Methods for the Modification and Evaluation of Cereal Proteins for the Substitution of Wheat Gluten in Dough Systems
Abstract
1. Introduction
2. Cereals and Pseudocereals as the Protein Source in Bread-Making
3. Protein Modification in Gluten-Free Dough
3.1. Chemical Modifications
3.2. Enzymatic Modifications
3.3. Physical Modifications
3.4. Genetic Modifications
4. Methodologies for the Measurements of the Gluten Substitute and the Gluten-Free Dough Properties
4.1. Characterization of Protein Modifications for Gluten-Free Doughs
4.1.1. Fourier Transform Infrared Spectroscopy
4.1.2. D-Electrophoresis
4.1.3. Microscopy
4.2. Measurement of Rheological Properties of Gluten-Free Doughs
4.3. Measurement of Thermal Properties of Gluten-Free Doughs
5. Future Perspectives
- In some cases, studies focus on finding the best set of variables and modifications to obtain higher quality products, often pairing modification techniques to save money and resources. However, this information cannot be used to fully understand the individual effects contributed by the techniques, and separate studies should be done to elucidate the effect that a treatment can have on the final product and its protein content.
- Non-covalent interactions have been assigned for the explanation of most improvements by non-chemical/enzymatic modifications. The full mechanism is not yet fully understood; hence, the critical protein/starch systems should be studied separately to fill the information gaps and introduce factors, such as other forces, overlooked by previous authors. Computational software can be used to generate simulations that model the forces and interactions between starch and protein.
- Genetic modification is still a relatively new method for the modification of gluten substitutes, and thus more studies should be performed to improve protein properties and functionalities. Amino-acid insertion or replacement can be an alternative to better approach the properties of gluten, keeping in mind to avoid the insertion of fractions or sequences that cause the allergic response in intolerant individuals.
- Pseudocereals have been studied as gluten-free alternatives; however, they have mostly been used as additives in flours containing other protein sources. Additional studies need to be done to give a verdict on their proteins’ susceptibility to chemical and physical modifications.
- Compared to a sample made from gluten, viscoelastic parameters can estimate the potential that a gluten-free product has. The obtainment of these values for each modification can help design methodologies to improve further the quality and approximation of the final product of a complete gluten imitation. Using the obtained values, an alternative might be creating software that compiles the texture, rheological, and thermal properties of gluten-free bread to generate a score that compares it with gluten bread properties.
- Given that gas retention plays a critical role in the efficacy of a gluten substitute in dough systems, the retention capacity of gluten-free bread should be tested. The use of equipment, such as alveographs, can simulate the fermentation process during breadmaking.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parzanese, I.; Qehajaj, D.; Patrinicola, F.; Aralica, M.; Chiriva-Internati, M.; Stifter, S.; Elli, L.; Grizzi, F. Celiac disease: From pathophysiology to treatment. World J. Gastrointest. Pathophysiol. 2017, 8, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Catassi, C.; Green, P.H.; Kelly, C.P.; Ahuja, V.; Makharia, G.K. Global prevalence of celiac disease: Systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, E.; Catassi, C. New clues in celiac disease epidemiology, pathogenesis, clinical manifestations, and treatment. Int. Rev. Immunol. 2011, 30, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Biesiekierski, J.R. What is gluten? J. Gastroenterol. Hepatol. 2017, 32, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.S.; Day, A.S.; Shaoul, R. Gluten in celiac disease more or less? Rambam Maimonides Med. J. 2019, 10, e0007. [Google Scholar] [CrossRef] [PubMed]
- Yano, H. Recent practical researches in the development of gluten-free breads. NPJ Sci. Food 2019, 3, 7. [Google Scholar] [CrossRef]
- Verheyen, C.; Albrecht, A.; Elgeti, D.; Jekle, M.; Becker, T. Impact of gas formation kinetics on dough development and bread quality. Food Res. Int. 2015, 76, 860–866. [Google Scholar] [CrossRef]
- Grand View Research. Gluten-Free Products Market Size, Share & Trends Analysis Report by Product (Bakery Products, Dairy/Dairy Alternatives), by Distribution Channel (Grcoery Stores, Mass Merchandiser), by Region, and Segment Forecasts, 2020–2027; Grand View Research: San Francisco, CA, USA, 2020. [Google Scholar]
- Espinosa, N.H.; Reyes, M.R.-; Jiménez, F.E.G.; Bretón, L.C.N.; Bribiesca, B.L.C. Importancia de las proteinas de almacenamiento en cereales (prolaminas). Vertientes Rev. Espec. Cienc. Salud 2015, 18, 3–7. [Google Scholar]
- Rasheed, F.; Newson, W.R.; Plivelic, T.S.; Kuktaite, R.; Hedenqvist, M.S.; Gällstedt, M.; Johansson, E. Structural architecture and solubility of native and modified gliadin and glutenin proteins: Non-crystalline molecular and atomic organization. RSC Adv. 2014, 4, 2051–2060. [Google Scholar] [CrossRef]
- Gorgitano, M.T.; Sodano, V. Gluten-free products: From dietary necessity to premium price extraction tool. Nutrients 2019, 11, 1997. [Google Scholar] [CrossRef]
- Makovicky, P.; Makovicky, P.; Lupan, I.; Samasca, G.; Sur, G.; Freeman, H.J. Perspective: Gluten-free products for patients with celiac disease should not contain trace levels. Adv. Nutr. 2017, 8, 409–411. [Google Scholar] [CrossRef]
- European Comission. Commission Implementing Regulation (EU) No 828/2014 of 30 July 2014 on the requirements for the provision of information to consumers on the absence or reduced presence of gluten in food (Text with EEA relevance). Off. J. Eur. Union 2014, 57, 5–8. [Google Scholar]
- United Sates Food and Drug Administration. Food labeling; gluten-free labeling of foods. Fed. Regist. 2013, 78, 47154–47179. [Google Scholar]
- Secretaría de Salud de México. Norma Oficial Mexicana NOM-086-SSA1-1994. Bienes y Servicios. Alimentos y bebidas no Alcoholicas con Modificaciones en su Composición. Especificaciones Nutrimentales. Available online: http://sidof.segob.gob.mx/notas/4890075 (accessed on 26 June 1996).
