitro Digestion of Lamb Chops
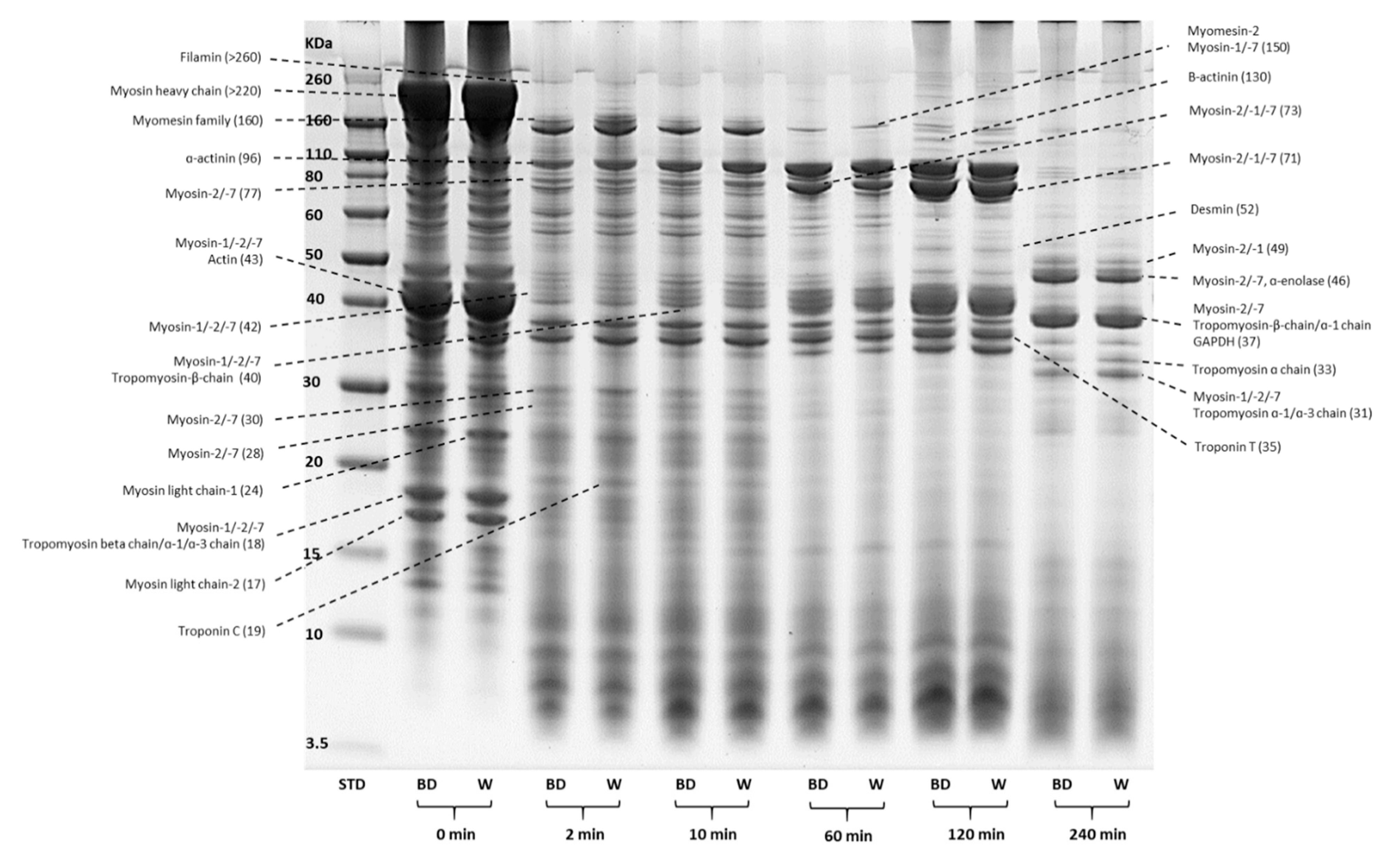
2.9.1. SDS-PAGE Gel Electrophoresis
2.9.2. Analysis of Free Amino Acids (FAAs)
2.9.3. Relative Protein Digestibility (%)
- (1)
- Relative digestibility SDS-PAGE = (1 − (Optical intensity of protein bands <10 kDa/Optical intensity of all protein bands)) × 100
- (2)
- Relative digestibility FAAs = ((Total FAAs (g) at 240 min − total FAAs (g) at 0 min)/Protein content of the sample) × 100
- (3)
- Relative digestibility Protein content = (1 − Protein content (g) in hydrolysate at 240 min/Protein content of the sample) × 100
2.10. Statistical Analysis
3. Results and Discussion
3.1. Meat Quality
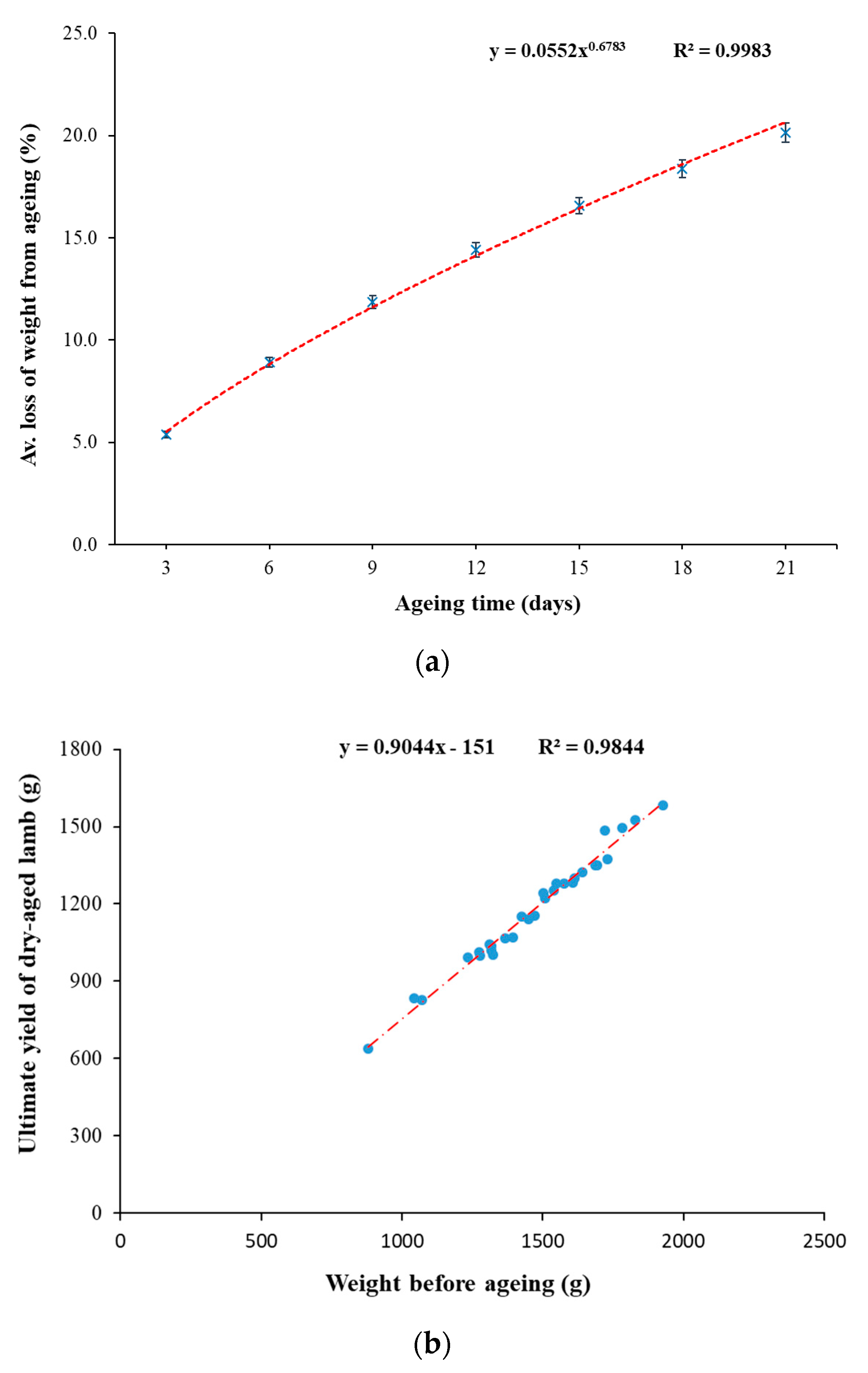
3.1.1. Weight Losses
- Y = % weight loss from ageing;
- X = Days of ageing
- Y = Dry-aged weight (g) at 21 days of ageing;
- X = Initial weight (g) before ageing
3.1.2. Proximate Content and pH
3.1.3. Surface Microorganism Growth
3.1.4. Instrumental Colour
3.1.5. Instrumental Texture Profile Analysis
3.2. Sensory Quality
3.2.1. Consumer Acceptability
3.2.2. Focus Group Perceptions of In-Bag Dry-Aged Lamb Chops
3.3. Protein and Lipid Oxidation
3.3.1. Protein Carbonyl Content
3.3.2. TBARS and FA Profile
3.4. Proteolysis
3.5. In Vitro Digestibility
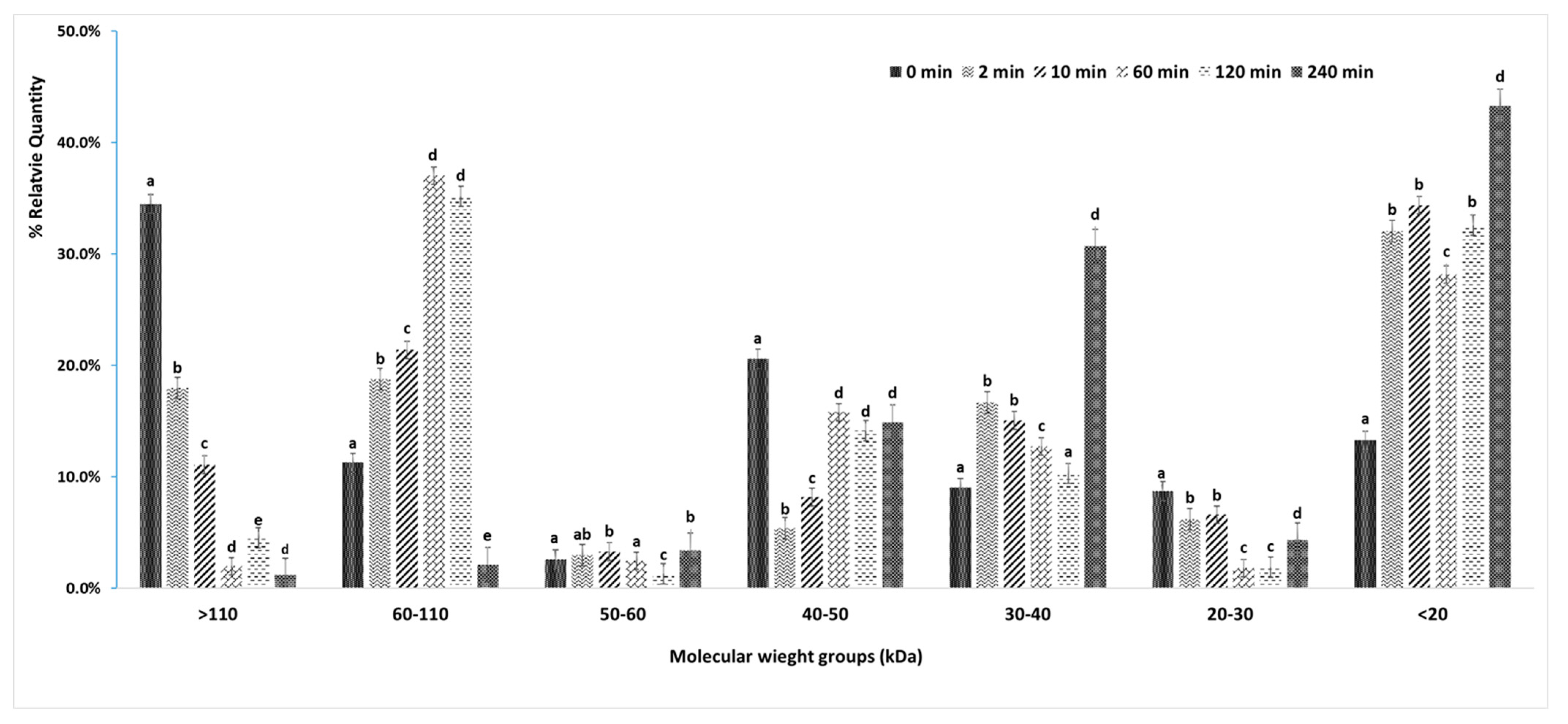
3.5.1. Changes in Protein Profiles (SDS-PAGE)
3.5.2. Changes in FAAs
3.5.3. Protein Content in Hydrolysate
3.5.4. Overall Relative Protein Digestibility (%)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Babol, J.; Bredie, W.L.P.; Nielsen, B.; Tománková, J.; Lundström, K. A comparative study of beef quality after ageing longissimus muscle using a dry ageing bag, traditional dry ageing or vacuum package ageing. Meat Sci. 2014, 97, 433–442. [Google Scholar] [CrossRef]
- Savell, J.W. Dry-Aging of Beef: Executive Summary; Center for Research and Knowledge Management, National Cattlemen’s Beef Association: Texas, Estados Unidos, 2008. [Google Scholar]
- Ahnström, M.L.; Seyfert, M.; Hunt, M.C.; Johnson, D.E. Dry aging of beef in a bag highly permeable to water vapour. Meat Sci. 2006, 73, 674–679. [Google Scholar] [CrossRef]
- Kim, Y.H.B.; Ma, D.; Setyabrata, D.; Farouk, M.M.; Lonergan, S.M.; Huff-Lonergan, E.; Hunt, M.C. Understanding postmortem biochemical processes and post-harvest aging factors to develop novel smart-aging strategies. Meat Sci. 2018, 144, 74–90. [Google Scholar] [CrossRef]
- Zhang, R.; Yoo, M.J.; Farouk, M. Quality and acceptability of fresh and long-term frozen in-bag dry-aged lean bull beef. J. Food Qual. 2019, 2019, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Benatti, P.; Peluso, G.; Nicolai, R.; Calvani, M. Polyunsaturated fatty acids: Biochemical, nutritional and epigenetic properties. J. Am. Coll. Nutr. 2004, 23, 281–302. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.B.; Kemp, R.; Samuelsson, L.M. Effects of dry-aging on meat quality attributes and metabolite profiles of beef loins. Meat Sci. 2016, 111, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Choe, J.; Kim, M.; Kim, H.C.; Yoon, J.W.; Oh, S.W.; Jo, C. Role of moisture evaporation in the taste attributes of dry- and wet-aged beef determined by chemical and electronic tongue analyses. Meat Sci. 2019, 151, 82–88. [Google Scholar] [CrossRef]
