Halophytes of the Mediterranean Basin—Underutilized Species with the Potential to Be Nutritious Crops in the Scenario of the Climate Change
Abstract
1. Introduction
2. Material and Methods
2.1. Relative Growth Rate
2.2. Analysis of Mineral Elements
2.3. Phenolic Compounds
2.4. Protein and Total Lipid Analysis
2.5. Data Analysis
3. Results
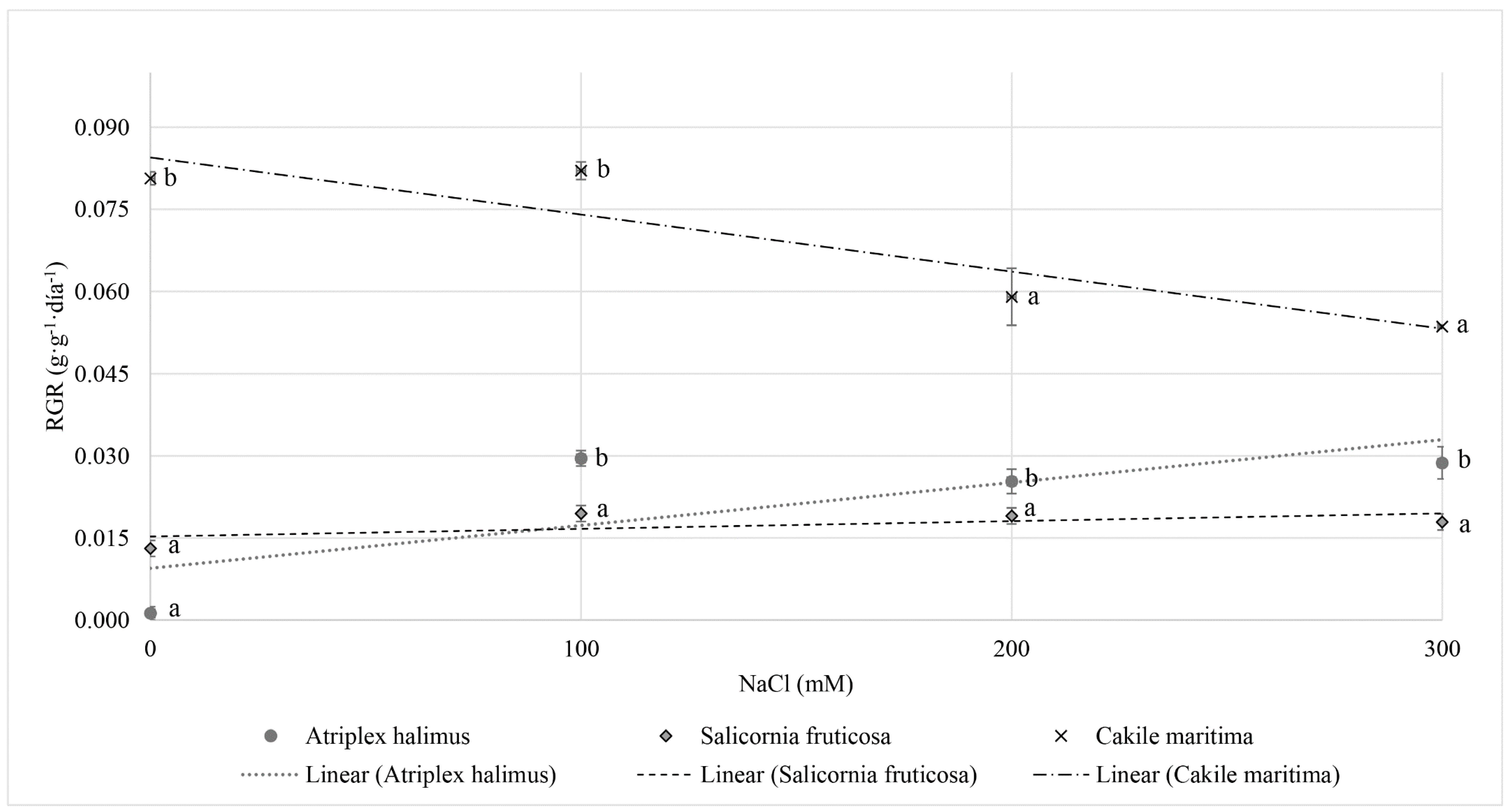
3.1. Relative Growth Rate
3.2. Mineral Elements
3.3. Phenolic Content
3.4. Protein and Lipid Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kaushal, S.S.; Likens, G.E.; Pace, M.L.; Utz, R.M.; Haq, S.; Gorman, J.; Grese, M. Freshwater salinization syndrome on a continental scale. Proc. Natl. Acad. Sci. USA 2018, 115, E574–E583. [Google Scholar] [CrossRef] [PubMed]
- Herbert, E.R.; Boon, P.; Burgin, A.J.; Neubauer, S.C.; Franklin, R.B.; Ardon, M.; Hopfensperger, K.N.; Lamers, L.P.M.; Gell, P.; Langley, J.A. A global perspective on wetland salinization: Ecological consequences of a growing threat to freshwater wetlands. Ecosphere 2015, 6, 1–43. [Google Scholar] [CrossRef]
- Wienhold, B.J. Soils, Land, and Food: Managing the Land during the Twenty-First Century. Soil Sci. 2003, 168, 748–749. [Google Scholar] [CrossRef]
- Khan, W.-D.; Tanveer, M.; Shaukat, R.; Ali, M.; Pirdad, F. Salt and Drought Stress Tolerance in Plants: Signaling and Communication in Plants; Springer: Berlin, Germany, 2020; ISBN 9783030402761. [Google Scholar]
- George, E.; Horst, W.J.; Neumann, E. Adaptation of Plants to Adverse Chemical Soil Conditions. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Elsevier Inc.: Hohenheim, Germany, 2011; pp. 409–472. ISBN 9780123849052. [Google Scholar]
- Shannon, M.C.; Grieve, C.M. Tolerance of vegetable crops to salinity. Sci. Hortic. 1998, 78, 5–38. [Google Scholar] [CrossRef]
- Flowers, T.; Hajibagheri, M.; Clipson, N. Halophytes. Q. Rev. Biol. 1986, 61, 313–337. [Google Scholar] [CrossRef]
- Flowers, T.J.; Muscolo, A. Introduction to the Special Issue: Halophytes in a changing world. AoB Plants 2015, 7, plv020. [Google Scholar] [CrossRef]
- Panta, S.; Flowers, T.; Lane, P.; Doyle, R.; Haros, G.; Shabala, S. Halophyte agriculture: Success stories. Environ. Exp. Bot. 2014, 107, 71–83. [Google Scholar] [CrossRef]
- Flowers, T.J.; Galal, H.K.; Bromham, L. Evolution of halophytes: Multiple origins of salt tolerance in land plants. Funct. Plant Biol. 2010, 37, 604–612. [Google Scholar] [CrossRef]
- Ksouri, R.; Megdiche, W.; Falleh, H.; Trabelsi, N.; Boulaaba, M.; Smaoui, A.; Abdelly, C. Influence of biological, environmental and technical factors on phenolic content and antioxidant activities of Tunisian halophytes. C. R. Biol. 2008, 331, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Benhammou, N.; Bekkara, F.A.; Kadifkova Panovska, T. Antioxidant activity of methanolic extracts and some bioactive compounds of Atriplex halimus. C. R. Chim. 2009, 12, 1259–1266. [Google Scholar] [CrossRef]