- Taylor, J.R.N.; Taylor, J.; Campanella, O.H.; Hamaker, B.R. Functionality of the storage proteins in gluten-free cereals and pseudocereals in dough systems. J. Cereal Sci. 2016, 67, 22–34. [Google Scholar] [CrossRef]
- Allen, B.; Orfila, C. The availability and nutritional adequacy of gluten-free bread and pasta. Nutrients 2018, 10, 1370. [Google Scholar] [CrossRef]
- Anton, A.A.; Artfield, S.D. Hydrocolloids in gluten-free breads: A review. Int. J. Food Sci. Nutr. 2008, 59, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, K.; Kamilah, H.; Shang, P.L.; Sulaiman, S.; Ariffin, F.; Alias, A.K. A review: Interaction of starch/non-starch hydrocolloid blending and the recent food applications. Food Biosci. 2017, 19, 110–120. [Google Scholar] [CrossRef]
- Zink, J.; Wyrobnik, T.; Prinz, T.; Schmid, M. Physical, chemical and biochemical modifications of protein-based films and coatings: An extensive review. Int. J. Mol. Sci. 2016, 17, 1376. [Google Scholar] [CrossRef]
- Lovegrove, A.; Edwards, C.H.; De Noni, I.; Patel, H.; El, S.N.; Grassby, T.; Zielke, C.; Ulmius, M.; Nilsson, L.; Butterworth, P.J.; et al. Role of polysaccharides in food, digestion, and health. Crit. Rev. Food Sci. Nutr. 2017, 57, 237–253. [Google Scholar] [CrossRef]
- de Mesa-Stonestreet, N.J.; Alavi, S.; Bean, S.R. Sorghum proteins: The concentration, isolation, modification, and food applications of kafirins. J. Food Sci. 2010, 75, R90–R104. [Google Scholar] [CrossRef]
- Espinosa-Ramírez, J.; Garzon, R.; Serna-Saldivar, S.O.; Rosell, C.M. Functional and nutritional replacement of gluten in gluten-free yeast-leavened breads by using β-conglycinin concentrate extracted from soybean flour. Food Hydrocoll. 2018, 84, 353–360. [Google Scholar] [CrossRef]
- Kieffer, R.; Wieser, H.; Henderson, M.H.; Graveland, A. Correlations of the breadmaking performance of wheat flour with rheological measurements on a micro-scale. J. Cereal Sci. 1998, 27, 53–60. [Google Scholar] [CrossRef]
- Mirmoghtadaie, L.; Aliabadi, S.S.; Hosseini, S.M. Recent approaches in physical modification of protein functionality. Food Chem. 2016, 199, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Kouassi-Koffi, J.D.; Ahi, A.P.; Faulet, B.M.; Gonnety, T.J.; Muresan, V.; Mudura, E.; Assemand, E. Essential steps of bread making process due to relevant rheological parameters of the raw material. Int. J. Pure Appl. Biosci. 2016, 4, 58–70. [Google Scholar] [CrossRef]
- Shewry, P. What is gluten—why is it special? Front. Nutr. 2019, 6, 101. [Google Scholar] [CrossRef]
- Mito, K.; Tatsuro, M.; Airi, M.; Tetsuya, A.; Masaharu, Y.; Koji, T.; Yasuyuki, S. The effects of mixing and fermentation times on chemical and physical properties of white pan bread. Food Sci. Technol. Res. 2017, 23, 181–191. [Google Scholar] [CrossRef]
- Barak, S.; Mudgil, D.; Khatkar, B.S. Influence of gliadin and glutenin fractions on rheological, pasting, and textural properties of dough. Int. J. Food Prop. 2014, 17, 1428–1438. [Google Scholar] [CrossRef]
- Parenti, A.; Guerrini, L.; Granchi, L.; Venturi, M.; Benedettelli, S.; Nistri, F.; Forestali, A.; Firenze, U.; Firenze, U.; Nistri, F.; et al. Control of mixing step in the bread production with weak wheat flour and sourdough. J. Agric. Eng. 2013, XLIV, 10–13. [Google Scholar] [CrossRef][Green Version]
- Sciarini, L.S.; Steffolani, M.E.; León, A.E. El rol del gluten en la panificación y el desafío de prescindir de su aporte en la elaboración de pan. Agriscientia 2016, 33, 61–74. [Google Scholar] [CrossRef]
- Mi, A.; Nawrocka, A.; Dziki, D. Identification of baking expansion phases of leavened dough using an experimental approach. Food Bioprocess Technol. 2016, 9, 892–903. [Google Scholar] [CrossRef][Green Version]
- Gerardo Rodriguez, J.E.; Ramírez Wong, B.; Carvajal Millan, E.; López Cervantes, J.; Vásquez Lara, F.; Silvas García, M.I. Viscoelastic characteristics of part-baked bread under different process conditions. Rev. Cienc. Biol. Salud 2018, XXI, 68–78. [Google Scholar] [CrossRef]
- Meybodi, N.M.; Mohammadifar, M.A.; Feizollahi, E. Gluten-free bread quality: A review of the improving factors. J. Food Qual. Hazards Control 2015, 2, 81–85. [Google Scholar]
- Matsushima, N.; Danno, G.; Takezawa, H.; Izumi, Y. Three-dimensional structure of maize a -zein proteins studied by small-angle X-ray scattering. Biochim. Biophys. Acta 1997, 1339, 14–22. [Google Scholar] [CrossRef]
- Schober, T.J.; Bean, S.R.; Boyle, D.L.; Park, S. Improved viscoelastic zein—Starch doughs for leavened gluten-free breads: Their rheology and microstructure. J. Cereal Sci. 2008, 48, 755–767. [Google Scholar] [CrossRef]
- Anderson, T.J.; Lamsal, B.P. Zein extraction from corn, corn products, and coproducts and modifications for various applications: A review. Cereal Chem. 2011, 88, 159–173. [Google Scholar] [CrossRef]