- Oh, H.; Lee, H.J.; Lee, J.; Jo, C.; Yoon, Y. Identification of Microorganisms Associated with the Quality Improvement of Dry-Aged Beef Through Microbiome Analysis and DNA Sequencing, and Evaluation of Their Effects on Beef Quality. J. Food Sci. 2019, 84, 2944–2954. [Google Scholar] [CrossRef]
- Lee, H.J.; Yoon, J.W.; Kim, M.; Oh, H.; Yoon, Y.; Jo, C. Changes in microbial composition on the crust by different air flow velocities and their effect on sensory properties of dry-aged beef. Meat Sci. 2019, 153, 152–158. [Google Scholar] [CrossRef]
- Martinaud, A.; Mercier, Y.; Marinova, P.; Tassy, C.; Gatellier, P.; Renerre, M. Comparison of oxidative processes on myofibrillar proteins from beef during maturation and by different model oxidation systems. J. Agric. Food Chem. 1997, 45, 2481–2487. [Google Scholar] [CrossRef]
- Park, D.; Xiong, Y.L.; Alderton, A.A.L.; Ooizumi, T. Biochemical changes in myofibrillar protein isolates exposed to three oxidizing systems. J. Agric. Food Chem. 2006, 54, 4445–4451. [Google Scholar] [CrossRef] [PubMed]
- Santé-Lhoutellier, V.; Engel, E.; Aubry, L.; Gatellier, P. Effect of animal (lamb) diet and meat storage on myofibrillar protein oxidation and in vitro digestibility. Meat Sci. 2008, 79, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.N.; Heinonen, M.; Baron, C.P.; Estévez, M. Protein oxidation in muscle foods: A review. Mol. Nutr. Food Res. 2010, 55, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Berardo, A.; Claeys, E.; Vossen, E.; Leroy, F.; De Smet, S. Protein oxidation affects proteolysis in a meat model system. Meat Sci. 2015, 106, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Sante-Lhoutellier, V.; Aubry, L.; Gatellier, P. Effect of oxidation on in vitro digestibility of skeletal muscle myofibrillar proteins. J. Agric. Food Chem. 2007, 55, 5343–5348. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [Green Version]
- Resconi, V.C.; Bueno, M.; Escudero, A.; Magalhaes, D.; Ferreira, V.; Campo, M.M. Ageing and retail display time in raw beef odour according to the degree of lipid oxidation. Food Chem. 2018, 242, 288–300. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Babol, J.; Wallby, A.; Lundström, K. Meat quality, microbiological status and consumer preference of beef gluteus medius aged in a dry ageing bag or vacuum. Meat Sci. 2013, 95, 229–234. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2010. [Google Scholar]
- Downes, F.P.; Ito, K. Compendium of Methods Microbiological Examination of Foods, 4th ed.; American Public Health Association: Washington, DC, USA, 2001. [Google Scholar]
- Zhang, R.; Yoo, M.J.; Gathercole, J.; Reis, M.G.; Farouk, M.M. Effect of animal age on the nutritional and physicochemical qualities of ovine bresaola. Food Chem. 2018, 254, 317–325. [Google Scholar] [CrossRef]
- McLafferty, I. Focus group interviews as a data collecting strategy. J. Adv. Nurs. 2004, 48, 187–194. [Google Scholar] [CrossRef]