- Mishra, A.; Patel, M.K.; Jha, B. Non-targeted metabolomics and scavenging activity of reactive oxygen species reveal the potential of Salicornia brachiata as a functional food. J. Funct. Foods 2015, 13, 21–31. [Google Scholar] [CrossRef]
- Bertin, R.L.; Gonzaga, L.V.; Borges, G.d.S.C.; Azevedo, M.S.; Maltez, H.F.; Heller, M.; Micke, G.A.; Tavares, L.B.B.; Fett, R. Nutrient composition and, identification/quantification of major phenolic compounds in Sarcocornia ambigua (Amaranthaceae) using HPLC-ESI-MS/MS. Food Res. Int. 2014, 55, 404–411. [Google Scholar] [CrossRef]
- Buhmann, A.; Papenbrock, J. An economic point of view of secondary compounds in halophytes. Funct. Plant Biol. 2013, 40, 952–967. [Google Scholar] [CrossRef] [PubMed]
- Randhir, R.; Lin, Y.T.; Shetty, K. Stimulation of phenolics, antioxidant and antimicrobial activities in dark germinated mung bean sprouts in response to peptide and phytochemical elicitors. Process Biochem. 2004, 39, 637–646. [Google Scholar] [CrossRef]
- Ventura, Y.; Wuddineh, W.A.; Myrzabayeva, M.; Alikulov, Z.; Khozin-Goldberg, I.; Shpigel, M.; Samocha, T.M.; Sagi, M. Effect of seawater concentration on the productivity and nutritional value of annual Salicornia and perennial Sarcocornia halophytes as leafy vegetable crops. Sci. Hortic. 2011, 128, 189–196. [Google Scholar] [CrossRef]
- Ksouri, R.; Megdiche, W.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol. Biochem. 2007, 45, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Giménez-Martínez, J.J.; Torija-Isasa, M.E. Nutritional composition of wild edible crucifer species. J. Food Biochem. 1999, 23, 283–294. [Google Scholar] [CrossRef]
- Khan, M.A.; Weber, D.J. Ecophysiology of High Salinity Tolerant Plants; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Hoi, K.D.; Hyun, K.T. Salicornia spp.-Derived Salt and Its Production Process. U.S. Patent No 8,420,152, 16 April 2013. [Google Scholar]
- Kim, H.-W.; Hwang, K.-E.; Song, D.-H.; Kim, Y.-J.; Ham, Y.-K.; Yeo, I.-J.; Jeong, T.-J.; Choi, Y.-S.; Kim, C.-J. Effects of Red and Green Glassworts (Salicornia herbacea L.) on Physicochemical and Textural Properties of Reduced-salt Cooked Sausages. Korean J. Food Sci. Anim. Resour. 2014, 34, 378. [Google Scholar] [CrossRef]
- Kim, H.L.; Kim, I.C. Sports Beverage Composition Comprising Salicornia Herbacia Extacts. KR20110002392, 1 July 2019. [Google Scholar]
- Lu, Z.; Hodges, R.M.; Mota-Urbina, C.J.; Gallawa, P.L.; Chaturvedi, R.; DeCianne, D.M.; Glenn, E.P.; Carl, N. Hodges. Salicornia Bigelovii (Chenopodiaceae)—A Seawater-Irrigated Crop with Versatile Commercial Products. In Proceedings of the 5th New Crops Symposium, Atlanta, Georgia, 10–13 November 2001; pp. 28–29. [Google Scholar]