- Oom, A.; Pettersson, A.; Taylor, J.R.N.; Stading, M. Rheological properties of kafirin and zein prolamins. J. Cereal Sci. 2008, 47, 109–116. [Google Scholar] [CrossRef]
- Dianda, N.; Rouf, T.B.; Bonilla, J.C.; Hedrick, V.; Kokini, J. Effect of solvent polarity on the secondary structure, surface and mechanical properties of biodegradable kafirin films. J. Cereal Sci. 2019, 90, 102856. [Google Scholar] [CrossRef]
- Xiao, J.; Li, Y.; Li, J.; Gonzalez, A.P.; Xia, Q.; Huang, Q. Structure, morphology, and assembly behavior of kafirin. J. Agric. Food Chem. 2015, 63, 216–224. [Google Scholar] [CrossRef]
- Li, A.; Jia, S.; Yobi, A.; Ge, Z.; Sato, S.J.; Zhang, C.; Angelovici, R.; Clemente, T.E.; Holding, D.R. Editing of an alpha-kafirin gene family increases digestibility and protein quality in sorghum. Plant Physiol. 2018, 177, 1425–1438. [Google Scholar] [CrossRef]
- Belton, P.S.; Delgadillo, I.; Halford, N.G.; Shewry, P.R. Kafirin structure and functionality. J. Cereal Sci. 2006, 44, 272–286. [Google Scholar] [CrossRef]
- Taylor, J.; Taylor, J.R.N. Making kafirin, the sorghum prolamin into a viable alternative protein source. J. Am. Oil Chem. Soc. 2018, 95, 969–990. [Google Scholar] [CrossRef]
- Gell, G.; Kovács, K.; Veres, G.; Korponay-Szabó, I.R.; Juhász, A. Characterization of globulin storage proteins of a low prolamin cereal species in relation to celiac disease. Sci. Rep. 2017, 7, 39876. [Google Scholar] [CrossRef]
- Mao, X.; Hua, Y.; Chen, G. Amino acid composition, molecular weight distribution and gel electrophoresis of walnut (Juglans regia L.) proteins and protein fractionations. Int. J. Mol. Sci. 2014, 15, 2003–2014. [Google Scholar] [CrossRef]
- Baxter, G.; Blanchard, C.; Zhao, J. Effects of glutelin and globulin on the physicochemical properties of rice starch and flour. J. Cereal Sci. 2014, 60, 414–420. [Google Scholar] [CrossRef]
- Rasheed, F.; Markgren, J.; Hedenqvist, M. Modeling to understand plant protein. Molecules 2020, 25, 873. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P.R.; Halford, N.G. Cereal seed storage proteins: Structures, properties and role in grain utilization. J. Exp. Bot. 2002, 53, 947–958. [Google Scholar] [CrossRef]
- Amagliani, L.; Regan, J.O.; Kelly, A.L.; Mahony, J.A.O. Composition and protein profile analysis of rice protein ingredients. J. Food Compos. Anal. 2017, 59, 18–26. [Google Scholar] [CrossRef]
- Renzetti, S.; Arendt, E.K. Effects of oxidase and protease treatments on the breadmaking functionality of a range of gluten-free flours. Eur. Food Res. Technol. 2009, 229, 307–317. [Google Scholar] [CrossRef]
- Schoenlechner, R. Properties of pseudocereals, selected specialty cereals and legumes for food processing with special attention to gluten-free products. Bodenkultur 2016, 67, 239–248. [Google Scholar] [CrossRef]
- Alonso-Miravalles, L.; O’Mahony, J.A. Composition, protein profile and rheological properties of pseudocereal-based protein-rich ingredients. Foods 2018, 7, 73. [Google Scholar] [CrossRef]
- Pirzadah, T.B.; Malik, B. Pseudocereals as super foods of 21st century: Recent technological interventions. J. Agric. Food Res. 2020, 2, 100052. [Google Scholar] [CrossRef]
- Turkut, G.M.; Cakmak, H.; Kumcuoglu, S.; Tavman, S. Effect of quinoa flour on gluten-free bread batter rheology and bread quality. J. Cereal Sci. 2016, 69, 174–181. [Google Scholar] [CrossRef]
- Geraghty, D.; Peifer, M.A.; Rubenstein, I.; Messing, J. The primary structure of a plant storage protein: Zein. Nucleic Acids Res. 1981, 9, 5163–5174. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, D.H.; Hoffmann, L., Jr.; Rooney, W.L.; Herald, T.J.; Bean, S.; Boyles, R.; Brenton, Z.W.; Kresovich, S. Genetic architecture of kernel composition in global sorghum germplasm. BMC Genom. 2017, 18, 15. [Google Scholar] [CrossRef] [PubMed]
- Dakhili, S.; Abdolalizadeh, L.; Hosseini, S.M.; Shojaee-Aliabadi, S.; Mirmoghtadaie, L. Quinoa protein: Composition, structure and functional properties. Food Chem. 2019, 299, 125161. [Google Scholar] [CrossRef]
- Navruz-Varli, S.; Sanlier, N. Nutritional and health benefits of quinoa (Chenopodium quinoa Willd.). J. Cereal Sci. 2016, 69, 371–376. [Google Scholar] [CrossRef]
- Janssen, F.; Pauly, A.; Rombouts, I.; Jansens, K.J.A.; Deleu, L.J.; Delcour, J.A. Proteins of amaranth (Amaranthus spp.), buckwheat (Fagopyrum spp.), and quinoa (Chenopodium spp.): A food science and technology perspective. Compr. Rev. Food Sci. Food Saf. 2017, 16, 39–58. [Google Scholar] [CrossRef]
- Grundy, M.M.L.; Momanyi, D.K.; Holland, C.; Kawaka, F.; Tan, S.; Salim, M.; Boyd, B.J.; Bajka, B.; Mulet-Cabero, A.I.; Bishop, J.; et al. Effects of grain source and processing methods on the nutritional profile and digestibility of grain amaranth. J. Funct. Foods 2020, 72, 104065. [Google Scholar] [CrossRef]