- Powell, R.A.; Single, H.M. Focus groups. Int. J. Qual. Health Care 1996, 8, 499–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabiee, F. Focus-group interview and data analysis. Proc. Nutr. Soc. 2004, 63, 655–660. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.-G.; Ahn, B.-W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods in Enzymology 1990, 186, 464–478. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [PubMed]
- Farouk, M.; Wu, G.; Frost, D.A.; Clerens, S.; Knowles, S.O. The in vitro digestibility of beef varies with its inherent ultimate pH. Food Funct. 2014, 5, 2759–2767. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yoo, M.J.; Farouk, M.M. Oxidative stability, proteolysis, and in vitro digestibility of fresh and long-term frozen stored in-bag dry-aged lean beef. Food Chem. 2020, 128601. [Google Scholar] [CrossRef] [PubMed]
- DeGeer, S.; Hunt, M.; Bratcher, C.; Crozier-Dodson, B.; Johnson, D.; Stika, J. Effects of dry aging of bone-in and boneless strip loins using two aging processes for two aging times. Meat Sci. 2009, 83, 768–774. [Google Scholar] [CrossRef]
- Dikeman, M.E.; Obuz, E.; Gök, V.; Akkaya, L.; Stroda, S. Effects of dry, vacuum, and special bag aging; USDA quality grade; and end-point temperature on yields and eating quality of beef Longissimus lumborum steaks. Meat Sci. 2013, 94, 228–233. [Google Scholar] [CrossRef]
- Kim, Y.H.B.; Meyers, B.; Kim, H.-W.; Liceaga, A.M.; LeMenager, R.P. Effects of stepwise dry/wet-aging and freezing on meat quality of beef loins. Meat Sci. 2017, 123, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Obuz, E.; Akkaya, L.; Gök, V.; Dikeman, M.E. Effects of blade tenderization, aging method and aging time on meat quality characteristics of Longissimus lumborum steaks from cull Holstein cows. Meat Sci. 2014, 96, 1227–1232. [Google Scholar] [CrossRef]
- Hulankova, R.; Kameník, J.; Saláková, A.; Závodský, D.; Borilova, G. The effect of dry aging on instrumental, chemical and microbiological parameters of organic beef loin muscle. LWT 2018, 89, 559–565. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Hopkins, D.L.; Bruce, H.; Li, D.; Baldi, G.; Bekhit, A.E.-D.A. Causes and contributing factors to “dark cutting” meat: Current trends and future directions: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 400–430. [Google Scholar] [CrossRef] [Green Version]
- Seideman, S.C.; Cross, H.R.; Smith, G.C.; Durland, P.R. Factors associated with fresh meat color: A review. J. Food Qual. 1984, 6, 211–237. [Google Scholar] [CrossRef]
- Renerre, M.; Labas, R. Biochemical factors influencing metmyoglobin formation in beef muscles. Meat Sci. 1987, 19, 151–165. [Google Scholar] [CrossRef]
- Sitz, B.M.; Calkins, C.R.; Feuz, D.M.; Umberger, W.J.; Eskridge, K.M. Consumer sensory acceptance and value of wet-aged and dry-aged beef steaks1. J. Anim. Sci. 2006, 84, 1221–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stenström, H.; Li, X.; Hunt, M.C.; Lundström, K. Consumer preference and effect of correct or misleading information after ageing beef longissimus muscle using vacuum, dry ageing, or a dry ageing bag. Meat Sci. 2014, 96, 661–666. [Google Scholar] [CrossRef]