- Wright, J. Edible Seashore: River Cottage Handbook; Bloomsbury Publishing: London, UK, 2009; ISBN 0747595313. [Google Scholar]
- Walker, D.J.; Lutts, S.; Sánchez-García, M.; Correal, E. Atriplex halimus L.: Its biology and uses. J. Arid Environ. 2014, 100, 111–121. [Google Scholar] [CrossRef]
- Epstein, E. Mineral Nutrition of Plants: Principles and Perspectives; Sinauer Associates, Inc.: Sunderland, MA, USA, 1972. [Google Scholar]
- Hunt, R.; Causton, D.R.; Shipley, B.; Askew, A.P. A modern tool for classical plant growth analysis. Ann. Bot. 2002, 90, 485–488. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley Sloane, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Jenks, M.A.; Hasegawa, P.M.; Jain, S.M. (Eds.) Advances in Molecular Breeding toward Drought and Salt Tolerant Crops, 1st ed.; Springer: Berlin, Germany, 2007; pp. 1–32. ISBN 9781402055775. [Google Scholar]
- He, Q.; Silliman, B.R.; Cui, B. Incorporating thresholds into understanding salinity tolerance: A study using salt-tolerant plants in salt marshes. Ecol. Evol. 2017, 7, 6326–6333. [Google Scholar] [CrossRef]
- Debez, A.; Braun, H.-P.; Pich, A.; Taamalli, W.; Koyro, H.-W.; Abdelly, C.; Huchzermeyer, B. Proteomic and physiological responses of the halophyte Cakile maritima to moderate salinity at the germinative and vegetative stages. J. Proteomics 2012, 75, 5667–5694. [Google Scholar] [CrossRef]
- Amor, N.N.; Jimenez, A.; Megdiche, W.; Lundqvist, M.; Sevilla, F.; Abdelly, C. Response of antioxidant systems to NaCl stress in the halophyte Cakile maritima. Physiol. Plant. 2006, 126, 446–457. [Google Scholar] [CrossRef]
- Megdiche, W.; Amor, N.B.; Debez, A.; Hessini, K.; Ksouri, R.; Zuily-Fodil, Y.; Abdelly, C. Salt tolerance of the annual halophyte Cakile maritima as affected by the provenance and the developmental stage. Acta Physiol. Plant. 2007, 29, 375–384. [Google Scholar] [CrossRef]
- Wided, M.; Nader, B.A.; Debez, A.; Kamel, H.; Riadh, K.; Chedly, A.; Ahmed, D.; Kamel, H.; Riadh, K.; Chedly, A. Physiological and biochemical traits involved in the genotypic variability to salt tolerance of Tunisian Cakile maritima. Afr. J. Ecol. 2009, 47, 774–783. [Google Scholar] [CrossRef]
- Debez, A.; Hamed, K.B.; Grignon, C.; Abdelly, C. Salinity effects on germination, growth, and seed production of the halophyte Cakile maritima. Plant Soil 2004, 262, 179–189. [Google Scholar] [CrossRef]
- Debez, A.; Saadaoui, D.; Ramani, B.; Ouerghi, Z.; Koyro, H.-W.; Huchzermeyer, B.; Abdelly, C. Leaf H +-ATPase activity and photosynthetic capacity of Cakile maritima under increasing salinity. Environ. Exp. Bot. 2006, 57, 285–295. [Google Scholar] [CrossRef]