- Kumar Maurya, N.; Arya, P. Amaranthus grain nutritional benefits: A review. J. Pharmacogn. Phytochem. 2018, 7, 2258–2262. [Google Scholar]
- Sytar, O.; Brestic, M.; Zivcak, M.; Phan Tran, L.-S. The contribution of buckwheat genetic resources to health and dietary diversity. Curr. Genom. 2016, 17, 193–206. [Google Scholar] [CrossRef]
- Taylor, J.; Anyango, J.O.; Muhiwa, P.J.; Oguntoyinbo, S.I.; Taylor, J.R.N. Comparison of formation of visco-elastic masses and their properties between zeins and kafirins. Food Chem. 2018, 245, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Federici, E.; Jones, O.G.; Selling, G.W.; Tagliasco, M.; Campanella, O.H. Effect of zein extrusion and starch type on the rheological behavior of gluten-free dough. J. Cereal Sci. 2020, 91, 102866. [Google Scholar] [CrossRef]
- Ozturk, O.K.; Mert, B. The effects of microfluidization on rheological and textural properties of gluten-free corn breads. Food Res. Int. 2018, 105, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Azizi, S.; Azizi, M.H.; Moogouei, R.; Rajaei, P. The effect of quinoa flour and enzymes on the quality of gluten-free bread. Food Sci. Nutr. 2020, 8, 2373–2382. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Inoue, N.; Sugimoto, R.; Matsumoto, K.; Nishioka, A. Dynamic viscoelasticity of protease-treated rice batters for gluten-free rice bread making. Biosci. Biotechnol. Biochem. 2018, 82, 484–488. [Google Scholar] [CrossRef]
- Raungrusmee, S.; Shrestha, S.; Sadiq, M.B.; Anal, A.K. Influence of resistant starch, xanthan gum, inulin and defatted rice bran on the physicochemical, functional and sensory properties of low glycemic gluten-free noodles. LWT Food Sci. Technol. 2020, 126, 109279. [Google Scholar] [CrossRef]
- Villanueva, M.; Harasym, J.; Muñoz, J.M.; Ronda, F. Rice flour physically modified by microwave radiation improves viscoelastic behavior of doughs and its bread-making performance. Food Hydrocoll. 2019, 90, 472–481. [Google Scholar] [CrossRef]
- Tunçil, Y.; Fevzioglu, M.; Tunçil, S.A.; Ejeta, G.; Campanella, O.H.; Hamaker, B.R. Transglutaminase shows better functionality on high digestible, high lysine sorghum-wheat composite dough and bread, compared to normal sorghum-wheat composites. Turk. J. Agric. Food Sci. Technol. 2019, 7, 877–882. [Google Scholar] [CrossRef]
- Elhassan, M.S.M.; Emmambux, M.N.; Taylor, J.R.N. Transgenic sorghum with suppressed synthesis of kafirin subclasses: Effects on flour and dough rheological characteristics. J. Cereal Sci. 2017, 75, 69–76. [Google Scholar] [CrossRef]
- Marston, K.; Khouryieh, H.; Aramouni, F. Effect of heat treatment of sorghum flour on the functional properties of gluten-free bread and cake. LWT Food Sci. Technol. 2017, 65, 637–644. [Google Scholar] [CrossRef]
- Elhassan, M.S.M.; Oguntoyinbo, S.I.; Taylor, J.; Taylor, J.R.N. Formation and properties of viscoelastic masses made from kafirin by a process of simple coacervation from solution in glacial acetic acid using water. Food Chem. 2018, 239, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Mattice, K.D.; Marangoni, A.G. Functionalizing zein through antisolvent precipitation from ethanol or aetic acid. Food Chem. 2020, 313, 126127. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Taylor, J.R.N.; Dutton, M.F.; de Kock, S. Glacial acetic acid—a novel food-compatible solvent for kafirin extraction. Cereal Chem. 2005, 82, 485–487. [Google Scholar] [CrossRef]
- Mainieri, D.; Marrano, C.A.; Prinsi, B.; Maffi, D.; Tschofen, M.; Espen, L.; Stöger, E.; Faoro, F.; Pedrazzini, E.; Vitale, A. Maize 16-kD 3-zein forms very unusual disulfide-bonded polymers in the endoplasmic reticulum: Implications for prolamin evolution. J. Exp. Bot. 2018, 69, 5013–5027. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.; Singh, J.; Bandral, J.D.; Gani, A.; Shams, R. Food hydrocolloids: Functional, nutraceutical and novel applications for delivery of bioactive compounds. Int. J. Biol. Macromol. 2020, 165, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Hamada, S.; Suzuki, K.; Aoki, N.; Suzuki, Y. Improvements in the qualities of gluten-free bread after using a protease obtained from aspergillus oryzae. J. Cereal Sci. 2013, 57, 91–97. [Google Scholar] [CrossRef]
- Agarwal, P.K. A biophysical perspective on enzyme catalysis. Biochemistry 2019, 58, 438–449. [Google Scholar] [CrossRef]
- Pérez-Carrillo, E.; Serna-Saldívar, S.O. Effect of protease treatment before hydrolysis with α-amylase on the rate of starch and protein hydrolysis of maize, whole sorghum, and decorticated sorghum. Cereal Chem. 2007, 84, 607–613. [Google Scholar] [CrossRef]