- Berger, J.; Kim, Y.H.B.; Legako, J.F.; Martini, S.; Lee, J.; Ebner, P.; Zuelly, S.M.S. Dry-aging improves meat quality attributes of grass-fed beef loins. Meat Sci. 2018, 145, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.D.; Nicholson, K.L.; Nicholson, J.D.W.; Harris, K.B.; Miller, R.K.; Griffin, D.B.; Savell, J.W. Dry versus wet aging of beef: Retail cutting yields and consumer palatability evaluations of steaks from US Choice and US Select short loins. Meat Sci. 2008, 79, 631–639. [Google Scholar] [CrossRef] [PubMed]
- O’Quinn, T.; Woerner, D.; Engle, T.; Chapman, P.; Legako, J.; Brooks, J.; Belk, K.; Tatum, J. Identifying consumer preferences for specific beef flavor characteristics in relation to cattle production and postmortem processing parameters. Meat Sci. 2016, 112, 90–102. [Google Scholar] [CrossRef]
- Wood, J. Fat deposition and the quality of fat tissue in meat animals. In Fats in Animal Nutrition; Wiseman, J., Ed.; Butterworth-Heinemann: Oxford, UK, 1984; pp. 407–435. [Google Scholar]
- Young, O.A.; Reid, D.H.; Smith, M.E.; Braggins, T.J. Sheepmeat odour and flavour. In Flavor of Meat and Meat Products; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 1994; pp. 71–97. [Google Scholar]
- Estévez, M. Protein carbonyls in meat systems: A review. Meat Sci. 2011, 89, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Ahn, D. Mechanism of lipid peroxidation in meat and meat products—A review. Food Sci. Biotechnol. 2005, 14, 152–163. [Google Scholar]
- Campo, M.M.; Nute, G.R.; Hughes, S.I.; Enser, M.; Wood, J.D.; Richardson, R.I. Flavour perception of oxidation in beef. Meat Sci. 2006, 72, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Busboom, J.R.; Nelson, M.L.; O’Fallon, J.; Ringkob, T.P.; Rogers-Klette, K.R.; Joos, D.; Piper, K. The influence of forage diets and aging on beef palatability. Meat Sci. 2010, 86, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Jo, C.; Tariq, M.R. Meat flavor precursors and factors influencing flavor precursors—A systematic review. Meat Sci. 2015, 110, 278–284. [Google Scholar] [CrossRef]
- Mottram, D.S. Flavour formation in meat and meat products: A review. Food Chem. 1998, 62, 415–424. [Google Scholar] [CrossRef]
- Watanabe, A.; Kamada, G.; Imanari, M.; Shiba, N.; Yonai, M.; Muramoto, T. Effect of aging on volatile compounds in cooked beef. Meat Sci. 2015, 107, 12–19. [Google Scholar] [CrossRef]
- Choe, J.; Park, B.; Lee, H.J.; Jo, C. Potential antioxidant and angiotensin I-converting enzyme inhibitory activity in crust of dry-aged beef. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Flores, M.; Toldrá, F. Microbial enzymatic activities for improved fermented meats. Trends Food Sci. Technol. 2011, 22, 81–90. [Google Scholar] [CrossRef]
- Wu, G.; Clerens, S.; Farouk, M. LC MS/MS identification of large structural proteins from bull muscle and their degradation products during post mortem storage. Food Chem. 2014, 150, 137–144. [Google Scholar] [CrossRef]
- Sitrin, M.D. Digestion and absorption of carbohydrates and proteins. In The Gastrointestinal System: Gastrointestinal, Nutritional and Hepatobiliary Physiology; Leung, P.S., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 137–158. [Google Scholar]