- Boughalleb, F.; Denden, M.; Tiba, B. Ben Anatomical changes induced by increasing NaCl salinity in three fodder shrubs, Nitraria retusa, Atriplex halimus and Medicago arborea. Acta Physiol. Plant. 2009, 31, 947–960. [Google Scholar] [CrossRef]
- Bankaji, I.; Sleimi, N.; López-Climent, M.F.; Perez-Clemente, R.M.; Gomez-Cadenas, A. Effects of Combined Abiotic Stresses on Growth, Trace Element Accumulation, and Phytohormone Regulation in Two Halophytic Species. J. Plant Growth Regul. 2014, 33, 632–643. [Google Scholar] [CrossRef]
- Bendaly, A.; Messedi, D.; Smaoui, A.; Ksouri, R.; Bouchereau, A.; Abdelly, C. Physiological and leaf metabolome changes in the xerohalophyte species Atriplex halimus induced by salinity. Plant Physiol. Biochem. 2016, 103, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Belkheiri, O.; Mulas, M. The effects of salt stress on growth, water relations and ion accumulation in two halophyte Atriplex species. Environ. Exp. Bot. 2013, 86, 17–28. [Google Scholar] [CrossRef]
- Katschnig, D.; Broekman, R.; Rozema, J. Salt tolerance in the halophyte Salicornia dolichostachya moss: Growth, morphology and physiology. Environ. Exp. Bot. 2013, 92, 32–42. [Google Scholar] [CrossRef]
- Pérez-Romero, J.A.; Idaszkin, Y.L.; Barcia-Piedras, J.M.; Duarte, B.; Redondo-Gómez, S.; Caçador, I.; Mateos-Naranjo, E. Disentangling the effect of atmospheric CO2 enrichment on the halophyte Salicornia ramosissima J. Woods physiological performance under optimal and suboptimal saline conditions. Plant Physiol. Biochem. 2018, 127, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Romero, J.A.; Duarte, B.; Barcia-Piedras, J.-M.; Rita Matos, A.; Redondo-Gómez, S.; Caçador, I.; Mateos-Naranjo, E. Investigating the physiological mechanisms underlying Salicornia ramosissima response to atmospheric CO2 enrichment under coexistence of prolonged soil flooding and saline excess. Plant Physiol. Biochem. 2019, 135, 149–159. [Google Scholar] [CrossRef]
- National Research Council. Saline Agriculture: Salt-Tolerant Plants for Developing Countries; National Academies Press: Washington, DC, USA, 1990. [Google Scholar]
- Ventura, Y.; Sagi, M. Halophyte crop cultivation: The case for Salicornia and Sarcocornia. Environ. Exp. Bot. 2013, 92, 144–153. [Google Scholar] [CrossRef]
- Burnier, M.; Wuerzner, G.; Bochud, M.; Aepli, S.; Arnold, M.; Conen, D.; Erne, P.; Hayoz, D.; Henzen, C.; Junker, T.; et al. Salt, blood pressure and cardiovascular risk: What is the most adequate preventive strategy? A Swiss perspective. Front. Physiol. 2015, 6, 227. [Google Scholar] [CrossRef]
- Comission, E. Food-Based Dietary Guidelines in Europe|EU Science Hub. Available online: https://ec.europa.eu/jrc/en/health-knowledge-gateway/promotion-prevention/nutrition/food-based-dietary-guidelines (accessed on 18 December 2020).