- Kouassi-Koffi, J.D.; Sturza, A.; Păucean, A.; Man, S.; Mureșan, A.E.; Petruț, G.; Mureșan, V.; Muste, S. Effect of glucose oxidase addition on the textural characteristics of wheat-maize dough and bread. Food Sci. Technol. 2019, 39, 127–133. [Google Scholar] [CrossRef]
- Razzaq, A.; Shamsi, S.; Ali, A.; Ali, Q.; Sajjad, M.; Malik, A.; Ashraf, M. Microbial proteases applications. Front. Bioeng. Biotechnol. 2019, 7, 1–20. [Google Scholar] [CrossRef]
- Kieliszek, M.; Misiewicz, A. Microbial transglutaminase and its application in the food industry. A review. Folia Microbiol. 2014, 59, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, T.; Yokoyama, K.; Ishikawa, K.; Ono, K.; Ejima, D.; Matsui, H.; Suzuki, E. Crystal structure of microbial transglutaminase from streptoverticillium mobaraense. J. Biol. Chem. 2002, 277, 44252–44260. [Google Scholar] [CrossRef] [PubMed]
- Mattice, K.D.; Marangoni, A.G. Physical properties of zein networks treated with microbial transglutaminase. Food Chem. 2021, 338, 128010. [Google Scholar] [CrossRef] [PubMed]
- Gómez, M.; Martínez, M.M. Changing flour functionality through physical treatments for the production of gluten-free baking goods. J. Cereal Sci. 2016, 67, 68–74. [Google Scholar] [CrossRef]
- Bemiller, J.N.; Huber, K.C. Physical modification of food starch functionalities. Annu. Rev. Food Sci. Technol. 2015, 6, 19–69. [Google Scholar] [CrossRef]
- Pucci, F.; Bourgeas, R.; Rooman, M. Predicting protein thermal stability changes upon point mutations using statistical potentials: Introducing HoTMuSiC. Sci. Rep. 2016, 6, 23257. [Google Scholar] [CrossRef]
- Miotto, M.; Olimpieri, P.P.; Di Rienzo, L.; Ambrosetti, F.; Corsi, P.; Lepore, R.; Tartaglia, G.G.; Milanetti, E. Insights on protein thermal stability: A graph representation of molecular interactions. Bioinformatics 2019, 35, 2569–2577. [Google Scholar] [CrossRef]
- Bohr, H.; Bohr, J. Microwave-enhanced folding and denaturation of globular proteins. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 2000, 61, 4310–4314. [Google Scholar] [CrossRef]
- Sobowale, S.; Bamgbose, A.; Adeboye, A. Effect of extrusion variables on the extrudate properties of wheatplantain noodle. J. Food Process. Technol. 2016, 7. [Google Scholar] [CrossRef]
- Madeka, H.; Kokini, J.L. Effect of glass transition and cross-linking on rheological properties of zein: Development of a preliminary state diagram. Cereal Chem. 1996, 73, 433–438. [Google Scholar]
- Du, Z.; Chen, F.; Liu, K.; Lai, S.; Zhang, L.; Bu, G.; Gao, X.; Liu, S. Effects of extruded soy protein on the quality of chinese steamed bread. J. Chem. 2016, 2016. [Google Scholar] [CrossRef]
- Kim, M.; Oh, I.; Jeong, S.; Lee, S. Particle size effect of rice flour in a rice-zein noodle system for gluten-free noodles slit from sheeted doughs. J. Cereal Sci. 2019, 86, 48–53. [Google Scholar] [CrossRef]
- Jambrak, A.R.; Mason, T.J.; Lelas, V.; Paniwnyk, L.; Herceg, Z. Effect of ultrasound treatment on particle size and molecular weight of whey proteins. J. Food Eng. 2014, 121, 15–23. [Google Scholar] [CrossRef]
- Zhang, H.; Claver, I.P.; Zhu, K.X.; Zhou, H. The effect of ultrasound on the functional properties of wheat gluten. Molecules 2011, 16, 4231–4240. [Google Scholar] [CrossRef]
- O’Sullivan, J.; Murray, B.; Flynn, C.; Norton, I. The effect of ultrasound treatment on the structural, physical and emulsifying properties of animal and vegetable proteins. Food Hydrocoll. 2016, 53, 141–154. [Google Scholar] [CrossRef]
- Sullivan, A.C.; Pangloli, P.; Dia, V.P. Impact of ultrasonication on the physicochemical properties of sorghum kafirin and in vitro pepsin-pancreatin digestibility of sorghum gluten-like flour. Food Chem. 2018, 240, 1121–1130. [Google Scholar] [CrossRef]
- Smith, L.J.; Fiebig, K.M.; Schwalbe, H.; Dobson, C.M. The concept of a random coil: Residual structure in peptides and denatured proteins. Fold. Des. 1996, 1, 95–106. [Google Scholar] [CrossRef]
- Zhang, C.; Wohlhueter, R.; Zhang, H. Genetically modified foods: A critical review of their promise and problems. Food Sci. Hum. Wellness 2016, 5, 116–123. [Google Scholar] [CrossRef]
- Richardson, T. Chemical modifications and genetic engineering of food proteins. J. Dairy Sci. 1985, 68, 2753–2762. [Google Scholar] [CrossRef]
- Khan, S.; Ullah, M.W.; Siddique, R.; Nabi, G.; Manan, S.; Yousaf, M.; Hou, H. Role of recombinant DNA technology to improve life. Int. J. Genom. 2016, 2016, 2405954. [Google Scholar] [CrossRef]
- Matuda, T.G.; Pessôa Filho, P.A.; Tadini, C.C. Experimental data and modeling of the thermodynamic properties of bread dough at refrigeration and freezing temperatures. J. Cereal Sci. 2011, 53, 126–132. [Google Scholar] [CrossRef]