- Krehbiel, C.R.; Matthews, J.C. Absorption of amino acids and peptides. In Amino Acids in Animal Nutrition, 2nd ed.; D’Mello, J.F., Ed.; CABI Publishing: Wallingford, UK, 2003; pp. 41–42. [Google Scholar]
- Flores, M.; Corral, S.; Cano-García, L.; Salvador, A.; Belloch, C. Yeast strains as potential aroma enhancers in dry fermented sausages. Int. J. Food Microbiol. 2015, 212, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Flores, M.; Durá, M.-A.; Marco, A.; Toldra, E. Effect of Debaryomyces spp. on aroma formation and sensory quality of dry-fermented sausages. Meat Sci. 2004, 68, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Fricker, G.; Drewe, J. Current concepts in intestinal peptide absorption. J. Pept. Sci. 1996, 2, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Corpet, D.E.; Yin, Y.; Zhang, X.; Rémésy, C.; Stamp, D.; Medline, A.; Thompson, L.; Bruce, W.R.; Archer, M.C. Colonic protein fermentation and promotion of colon carcinogenesis by thermolyzed casein. Nutr. Cancer 1995, 23, 271–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silvester, K.R.; Cummings, J.H. Does digestibility of meat protein help explain large bowel cancer risk? Nutr. Cancer 1995, 24, 279–288. [Google Scholar] [CrossRef] [PubMed]
|
|
|
|
| W 1 | BD 1 | SED 2 | p-Ageing | |
|---|---|---|---|---|
| pH | 5.92 a | 6.04 b | 0.02 | <0.0001 |
| % Moisture | 73.25 a | 67.30 b | 0.37 | <0.0001 |
| % Crude fat | 5.93 | 6.82 | 0.46 | 0.058 |
| % Muscle protein | 16.54 a | 20.22 b | 0.24 | <0.0001 |
| % Cook loss | 27.79 a | 16.94 b | 0.67 | <0.0001 |
| % Total loss | 28.48 a | 36.52 b | 0.56 | <0.0001 |
| Meat Surface Microbial Count (Mean log cfu/g) | ||||
| Aerobic bacteria | 5.16 a | 2.68 b | 0.75 | 0.030 |
| Lactic acid bacteria | 2.64 | n.d. | - | - |
| Moulds | n.d. 3 | n.d. | - | - |
| Yeast | 2.38 a | 3.75 b | 0.47 | 0.043 |
| Enterobacteriaceae | 2.36 | n.d. | - | - |
| Escherichia coli (log MPN/g) | n.d. | n.d. | - | - |
| W 1 | BD 1 | SED 2 | p-Ageing | p-Muscle (Across Treatments) | p-Ageing (Across Muscles) | |
|---|---|---|---|---|---|---|
| L* | ||||||
| SM3 | 44.01 ax | 42.57 b | 0.56 | 0.017 | <0.0001 | <0.0001 |
| BF3 | 44.52 ax | 42.66 b | <0.0001 | |||
| VL3 | 45.78 ay | 43.40 b | <0.0001 | |||
| RF3 | 45.70 ay | 43.63 b | 0.002 | |||
| p-muscle | 0.002 | 0.202 | ||||
| a* | ||||||
| SM | 14.92 axy | 13.84 bx | 0.52 | 0.041 | <0.0001 | <0.0001 |
| BF | 15.95 az | 14.40 bxy | 0.007 | |||
| VL | 15.78 xz | 14.97 y | 0.092 | |||
| RF | 14.39 ay | 12.43 bz | 0.001 | |||
| p-muscle | 0.004 | < 0.001 | ||||
| b* | ||||||
| SM | 12.62 ax | 11.66 bx | 0.39 | 0.011 | <0.0001 | <0.0001 |
| BF | 13.59 ay | 12.32 bxy | 0.003 | |||
| VL | 13.75 ay | 12.64 by | 0.003 | |||
| RF | 13.13 ax | 11.17 bz | <0.0001 | |||
| p-muscle | 0.009 | 0.003 | ||||
| Chroma | ||||||
| SM | 19.56 ax | 18.11 bx | 0.63 | 0.020 | <0.0001 | <0.0001 |
| BF | 20.96 ay | 18.96 bxy | 0.004 | |||
| VL | 20.93 ay | 19.59 by | 0.024 | |||
| RF | 19.49 ax | 16.73 bz | <0.001 | |||
| p-muscle | 0.009 | < 0.001 | ||||
| Hue | ||||||
| SM | 40.34 x | 40.20 x | 0.47 | 0.803 | <0.0001 | 0.216 |