- Sociedad Española de Nutrición Comunitaria. Guías alimentarias para la población española (SENC, diciembre 2016); la nueva pirámide de la alimentación saludable. Nutr. Hosp. 2016, 33, 1–48. [Google Scholar]
- Barreira, L.; Resek, E.; Rodrigues, M.J.; Rocha, M.I.; Pereira, H.; Bandarra, N.; da Silva, M.M.; Varela, J.; Custódio, L. Halophytes: Gourmet food with nutritional health benefits? J. Food Compos. Anal. 2017, 59, 35–42. [Google Scholar] [CrossRef]
- El-Said, G.F.; El-Sikaily, A. Chemical composition of some seaweed from Mediterranean Sea coast, Egypt. Environ. Monit. Assess. 2013, 185, 6089–6099. [Google Scholar] [CrossRef]
- Antunes, M.D.; Gago, C.; Branquinho, A.R.; Julião, M.; Guerreiro, A.; Miguel, G.; Faleiro, M.L.; Panagopoulos, T. Behavior of “Green salt” from Salicornia ramosissima and Sarcocornia perennis through storage. Acta Hortic. 2018, 1194, 777–783. [Google Scholar] [CrossRef]
- Renna, M.; Gonnella, M. The use of the sea fennel as a new spice-colorant in culinary preparations. Int. J. Gastron. Food Sci. 2012, 1, 111–115. [Google Scholar] [CrossRef]
- Sánchez-Faure, A.; Calvo, M.M.; Pérez-Jiménez, J.; Martín-Diana, A.B.; Rico, D.; Montero, M.P.; Gómez-Guillén, M.d.C.; López-Caballero, M.E.; Martínez-Alvarez, O. Exploring the potential of common iceplant, seaside arrowgrass and sea fennel as edible halophytic plants. Food Res. Int. 2020, 137, 109613. [Google Scholar] [CrossRef]
- Lv, S.; Jiang, P.; Chen, X.; Fan, P.; Wang, X.; Li, Y. Multiple compartmentalization of sodium conferred salt tolerance in Salicornia europaea. Plant Physiol. Biochem. 2012, 51, 47–52. [Google Scholar] [CrossRef]
- Ushakova, S.A.; Kovaleva, N.P.; Gribovskaya, I.V.; Dolgushev, V.A.; Tikhomirova, N.A. Effect of NaCl concentration on productivity and mineral composition of Salicornia europaea as a potential crop for utilization NaCl in LSS. Adv. Space Res. 2005, 36, 1349–1353. [Google Scholar] [CrossRef]
- Tikhomirova, N.A.; Ushakova, S.A.; Shklavtsova, E.S.; Anishchenko, O.V.; Mikheeva, Y.A.; Tikhomirov, A.A. Effects of PAR Intensity and NaCl Concentration on Growth of Salicornia europaea Plants as Relevant to Artificial Ecological Systems. Russ. J. Plant Physiol. 2016, 63, 504–513. [Google Scholar] [CrossRef]
- Khan, M.A.; Ungar, I.A.; Showalter, A.M. Effects of salinity on growth, water relations and ion accumulation of the subtropical perennial halophyte, Atriplex griffithii var. stocksii. Ann. Bot. 2000, 85, 225–232. [Google Scholar] [CrossRef]
- Rupérez, P. Mineral content of edible marine seaweeds. Food Chem. 2002, 79, 23–26. [Google Scholar] [CrossRef]
- Triana, M.H. Recomendaciones nutricionales para el ser humano: Actualización. Rev. Cuba. Investig. Biomed. 2004, 23, 266–292. [Google Scholar]
- Nutrient Lists from Standard Reference Legacy (2018)|Food and Nutrition Information Center|NAL|USDA. Available online: https://www.nal.usda.gov/fnic/nutrient-lists-standard-reference-legacy-2018 (accessed on 25 November 2020).
- Guilbert, J.J. The world health report 2002—Reducing risks, promoting healthy life. Educ. Health 2003, 16, 230. [Google Scholar] [CrossRef]
- Padash, A.; Shahabivand, S.; Behtash, F.; Aghaee, A. A practicable method for zinc enrichment in lettuce leaves by the endophyte fungus Piriformospora indica under increasing zinc supply. Sci. Hortic. 2016, 213, 367–372. [Google Scholar] [CrossRef]
- Sularz, O.; Smoleń, S.; Koronowicz, A.; Kowalska, I.; Leszczyńska, T. Chemical composition of lettuce (Lactuca sativa L.) biofortified with iodine by KIO3, 5-Iodo-, and 3.5-diiodosalicylic acid in a hydroponic cultivation. Agronomy 2020, 10, 1022. [Google Scholar] [CrossRef]
- Wong, K.W.; Yap, C.K.; Nulit, R.; Omar, H.; Aris, A.Z.; Cheng, W.H.; Latif, M.T.; Leow, C.S. Zn in vegetables: A review and some insights. Integr. Food Nutr. Metab. 2019, 6, 1–7. [Google Scholar] [CrossRef]
- Jallali, I.; Megdiche, W.; M’Hamdi, B.; Oueslati, S.; Smaoui, A.; Abdelly, C.; Ksouri, R. Changes in phenolic composition and antioxidant activities of the edible halophyte Crithmum maritimum L. with physiological stage and extraction method. Acta Physiol. Plant. 2012, 34, 1451–1459. [Google Scholar] [CrossRef]
- Adebooye, O.C.; Vijayalakshmi, R.; Singh, V. Peroxidase activity, chlorophylls and antioxidant profile of two leaf vegetables (Solanum nigrum L. and Amaranthus cruentus L.) under six pretreatment methods before cooking. Int. J. Food Sci. Technol. 2008, 43, 173–178. [Google Scholar] [CrossRef]
- Boestfleisch, C.; Wagenseil, N.B.; Buhmann, A.K.; Seal, C.E.; Wade, E.M.; Muscolo, A.; Papenbrock, J. Manipulating the antioxidant capacity of halophytes to increase their cultural and economic value through saline cultivation. AoB Plants 2014, 6, 1–16. [Google Scholar] [CrossRef]
- Barrett-Lennard, E.G.; Bathgate, A.; Malcolm, C.V. Saltland Pastures in Australia: A Practical Guide; Bulletin No. 4312; WA Government. Department of Agriculture and Food: Perth, Australia, 2016; ISBN 192086007X.