- Weipert, D. The benefits of basic rheometry in studying dough rheology. Cereal Chem. 1990, 67, 311–317. [Google Scholar]
- Jackson, M.; Mantsch, H.H. The use and misuse of FTIR spectroscopy in the determination of protein structure. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 95–120. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, S.; Kong, J.; Dong, A.; Yu, S. Obtaining information about protein secondary structures in aqueous solution using Fourier transform IR spectroscopy. Nat. Protoc. 2015, 10, 382–396. [Google Scholar] [CrossRef]
- Garidel, P.; Schott, H. Fourier-transform midinfrared spectroscopy for analysis and screening of liquid protein formulations. Part 2: Detailed analysis and applications. Bioprocess Int. 2006, 4, 48–55. [Google Scholar]
- Nowakowski, A.B.; Wobig, W.J.; Petering, D.H. Native SDS-PAGE: High resolution electrophoretic separation of proteins with retention of native properties including bound metal ions. Metallomics 2014, 6, 1068–1078. [Google Scholar] [CrossRef]
- Weber, K.; Osborn, M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J. Biol. Chem. 1969, 244, 4406–4412. [Google Scholar] [CrossRef]
- Magdeldin, S.; Enany, S.; Yoshida, Y.; Xu, B.; Zhang, Y.; Zureena, Z.; Lokamani, I.; Yaoita, E.; Yamamoto, T. Basics and recent advances of two dimensional-polyacrylamide gel electrophoresis. Clin. Proteom. 2014, 11, 16. [Google Scholar] [CrossRef]
- Fazaeli, M.; Tahmasebi, M.; Emam-djomeh, Z. Characterization of food texture: Application of microscopic technology. In Current Microscopy Contributions to Advances in Science and Technology; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2012; pp. 855–871. ISBN 978-84-939843-6-6. [Google Scholar]
- Dürrenberger, M.B.; Handschin, S.; Conde-petit, B.; Escher, F. Visualization of food structure by confocal laser scanning microscopy (CLSM). LWT Food Sci. Technol. 2001, 34, 11–17. [Google Scholar] [CrossRef]
- Sharma, S.; Jaiswal, S.; Duffy, B.; Jaiswal, A.K. Nanostructured materials for food applications: Spectroscopy, microscopy and physical properties. Bioengineering 2019, 6, 26. [Google Scholar] [CrossRef]
- Serna-Saldivar, S.O. Cereal Grains: Properties, Processing, and Nutritional Attributes, 1st ed.; CRC Press (Taylor & Francis Group): Boca Raton, FL, USA, 2010; ISBN 9780429112119. [Google Scholar]
- Weitz, D.; Wyss, H.; Larsen, R. Oscillatory rheology: Measuring the viscoelastic behaviour of soft materials. GIT Lab. J. Eur. 2007, 11, 68–70. [Google Scholar]
- Mandala, I.G. Viscoelastic properties of starch and non-starch thickeners in simple mixtures or model food. In Viscoelasticity—From Theory to Biological Applications; de Vicente, J., Ed.; IntechOpen: London, UK, 2012; p. 13. [Google Scholar]
- Janmey, P.A.; Schliwa, M. Rheology. Curr. Biol. 2008, 18, R639–R641. [Google Scholar] [CrossRef] [PubMed]
- Abang Zaidel, D.; Chin, N.; Abd Rahman, R.; Karim, R. Development of gluten extensibility measurement using tensile test. Pertanika J. Sci. Technol. 2007, 16, 49–59. [Google Scholar]
- Scheuer, P.M.; di Luccio, M.; Zibetti, A.W.; de Miranda, M.Z.; de Francisco, A. Reltionship between instrumental and sensory texture profile of bread loaves made with whole-wheat flour and fat replacer. J. Texture Stud. 2016, 47, 14–23. [Google Scholar] [CrossRef]
- Sheikholeslami, Z.; Mahfouzi, M.; Karimi, M.; Hejrani, T.; Ghiafehdavoodi, M.; Ghodsi, M. Evaluating the traditional bread properties with new formula: Affected by triticale and cress seed gum. Food Sci. Technol. Int. 2020. [Google Scholar] [CrossRef]
- Nyembwe, P.M.; de Kock, H.L.; Taylor, J.R.N. Potential of defatted marama flour-cassava starch composites to produce functional gluten-free bread-type dough. LWT 2018, 92, 429–434. [Google Scholar] [CrossRef]
- Witczak, T.; Juszczak, L.; Ziobro, R.; Korus, J. Rheology of gluten-free dough and physical characteristics of bread with potato protein. J. Food Process Eng. 2017, 40, e12491. [Google Scholar] [CrossRef]
- Jagelaviciute, J.; Cizeikiene, D. The influence of non-traditional sourdough made with quinoa, hemp and chia flour on the characteristics of gluten-free maize/rice bread. LWT 2021, 137, 110457. [Google Scholar] [CrossRef]
- Srikanlaya, C.; Therdthai, N.; Ritthiruangdej, P.; Zhou, W. Effect of hydroxypropyl methylcellulose, whey protein concentrate and soy protein isolate enrichment on characteristics of gluten-free rice dough and bread. Int. J. Food Sci. Technol. 2018, 53, 1760–1770. [Google Scholar] [CrossRef]
- Falcao-Rodrigues, M.M.; Moldao-Martins, M.; Beirao-da-Costa, M.L. Thermal properties of gluten proteins of two soft wheat varieties. Food Chem. 2005, 93, 459–465. [Google Scholar] [CrossRef]