| BF | 40.47 x | 40.67 x | 0.611 | |||
| VL | 41.11 ax | 40.25 bx | 0.017 | |||
| RF | 42.49 y | 42.12 y | 0.501 | |||
| p-muscle | < 0.0001 | < 0.001 |
| W 1 | BD 1 | SED 2 | p-Ageing | p-Muscle (Across Treatments) | p-Ageing (Across Muscles) | |
|---|---|---|---|---|---|---|
| Hardness (N) | ||||||
| SM3 | 21.14 a | 25.27 b | 1.66 | 0.015 | 0.366 | <0.001 |
| BF3 | 22.53 | 26.50 | 0.067 | |||
| VL3 | 22.48 a | 27.19 b | 0.004 | |||
| RF3 | 22.17 | 24.45 | 0.120 | |||
| p-muscle | 0.680 | 0.465 | ||||
| Chewiness (N) | ||||||
| SM | 7.47 | 8.46 | 0.69 | 0.134 | 0.017 | <0.001 |
| BF | 8.22 a | 9.89 b | 0.048 | |||
| VL | 7.67 a | 9.46 b | 0.014 | |||
| RF | 7.27 | 7.87 | 0.309 | |||
| p-muscle | 0.414 | 0.051 | ||||
| Springiness | ||||||
| SM | 0.62 xy | 0.63 x | 0.02 | 0.514 | <0.0001 | 0.427 |
| BF | 0.65 x | 0.65 x | 0.979 | |||
| VL | 0.61 y | 0.62 xy | 0.463 | |||
| RF | 0.59 y | 0.60 y | 0.657 | |||
| p-muscle | 0.012 | 0.010 | ||||
| Cohesiveness | ||||||
| SM | 0.56 a | 0.53 b | 0.01 | 0.002 | 0.597 | 0.001 |
| BF | 0.55 | 0.54 | 0.360 | |||
| VL | 0.55 | 0.54 | 0.497 | |||
| RF | 0.55 | 0.53 | 0.057 | |||
| p-muscle | 0.373 | 0.354 | ||||
| Adhesiveness | ||||||
| SM | −11.56 | −18.13 | 4.10 | 0.229 | 0.054 | 0.335 |
| BF | −9.18 | −8.35 | 0.229 | |||
| VL | −10.93 | −12.31 | 0.736 | |||
| RF | −7.08 | −8.44 | 0.593 | |||
| p-muscle | 0.439 | 0.127 | ||||
| Resilience | ||||||
| SM | 0.23 a | 0.21 b | 0.01 | 0.017 | 0.284 | 0.048 |
| BF | 0.22 | 0.22 | 0.845 | |||
| VL | 0.22 | 0.22 | 0.465 | |||
| RF | 0.22 | 0.21 | 0.356 | |||
| p-muscle | 0.123 | 0.443 |
| W 1 | BD 1 | SED 2 | p-Ageing | |
|---|---|---|---|---|
| Degree of Liking | 6.75 | 6.68 | 0.19 | 0.682 |
| % preferred 3 | 44.74 | 40.35 | ||
| Eating Quality Rating | 3.14 | 3.10 | 0.13 | 0.750 |
| % rating 4 | 0.670 | |||
| Unsatisfactory as an everyday product | 2.65 | 4.42 | ||
| Good everyday product | 29.20 | 23.89 | ||
| Slightly better than an everyday product | 31.86 | 37.17 | ||
| Almost a premium product | 24.78 | 26.55 | ||
| A premium product | 11.50 | 7.96 |
| W 1 | BD 1 | SED 2 | p-Ageing | |
|---|---|---|---|---|
| Protein Carbonyl (nmol/mg Protein) | 2.20 | 2.31 | 0.08 | 0.151 |
| TBARS 3 (mg MDA 3/kg Meat) | 0.38 a | 1.30 b | 0.13 | <0.0001 |
| Fatty Acid Profile (mg/g dry Matter) | ||||
| 10:0 | 0.18 | 0.17 | 0.02 | 0.631 |
| 12:0 | 0.35 | 0.33 | 0.03 | 0.539 |
| 14:0 | 2.56 | 2.44 | 0.22 | 0.580 |
| 14:1 | 0.09 | 0.09 | 0.01 | 0.490 |
| 15:0 | 0.27 | 0.25 | 0.02 | 0.390 |
| 16:0 | 12.71 | 12.44 | 0.72 | 0.706 |
| 16:1 | 1.00 | 1.01 | 0.07 | 0.879 |
| 17:0 | 1.10 | 1.07 | 0.05 | 0.542 |
| 17:1 | 0.38 | 0.37 | 0.02 | 0.490 |
| 18:0 | 10.89 | 10.60 | 0.57 | 0.606 |
| 18:1 (n 3—9, cis & trans) | 18.37 | 18.22 | 1.06 | 0.887 |
| 18:2 (n—6, cis & trans) | 2.00 | 2.02 | 0.07 | 0.715 |
| 18:3 (n—6) | 0.21 | 0.21 | 0.02 | 0.848 |
| 18:3 (n—3) | 1.31 | 1.29 | 0.05 | 0.713 |
| 20:0 | 0.19 | 0.20 | 0.01 | 0.272 |
| 20:2 (n—6) | 0.15 | 0.16 | 0.01 | 0.429 |
| 20:6 (n—6) | 0.23 | 0.23 | 0.01 | 0.873 |
| 20:5 (n—3) | 0.69 | 0.70 | 0.02 | 0.801 |
| 22:0 | 0.27 | 0.27 | 0.01 | 0.900 |
| 24:0 | 0.20 | 0.20 | 0.01 | 0.946 |