- Andueza, D.; Muñoz, F.; Delgado, I.; Correal, E. Intraspecific variation in Atriplex halimus: Chemical composition of edible biomass. Options Mediterr. 2005, 377–381. [Google Scholar]
- El-Shatnawi, M.K.J.; Turuk, M. Dry matter accumulation and chemical content of saltbush (atriplex halimus) grown in mediterranean desert shrublands. N. Z. J. Agric. Res. 2002, 45, 139–144. [Google Scholar] [CrossRef]
- Obeidat, B.S.; Mahmoud, K.Z.; Maswadeh, J.A.; Bsoul, E.Y. Effects of feeding Atriplex halimus L. on growth performance and carcass characteristics of fattening Awassi lambs. Small Rumin. Res. 2016. [Google Scholar] [CrossRef]
- Min, J.-G.; Lee, D.-S.; Kim, T.-J.; Park, J.-H.; Cho, T.-Y.; Park, D.-I. Chemical Composition of Salicornia Herbacea L. Prev. Nutr. Food Sci. 2002, 7, 105–107. [Google Scholar] [CrossRef]
- Yepes, L.; Chelbi, N.; Vivo, J.-M.M.; Franco, M.; Agudelo, A.; Carvajal, M.; Martínez-Ballesta, M.d.C. Analysis of physiological traits in the response of Chenopodiaceae, Amaranthaceae, and Brassicaceae plants to salinity stress. Plant Physiol. Biochem. 2018, 132, 145–155. [Google Scholar] [CrossRef]
- Maciel, E.; Domingues, P.; Domingues, M.R.M.; Calado, R.; Lillebø, A. Halophyte plants from sustainable marine aquaponics are a valuable source of omega-3 polar lipids. Food Chem. 2020, 320, 126560. [Google Scholar] [CrossRef]
- Chalbi, N.; Hessini, K.; Gandour, M.; Mohamed, S.; Smaoui, A.; Abdelly, C.; Youssef, N. Ben Are changes in membrane lipids and fatty acid composition related to salt-stress resistance in wild and cultivated barley? J. Plant Nutr. Soil Sci. 2013, 176, 138–147. [Google Scholar] [CrossRef]
| Species | Treatments (Mm) | Na | K | Ca | P | Mg | S |
|---|---|---|---|---|---|---|---|
| Atriplex halimus | 0 | 989.37 ± 192.08 Ba | 5725.03 ± 953.91 Bb | 565.42 ± 83.93 ABb | 140.19 ± 19.00 Ab | 224.45 ± 36.20 Bb | 127.50 ± 19.87 Ab |
| 100 | 4472.24 ± 665.96 Ab | 2158.81 ± 251.95 Ba | 93.60 ± 12.28 Aa | 73.78 ± 9.29 Aa | 70.11 ± 8.48 Aa | 57.39 ± 7.33 Aa | |
| 200 | 6507.30 ± 119.09 Bc | 2029.97 ± 585.11 Aa | 79.19 ± 13.19 Aa | 85.92 ± 10.62 Aa | 70.94 ± 17.06 Aa | 80.18 ± 9.49 Aab | |
| 300 | 7369.31 ± 162.34 Cc | 1821.36 ± 44.51 ABa | 133.83 ± 1.37 Aa | 123.29 ± 1.65 Bab | 174.46 ± 3.46 Ab | 94.31 ± 1.69 Aab | |
| Cakile maritima | 0 | 23.71 ± 0.29 Aa | 2940.52 ± 14.48 Ad | 787.74 ± 5.91 Bc | 209.10 ± 0.51 Ba | 116.89 ± 0.99 Ac | 243.37 ± 0.59 Bd |
| 100 | 2833.61 ± 16.27 Ab | 1047.22 ± 20.90 Aa | 370.31 ± 6.61 Bb | 291.72 ± 8.44 Bc | 69.33 ± 1.50 Ab | 179.71 ± 4.03 Bc | |