- Bourekoua, H.; Różyło, R.; Benatallah, L.; Wójtowicz, A.; Łysiak, G.; Zidoune, M.N.; Sujak, A. Characteristics of gluten-free bread: Quality improvement by the addition of starches/hydrocolloids and their combinations using a definitive screening design. Eur. Food Res. Technol. 2018, 244, 345–354. [Google Scholar] [CrossRef]
- Farkas, J.; Mohásci-Farkas, C. Application of differential scanning calorimetryin food research and food quality assurance. J. Therm. Anal. 1996, 47, 1787–1803. [Google Scholar] [CrossRef]
- Woishnis, W.A.; Ebnesajjad, S. Introduction to Plastics and Elastomers. In Chemical Resistance of Thermoplastics; Woishnis, W.A., Ebnesajjad, S., Eds.; William Andrew Publishing: Norwich, NY, USA, 2012; pp. xxi–xxxiv. ISBN 978-1-4557-7896-6. [Google Scholar]
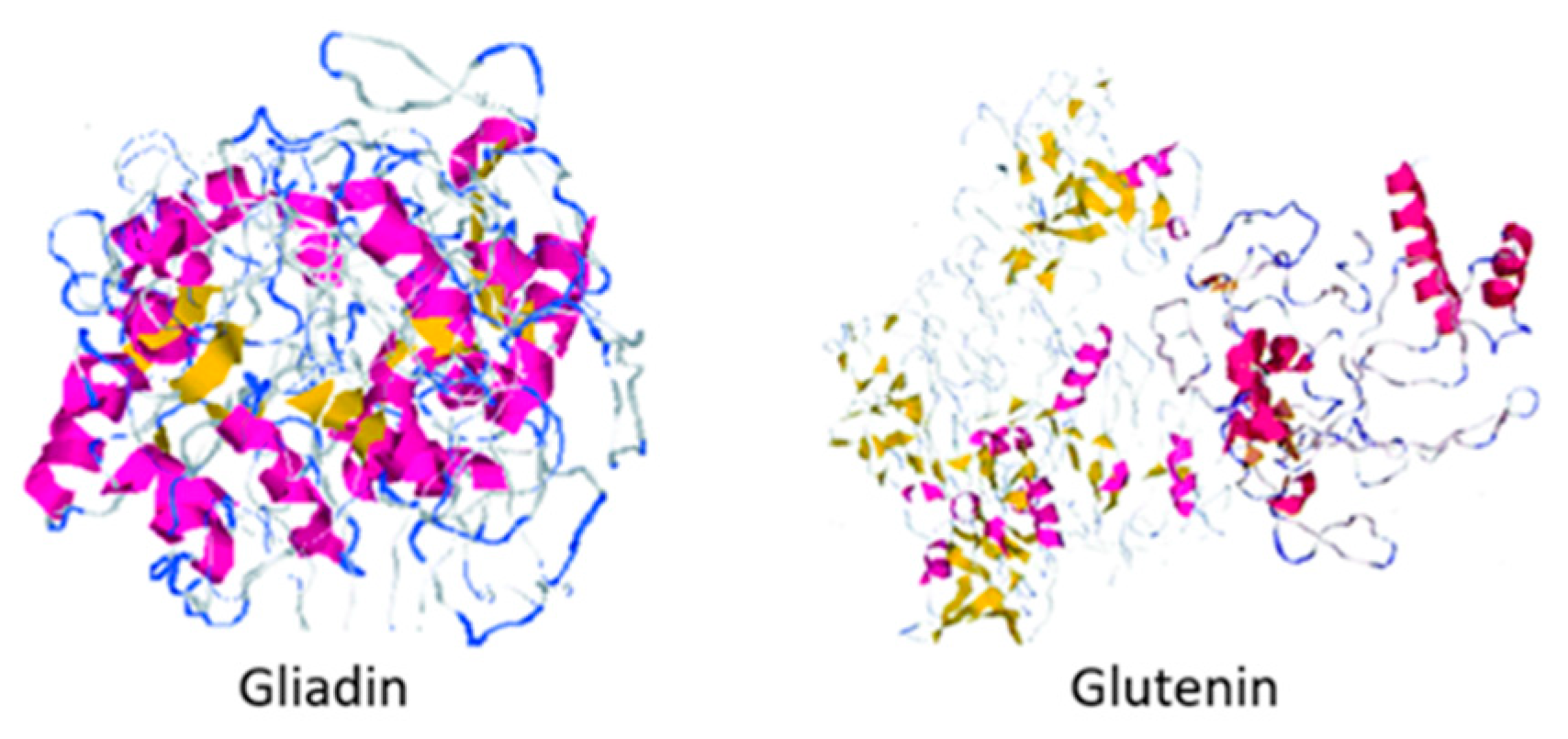

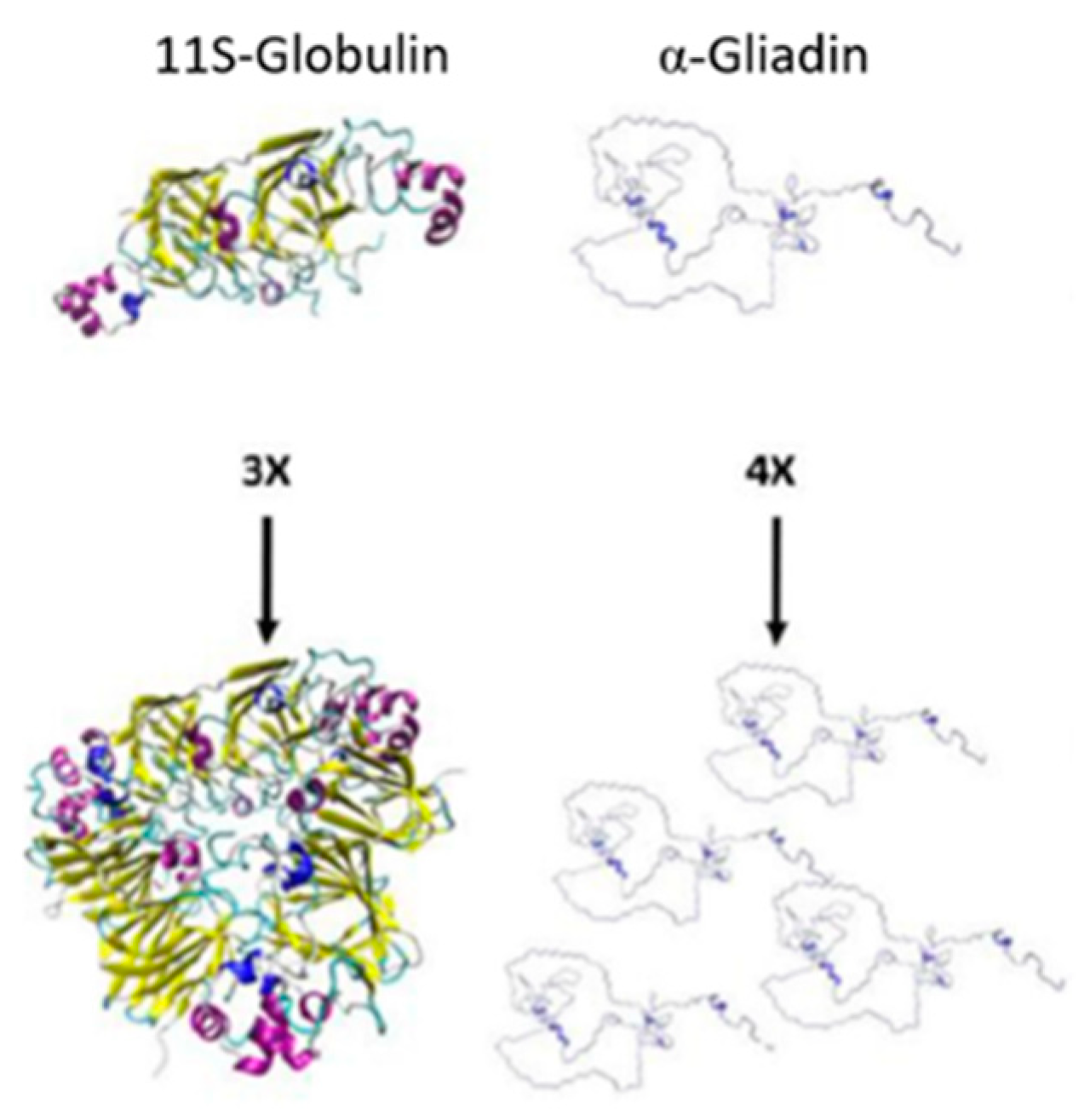


| Cereal/Pseudo-Cereal | Seed Protein Content | Major Storage Proteins | Predominant Amino Acid Content | References |
|---|---|---|---|---|
| Maize | 6.0–12.0% | α, β, γ, δ-Zein | Glutamine 22.5% | Alonso-Miravalles, 2018 [52] Espinosa, 2015 [9] Geraghty, 1981 [55] |
| Leucine 20.9% | ||||
| Proline 9.6% | ||||
| Alanine 8.8% | ||||
| Phenylalanine 8.2% | ||||
| Rice | 6.0–8.0% | 12S-Globulin 11S-Globulin | Glutamine 16.7% | Amagliani, 2017 [49] Shewry, 2002 [48] |
| Aspartate 8.4% | ||||
| Arginine 8.1% | ||||
| Leucine 7.7% | ||||
| Valine 5.3% | ||||
| Sorghum | 6.0–18.0% | α, β, γ, δ-Kafirin | Glutamine 28.2% | de Mesa-Stonestreet, 2010 [22] Espinosa, 2015 [9] Xiao, 2015 [40] Rhodes, 2017 [56] |
| Leucine 17.5% | ||||
| Alanine 11.8% | ||||
| Proline 10.2% | ||||
| Phenylalanine 6.6% | ||||
| Quinoa | 13.8–16.5% | 11S-Globulin 2S-Albumin | Glutamine 13.2% | Dakhili, 2019 [57] Navruz-Varli, 2016 [58] Janssen, 2017 [59] |
| Aspartate 8.0% | ||||
| Arginine 7.7% | ||||
| Leucine 5.9% | ||||
| Proline 5.5% | ||||
| Amaranth | 12.0–22.0% | 11S-Globulin 7S-Globulin | Glutamine 16.2% | Grundy, 2020 [60] Kumar Maurya, 2018 [61] Janssen, 2017 [59] |
| Glycine 11.7% | ||||
| Aspartate 9.0% | ||||
| Serine 8.2% | ||||
| Arginine 7.6% | ||||
| Buckwheat | 11.0–15.0% | 13S-Globulin 8S-Globulin 2S-Globulin 2S-Albumin | Glutamine 19.4% | Alonso-Miravalles, 2018 [52] Janssen, 2017 [59] Sytar, 2016 [62] |
| Arginine 11.2% | ||||
| Aspartate 9.5% | ||||
| Proline 7.9% | ||||
| Leucine 5.9% |
| Cereal | Protein Modification Type | Description of Protein Modification | Results of Modification | Reference |
|---|---|---|---|---|
| Maize | Chemical | Defatting of zein with hexane and extraction with 70% (w/w) aqueous ethanol. | Obtainment of high α-prolamin. Increase of firmness with the presence of α-zein. | Taylor, 2018 [63] |
| Physical | Extrusion of zein at 90, 120, 140, and 160 °C. Addition of corn, rice, or potato starch. | Improvement of elasticity. Higher elasticity and peak stress with rice starch. | Federici, 2020 [64] | |
| Physical/chemical | Microfluidization of corn gluten meal (62% zein). Addition of guar gum or hydroxypropyl methylcellulose (HPMC). | Decrease of specific volume and porosity with microfluidization. Higher elastic moduli. | Ozturk, 2018 [65] | |
| Rice | Chemical/enzymatic | Addition of corn starch, inulin, xanthan gum, transglutaminase, protease, lipase, and quinoa flour to rice flour. | A small increase in specific volume with protease. Decrease of staling. No significant change in springiness. | Azizi, 2020 [66] |
| Enzymatic | Addition of proteases (papain, Protin SD-AY, Protin SD-NY, or Newlase F) to rice flour. | Increase of specific volume except with Newlase F. Improvement of viscoelastic moduli and elastic behavior. | Honda, 2018 [67] | |
| Chemical | Addition of xanthan gum and inulin to rice starch and bran. | Increase of firmness. Obtainment of tensile strength and elasticity. Porous microstructure. | Raungrusmee, 2020 [68] | |
| Physical | Microwave radiation of 20 and 30% moisture content rice flour. | Improvement of viscoelastic moduli and specific volume. Decrease of firmness and springiness. | Villanueva, 2019 [69] | |
| Sorghum | Enzymatic | Addition of transglutaminase to 10, 20, and 30% sorghum substituted wheat flours. | Increase of firmness and elasticity. No changes in volume. | Tunçil, 2019 [70] |
| Genetic | RNA interference technology suppressed α, γ, and δ kafirin. | Higher maximum force and viscosity. | Elhassan, 2017 [71] | |
| Chemical | Defatting of kafirin with hexane and extraction with 70% (w/w) aqueous ethanol. Extraction of γ-kafirin with 0.05M sodium lactate containing 2% (v/v) 2-mercaptoethanol. | Obtainment of high α-prolamin. Increase in the firmness of bread and a decrease in % stress recovery with the absence of γ-kafirin. | Taylor, 2018 [63] | |
| Physical | Thermal treatments on sorghum flour (90 and 125 °C) during 15, 30, and 45 min. | Specific volume increases. Peak viscosity increased with time. Decrease in firmness with time. | Marston, 2017 [72] |