| 22:6 (n—3) | 0.36 | 0.35 | 0.01 | 0.725 |
| UFAs 3 | 24.80 | 24.65 | 1.24 | 0.905 |
| SFAs 3 | 28.72 | 27.96 | 1.47 | 0.609 |
| MUFAs 3 | 19.84 | 19.68 | 1.14 | 0.890 |
| n—3 | 2.36 | 2.34 | 0.05 | 0.756 |
| n—6 | 2.59 | 2.62 | 0.08 | 0.734 |
| PUFAs 3 | 4.95 | 4.96 | 0.12 | 0.928 |
| % UFAs | 46.40 | 46.92 | 0.47 | 0.266 |
| % SFAs | 53.60 | 53.08 | 0.47 | 0.266 |
| % MUFAs | 36.87 | 37.15 | 0.54 | 0.609 |
| % n—3 | 4.56 | 4.64 | 0.22 | 0.721 |
| % n—6 | 4.96 | 5.14 | 0.20 | 0.390 |
| % PUFAs | 9.52 | 9.77 | 0.40 | 0.534 |
| 0 Min | 2 Min | 10 min | 60 Min | 120 Min | 240 Min | SED 2 | p-Ageing (Across Digestion Time) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W 1 | BD 1 | p-Ageing | W | BD | p-Ageing | W | BD | p-Ageing | W | BD | p-Ageing | W | BD | p-Ageing | W | BD | p-Ageing | |||
| Essential amino acids | ||||||||||||||||||||
| Histidine | 0.36 | 0.46 | 0.252 | 0.35 | 0.41 | 0.202 | 0.37 | 0.40 | 0.481 | 0.42 a | 0.60 b | 0.021 | 0.38 a | 0.57 b | 0.001 | 0.73 a | 0.87 b | 0.034 | 0.06 | <0.0001 |
| Isoleucine | 1.24 a | 1.57 b | 0.018 | 0.87 | 0.94 | 0.570 | 0.87 | 0.92 | 0.458 | 0.97 a | 1.21 b | 0.049 | 1.00 a | 1.31 b | 0.005 | 1.80 | 2.01 | 0.153 | 0.10 | <0.0001 |
| Leucine | 1.01 | 0.98 | 0.907 | 1.49 | 1.60 | 0.574 | 1.51 | 1.59 | 0.507 | 1.77 | 2.18 | 0.075 | 1.89 a | 2.49 b | 0.005 | 12.13 | 12.75 | 0.591 | 0.50 | 0.150 |
| Lysine | 1.01 a | 1.19 b | 0.001 | 1.27 | 1.39 | 0.528 | 1.46 | 1.59 | 0.463 | 1.42 a | 2.01 b | 0.021 | 1.37 a | 2.10 b | 0.002 | 13.56 | 13.36 | 0.922 | 0.88 | 0.505 |
| Methionine | 0.66 | 0.72 | 0.491 | 0.66 | 0.71 | 0.571 | 0.59 | 0.59 | 0.996 | 0.73 | 0.82 | 0.410 | 0.79 a | 0.97 b | 0.030 | 1.89 | 2.05 | 0.438 | 0.11 | 0.063 |
| Phenylalanine | 1.60 | 2.00 | 0.144 | 1.00 | 1.03 | 0.669 | 0.99 | 1.01 | 0.632 | 1.25 | 1.38 | 0.180 | 1.46 a | 1.70 b | 0.025 | 10.41 | 10.91 | 0.632 | 0.44 | 0.218 |
| Threonine | 1.34 | 1.73 | 0.153 | 0.56 | 0.63 | 0.285 | 0.63 | 0.77 | 0.305 | 0.67 a | 0.98 b | 0.045 | 0.65 a | 1.01 b | 0.007 | 0.82 a | 1.05 b | 0.004 | 0.14 | <0.0001 |
| Tryptophan | 0.24 | 0.30 | 0.238 | 0.53 | 0.55 | 0.424 | 0.55 | 0.56 | 0.704 | 0.63 | 0.68 | 0.125 | 0.67 a | 0.75 b | 0.011 | 2.55 | 2.58 | 0.898 | 0.11 | 0.372 |
| Valine | 1.01 | 1.27 | 0.087 | 1.16 | 1.26 | 0.518 | 1.23 | 1.29 | 0.532 | 1.32 a | 1.70 b | 0.038 | 1.36 a | 1.85 b | 0.005 | 1.92 a | 2.34 b | 0.028 | 0.14 | <0.0001 |
| Non-essential amino acids | ||||||||||||||||||||
| Alanine | 1.97 | 2.21 | 0.547 | 3.25 | 3.57 | 0.405 | 3.38 | 3.51 | 0.771 | 3.78 | 4.36 | 0.242 | 3.92 a | 4.93 b | 0.034 | 3.91 | 4.60 | 0.101 | 0.39 | 0.004 |
| Arginine | 1.35 | 1.63 | 0.152 | 1.04 | 1.16 | 0.380 | 1.02 | 1.08 | 0.599 | 1.25 a | 1.49 b | 0.034 | 1.40 | 1.64 | 0.155 | 20.02 | 20.12 | 0.963 | 0.91 | 0.643 |
| Asparagine | 0.45 | 0.59 | 0.124 | 0.46 | 0.53 | 0.227 | 0.52 | 0.55 | 0.585 | 0.82 | 0.82 | 0.768 | 1.24 a | 1.73 a | 0.004 | 1.46 a | 1.84 b | 0.008 | 0.11 | <0.0001 |
| Aspartic acid | 0.35 a | 0.64 b | 0.043 | 0.49 | 0.52 | 0.688 | 0.70 | 0.76 | 0.446 | 0.60 a | 1.00 b | 0.004 | 0.63 a | 1.15 b | 0.000 | 0.67 a | 1.11 b | 0.000 | 0.09 | <0.0001 |