| 200 | 3422.74 ± 84.77 Ac | 1295.66 ± 34.23 Ab | 305.52 ± 7.95 Ba | 236.29 ± 3.85 Bb | 57.26 ± 1.60 Aa | 154.84 ± 1.97 Bb | |
| 300 | 3528.19 ± 49.49 Ac | 1469.93 ± 11.92 Ac | 320.34 ± 4.75 Ba | 216.63 ± 4.09 Cab | 55.79 ± 0.67 Aa | 141.32 ± 1.72 Ba | |
| Salicornia fruticosa | 0 | 967.90 ± 88.80 Ba | 4592.69 ± 460.27 ABb | 341.42 ± 33.38 Ab | 96.38 ± 9.79 Aa | 177.71 ± 17.97 ABa | 79.53 ± 7.33 Aa |
| 100 | 2733.93 ± 204.68 Ab | 1139.77 ± 85.95 Aa | 68.41 ± 22.53 Aa | 120.11 ± 29.25 Aa | 120.94 ± 12.56 Ba | 59.35 ± 2.97 Aa | |
| 200 | 3577.05 ± 146.44 Ac | 1350.47 ± 238.35 Aa | 57.15 ± 4.20 Aa | 114.61 ± 31.05 Aa | 98.13 ± 18.88 Aa | 54.69 ± 6.13 Aa | |
| 300 | 4314.28 ± 242.84 Bc | 2029.20 ± 198.43 Ba | 142.94 ± 37.82 Aa | 87.87 ± 11.32 Aa | 179.63 ± 55.45 Aa | 86.61 ± 12.10 Aa |
| Species | Treatments (Mm) | Fe | Zn | Mo | B | Mn |
|---|---|---|---|---|---|---|
| Atriplex halimus | 0 | 0.41 ± 0.07 Aa | 0.41 ± 0.05 Aa | 0.03 ± 0.00 Ab | 2.50 ± 0.40 Ab | 0.65 ± 0.10 Ab |
| 100 | 0.36 ± 0.05 Aa | 0.23 ± 0.03 Aa | 0.02 ± 0.00 Aa | 1.52 ± 0.20 Aab | 0.25 ± 0.03 Aa | |
| 200 | 0.41 ± 0.05 Aa | 0.28 ± 0.07 Aa | 0.02 ± 0.00 Aab | 1.35 ± 0.08 Aab | 0.30 ± 0.07 Aa | |
| 300 | 0.63 ± 0.01 Bb | 0.30 ± 0.00 Aa | 0.02 ± 0.00 Aab | 1.49 ± 0.03 Aa | 0.64 ± 0.01 Ab | |
| Cakile maritima | 0 | 1.14 ± 0.04 Ba | 2.36 ± 0.03 Ba | 0.07 ± 0.00 Ba | 2.66 ± 0.00 Aa | 0.68 ± 0.00 Aa |
| 100 | 0.69 ± 0.00 Aa | 2.17 ± 0.09 Ba | 0.05 ± 0.00 Ba | 1.80 ± 0.03 Aa | 0.61 ± 0.01 ABa | |
| 200 | 0.63 ± 0.00 Aa | 2.31 ± 0.03 Ba | 0.05 ± 0.00 Aa | 1.67 ± 0.02 Aa | 0.56 ± 0.01 Aa | |
| 300 | 0.57 ± 0.01 ABa | 2.96 ± 0.58 Ba | 0.05 ± 0.00 Ba | 1.67 ± 0.00 Aa | 0.56 ± 0.01 Aa | |
| Salicornia fruticosa | 0 | 0.47 ± 0.05 Ac | 0.36 ± 0.03 Aa | 0.02 ± 0.00 Ac | 1.65 ± 0.16 Ac | 0.63 ± 0.06 Ac |
| 100 | 0.86 ± 0.27 Ab | 1.07 ± 0.43 ABa | 0.04 ± 0.01 ABa | 2.07 ± 0.50 Ab | 1.28 ± 0.40 Bb | |
| 200 | 0.70 ± 0.29 Aab | 0.96 ± 0.40 Aa | 0.03 ± 0.01 Ab | 1.90 ± 0.58 Aa | 1.06 ± 0.45 Aa | |
| 300 | 0.44 ± 0.06 Aa | 0.32 ± 0.03 Aa | 0.02 ± 0.00 Aab | 1.44 ± 0.26 Aa | 0.62 ± 0.16 Aa |
| Species | Treatment (mM) | Sinapic Acid Derivatives | Flavonoid Glycosides | Chlorogenic Acid Derivatives | Total |
|---|---|---|---|---|---|
| Atriplex halimus | 0 | 0.41 ± 0.01 cB | 0.98 ± 0.02 aA | 0.49 ± 0.01 bA | 1.88 ± 0.04 bB |
| 100 | 0.20 ± 0.06 bA | 1.33 ± 0.05 bA | 0.18 ± 0.01 aA | 1.71 ± 0.01 bA | |