| Tested Sample | Sample Pretreatment | Microscope | Microscopic Features | Reference |
|---|---|---|---|---|
| Genetic suppression of γ-kafirin | Addition of three drops 0.02% Acid Fuchsin dye in 1% acetic acid Heated at 60 °C 1 min | Zeiss 510 META system CLSM Excitation wavelength: 405 nm | Protein matrix density and microstructure | Elhassan, 2017 [71] |
| Extrusion of zein | Sputter coated with Pt Flash-frozen in liquid nitrogen at −185 °C | FEI NOVA nanoSEM Field Emission SEM | Morphology of zein Detection of fibrous microstructures. | Federici, 2020 [64] |
| Microfluidization of corn gluten meal | Freeze-dried 48 h Coated with Au-Pd by sputter coater device | Quanta 400F Field Emission SEM Voltage: 20 kV | Observe the aggregation tendency of zein. | Ozturk, 2018 [65] |
| Addition of hydrocolloids to rice bran | Coated with Au | JSM 6310F SEM Voltage: 5 kV | Observe the porosity of the microstructure. | Raungrusmee, 2020 [68] |
| Chemical extraction of γ-kafirin and zein | No pretreatment | Zeiss 510 META system CLSM Excitation wavelength: 488 nm | Morphology of zein and kafirin Detection of fibrous microstructures. | Taylor, 2018 [63] |
| Sample Modification | Sample Pretreatment | Rheometer Characteristics | Studied Parameters | Reference |
|---|---|---|---|---|
| Genetic suppression of γ-kafirin (dough) | No pretreatment | Physica MCR 101 rheometer Parallel plate geometry 25 mm diameter/2 mm gap 25–150 °C at 6.25 °C/min 6.3 rad/s 0.01–100% strain rate | G’ G” tan δ Transgenic sorghum > control sorghum | Elhassan, 2017 [71] |
| Extrusion of zein (dough) | 15 min rest at room temperature inside a polystyrene box with a water beaker | TA Instruments ARG-2 Model rheometer Parallel plate geometry 40 mm diameter Above 35 °C | α: 0.43 | Federici, 2020 [64] |
| Protease addition to rice flour (batter) | No pretreatment | Physica MCR 301 rotational rheometer Coaxial cylinder 25 mm inner diameter/26 mm outer diameter 30–90 °C at 2.5 °C/min 10 rad/s angular velocity 0.1% strain | G’: 2160 Pa G”: 584 Pa tan δ: 0.27 | Honda, 2018 [67] |
| Microfluidization of corn gluten meal (dough) | 5 min rest at room temperature | TA Instruments AR2000ex rheometer Parallel plate geometry 20 mm diameter/2 mm gap 25 °C 6.283 rad/s 0.01–10% strain rate | G’: 140,000 Pa G”: 40,000 Pa | Ozturk, 2018 [65] |
| Transglutaminase addition to sorghum/wheat flour (dough) | No pretreatment | TA Instruments ARG-2 Model rheometer Parallel plate geometry 40 mm diameter 0.01–50 rad/s 0.5% | G*: 60,000 Pa δ: 18 | Tunçil, 2018 [70] |
| Microwave radiation to rice flour (dough) | 5 min rest at room temperature | Malvern Instruments Kinexus Pro+ rheometer Parallel plate geometry 40 mm diameter/1 mm gap 25 °C | G’: 3238 Pa G”: 1485 Pa tan δ: 0.49 | Villanueva, 2019 [69] |
| Sample Modification | Sample Pretreatment | Equipment | Studied Parameter | Reference |
|---|---|---|---|---|
| Chemical extraction of γ-kafirin and zein | Dried in a desiccator for 14 days | DSC 25–280 °C scans Heat rate of 10 °C/min under nitrogen pressure (40 bar) | Tg Kafirin Tg > Zein Tg | Taylor, 2018 [63] |
| Genetic suppression of γ-kafirin | Addition of deionized distilled water for a total weight of 36 mg | DSC 30–120 °C scans Heat rate of 10 °C/min under nitrogen at normal air pressure A flow rate of 30 mL/min | ΔH of gelatinization: 3.1 J/g | Elhassan, 2017 [71] |
| Addition of hydrocolloids to rice bran | No pretreatment | DSC 25–200 °C scans Heat rate of 10 °C/min Cool rate of 25 °C | ΔH of gelatinization: −6691.51 J/g | Raungrusmee, 2020 [68] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinoza-Herrera, J.; Martínez, L.M.; Serna-Saldívar, S.O.; Chuck-Hernández, C. Methods for the Modification and Evaluation of Cereal Proteins for the Substitution of Wheat Gluten in Dough Systems. Foods 2021, 10, 118. https://doi.org/10.3390/foods10010118
Espinoza-Herrera J, Martínez LM, Serna-Saldívar SO, Chuck-Hernández C. Methods for the Modification and Evaluation of Cereal Proteins for the Substitution of Wheat Gluten in Dough Systems. Foods. 2021; 10(1):118. https://doi.org/10.3390/foods10010118
Chicago/Turabian StyleEspinoza-Herrera, Javier, Luz María Martínez, Sergio O. Serna-Saldívar, and Cristina Chuck-Hernández. 2021. "Methods for the Modification and Evaluation of Cereal Proteins for the Substitution of Wheat Gluten in Dough Systems" Foods 10, no. 1: 118. https://doi.org/10.3390/foods10010118
APA StyleEspinoza-Herrera, J., Martínez, L. M., Serna-Saldívar, S. O., & Chuck-Hernández, C. (2021). Methods for the Modification and Evaluation of Cereal Proteins for the Substitution of Wheat Gluten in Dough Systems. Foods, 10(1), 118. https://doi.org/10.3390/foods10010118