| Cysteine | 0.04 | 0.04 | 0.236 | 0.69 a | 0.83 b | 0.014 | 0.85 | 0.98 | 0.412 | 0.91 a | 1.19 b | 0.046 | 0.91 a | 1.30 b | 0.002 | 1.18 | 1.38 | 0.233 | 0.11 | <0.0001 |
| Glutamic acid | 0.93 | 1.16 | 0.354 | 1.13 | 1.24 | 0.534 | 1.25 | 1.17 | 0.630 | 1.36 | 1.59 | 0.139 | 1.47 a | 1.81 b | 0.007 | 1.36 a | 1.69 b | 0.044 | 0.16 | 0.005 |
| Glutamine | 3.67 | 4.18 | 0.447 | 2.97 | 3.26 | 0.567 | 2.94 | 3.22 | 0.672 | 3.68 | 4.14 | 0.367 | 3.43 | 4.58 | 0.056 | 3.68 | 4.50 | 0.258 | 0.59 | 0.017 |
| Glycine | 1.19 | 1.28 | 0.603 | 2.80 | 2.92 | 0.659 | 2.71 | 2.91 | 0.254 | 2.94 | 3.19 | 0.232 | 2.92 a | 3.50 b | 0.008 | 3.29 | 3.78 | 0.054 | 0.20 | 0.001 |
| Hydroxyproline | 0.11 | 0.11 | 0.847 | 0.04 | 0.05 | 0.302 | 0.04 | 0.04 | 0.580 | 0.06 | 0.05 | 0.218 | 0.05 | 0.06 | 0.094 | 0.05 | 0.06 | 0.067 | 0.01 | 0.051 |
| Proline | 0.50 a | 0.71 b | 0.045 | 0.42 | 0.45 | 0.534 | 0.47 | 0.51 | 0.468 | 0.50 a | 0.65 b | 0.031 | 0.60 a | 0.85 b | 0.007 | 0.50 a | 0.67 b | 0.014 | 0.06 | <0.0001 |
| Serine | 1.20 | 1.46 | 0.259 | 1.38 | 1.50 | 0.530 | 1.50 | 1.56 | 0.668 | 1.62 | 2.04 | 0.055 | 1.70 a | 2.24 b | 0.003 | 1.69 a | 2.17 b | 0.008 | 0.17 | <0.0001 |
| Tyrosine | 0.93 | 1.13 | 0.361 | 0.93 | 0.99 | 0.603 | 0.93 | 0.98 | 0.384 | 1.11 | 1.29 | 0.129 | 1.15 a | 1.43 b | 0.008 | 8.63 | 9.01 | 0.657 | 0.37 | 0.203 |
| EAAs 3 | 8.48 | 10.23 | 0.092 | 7.88 | 8.52 | 0.498 | 8.20 | 8.72 | 0.460 | 9.23 | 11.56 | 0.044 | 9.56 | 12.75 | 0.005 | 45.81 | 47.92 | 0.682 | 2.24 | 0.063 |
| % EAAs | 40.43 | 40.65 | 0.895 | 33.53 | 33.39 | 0.874 | 33.56 | 33.73 | 0.877 | 33.46 | 34.55 | 0.484 | 32.98 | 33.57 | 0.569 | 49.51 | 48.53 | 0.258 | 1.15 | 0.790 |
| Total AAs 3 | 21.17 | 25.36 | 0.211 | 23.48 | 25.54 | 0.458 | 24.51 | 25.99 | 0.547 | 27.82 | 33.37 | 0.052 | 28.99 a | 37.96 b | 0.003 | 92.25 | 98.84 | 0.499 | 4.59 | 0.014 |
| W 1 | BD 1 | p-Ageing | SED 2 | |
|---|---|---|---|---|
| Relative digestibility SDS-PAGE | 63.96 | 65.24 | 0.776 | 4.36 |
| Relative digestibility FAAs | 7.11 | 7.35 | 0.802 | 0.92 |
| Relative digestibility protein content | 70.18 | 70.75 | 0.570 | 0.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Yoo, M.J.Y.; Realini, C.E.; Staincliffe, M.; Farouk, M.M. In-Bag Dry- vs. Wet-Aged Lamb: Quality, Consumer Acceptability, Oxidative Stability and In Vitro Digestibility. Foods 2021, 10, 41. https://doi.org/10.3390/foods10010041
Zhang R, Yoo MJY, Realini CE, Staincliffe M, Farouk MM. In-Bag Dry- vs. Wet-Aged Lamb: Quality, Consumer Acceptability, Oxidative Stability and In Vitro Digestibility. Foods. 2021; 10(1):41. https://doi.org/10.3390/foods10010041
Chicago/Turabian StyleZhang, Renyu, Michelle J. Y. Yoo, Carolina E. Realini, Maryann Staincliffe, and Mustafa M. Farouk. 2021. "In-Bag Dry- vs. Wet-Aged Lamb: Quality, Consumer Acceptability, Oxidative Stability and In Vitro Digestibility" Foods 10, no. 1: 41. https://doi.org/10.3390/foods10010041
APA StyleZhang, R., Yoo, M. J. Y., Realini, C. E., Staincliffe, M., & Farouk, M. M. (2021). In-Bag Dry- vs. Wet-Aged Lamb: Quality, Consumer Acceptability, Oxidative Stability and In Vitro Digestibility. Foods, 10(1), 41. https://doi.org/10.3390/foods10010041