| 200 | 0.02 ± 0.00 aA | 1.10 ± 0.01 aA | 0.24 ± 0.00 aA | 1.37 ± 0.01 aA | |
| 300 | 0.17 ± 0.02 abA | 1.57 ± 0.05 cA | 0.52 ± 0.03 bA | 2.27 ± 0.09 cB | |
| Cakile maritima | 0 | 3.22 ± 0.17 cC | 1.50 ± 0.08 aC | 0.45 ± 0.04 cB | 5.17 ± 0.26 cC |
| 100 | 2.02 ± 0.06 bB | 1.77 ± 0.06 aA | 0.20 ± 0.01 abA | 3.99 ± 0.12 bA | |
| 200 | 2.08 ± 0.05 bB | 1.70 ± 0.04 aB | 0.27 ± 0.01 bA | 4.05 ± 0.09 bB | |
| Salicornia fruticosa | 0 | 0.00 ± 0.00 aA | 0.38 ± 0.01 aB | 0.11 ± 0.00 aB | 0.49 ± 0.01 aA |
| 100 | 0.00 ± 0.00 aA | 1.55 ± 0.07 cA | 0.24 ± 0.01 cA | 1.79 ± 0.08 cA | |
| 200 | 0.00 ± 0.00 aA | 0.32 ± 0.01 aAB | 0.10 ± 0.00 aA | 0.42 ± 0.01 aA | |
| 300 | 0.00 ± 0.00 aA | 0.75 ± 0.02 bB | 0.20 ± 0.00 bA | 0.96 ± 0.02 bA |
| Species | Treatments (mM) | Protein Content | Lipid Content |
|---|---|---|---|
| Atriplex halimus | 0 | 292.7 ± 6.7 aB | 10.4 ± 0.4 aA |
| 100 | 316.4 ± 0.8 bC | 10.0 ± 0.8 aA | |
| 200 | 281.0 ± 2.8 aB | 10.7 ± 2.0 aA | |
| 300 | 320.4 ± 1.3 bB | 11.3 ± 0.4 aA | |
| Cakile maritima | 0 | 128.0 ± 21.9 aA | 21.0 ± 0.7 aB |
| 100 | 143.6 ± 5.5 aA | 23.1 ± 6.8 aAB | |
| 200 | 132.5 ± 25.0 aA | 19.2 ± 0.6 aB | |
| 300 | 168.2 ± 47.4 aA | 19.6 ± 0.5 aB | |
| Salicornia fruticosa | 0 | 271.2 ± 6.9 bB | 9.9 ± 0.6 aA |
| 100 | 250.9 ± 4.6 abB | 10.1 ± 0.9 aA | |
| 200 | 231.9 ± 3.1 aB | 12.1 ± 1.1 aA | |
| 300 | 263.5 ± 2.6 bAB | 12.2 ± 0.3 aA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agudelo, A.; Carvajal, M.; Martinez-Ballesta, M.d.C. Halophytes of the Mediterranean Basin—Underutilized Species with the Potential to Be Nutritious Crops in the Scenario of the Climate Change. Foods 2021, 10, 119. https://doi.org/10.3390/foods10010119
Agudelo A, Carvajal M, Martinez-Ballesta MdC. Halophytes of the Mediterranean Basin—Underutilized Species with the Potential to Be Nutritious Crops in the Scenario of the Climate Change. Foods. 2021; 10(1):119. https://doi.org/10.3390/foods10010119
Chicago/Turabian StyleAgudelo, Agatha, Micaela Carvajal, and María del Carmen Martinez-Ballesta. 2021. "Halophytes of the Mediterranean Basin—Underutilized Species with the Potential to Be Nutritious Crops in the Scenario of the Climate Change" Foods 10, no. 1: 119. https://doi.org/10.3390/foods10010119
APA StyleAgudelo, A., Carvajal, M., & Martinez-Ballesta, M. d. C. (2021). Halophytes of the Mediterranean Basin—Underutilized Species with the Potential to Be Nutritious Crops in the Scenario of the Climate Change. Foods, 10(1), 119. https://doi.org/10.3390/foods10010119







