New Mixed Y0.5R0.5VO4 and RVO4:Bi Materials: Synthesis, Crystal Structure and Some Luminescence Properties
Abstract
1. Introduction
2. Results and Discussion
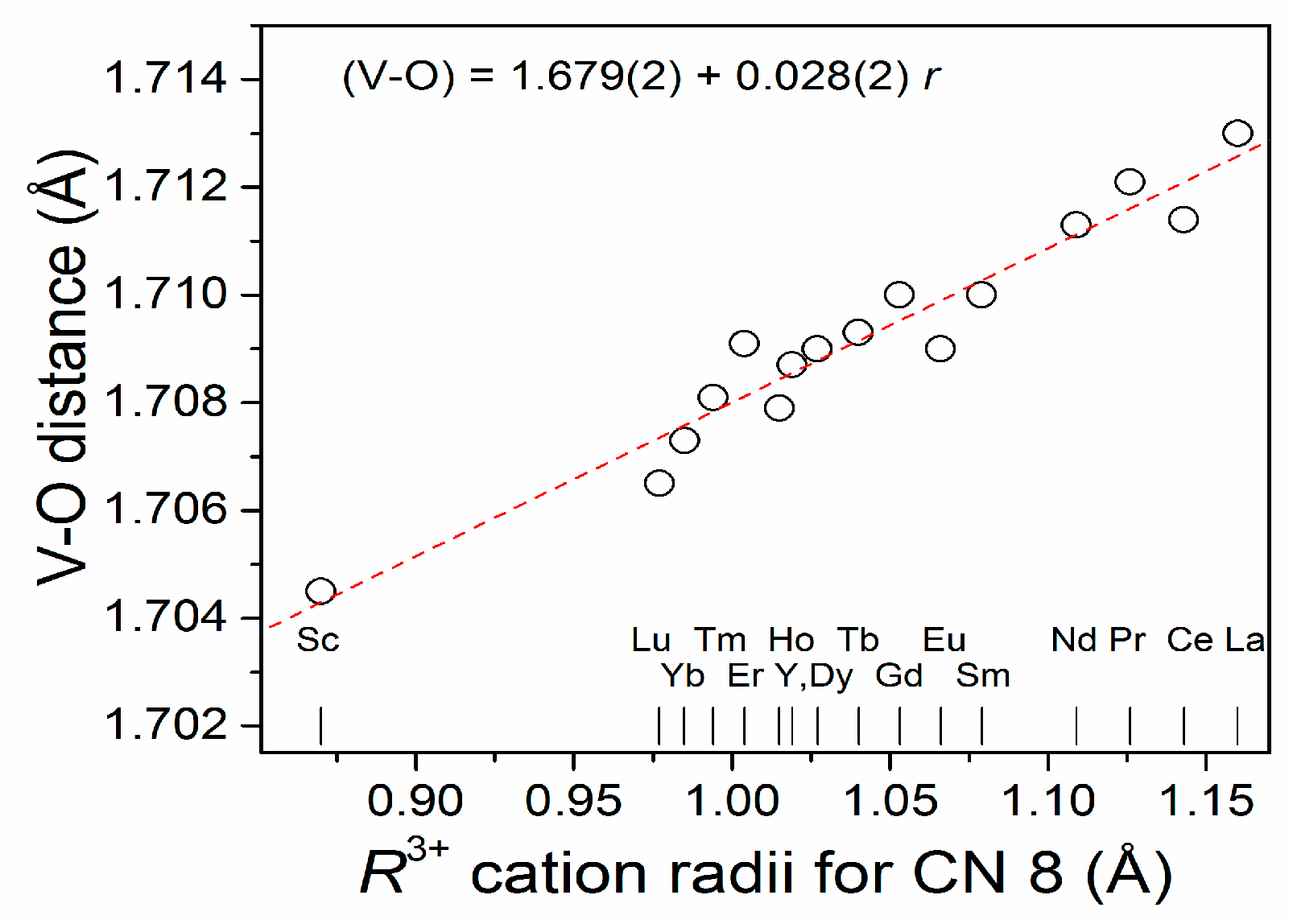
2.1. Crystal Structure Results
2.1.1. Mixed Vanadates Y0.5R0.5VO4
2.1.2. Bi3+-Doped Vanadates RVO4 (R = La, Gd, Y, Y0.5Lu0.5, Lu)
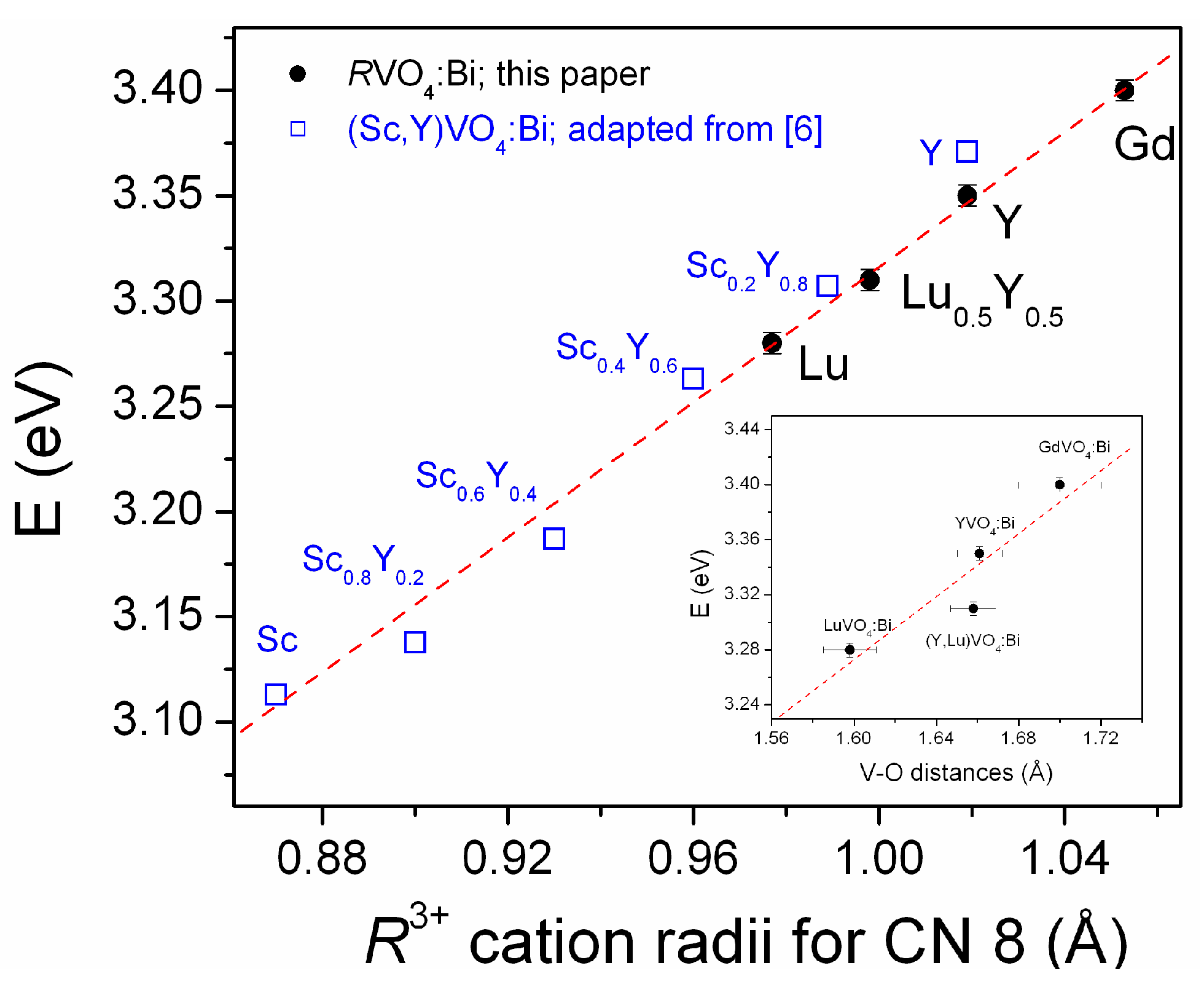
2.2. Luminescence and Optical Studies of the Bi-Doped Materials
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kolitsch, U.; Holtstam, D. Crystal chemistry of REEXO4 compounds (X = P, As, V). II. Review of REEXO4 compounds and their stability fields. Eur. J. Mineral. 2004, 16, 117–126. [Google Scholar] [CrossRef]
- Koechner, W. Solid-State Laser Engineering; Springer: Berlin/Heidelberg, Germany, 2006; p. 69. ISBN 978-0-387-29094-2. [Google Scholar]
- Kalai Selvan, R.; Gedanken, A.; Anilkumar, P.; Manikandan, G.; Karunakaran, C. Synthesis and characterization of rare earth orthovanadate (RVO4; R = La, Ce, Nd, Sm, Eu & Gd) Nanorods/Nanocrystals/Nanospindles by a facile sonochemical method and their catalytic properties. J. Clust. Sci. 2009, 20, 291–305. [Google Scholar] [CrossRef]
- Kaminskii, A.A. Tetragonal vanadates REVO4 (RE = Ln (Ce–Lu), Y)—A novel class of SRS-active crystals. Dokl. Phys. 2013, 58, 165–168. [Google Scholar] [CrossRef]
- Chen, L.; Chen, K.J.; Lin, C.C.; Chu, C.I.; Hu, S.F.; Lee, M.H.; Liu, R.S. Combinatorial approach to the development of a single mass YVO4:Bi3+, Eu3+ Phosphor with red and green dual colors for high color rendering white light–emitting diodes. J. Comb. Chem. 2010, 12, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Kang, F.; Zhang, H.; Wondraczek, L.; Yang, X.; Zhang, Y.; Lei, D.Y.; Peng, M. Band-gap modulation in single Bi3+-doped yttrium–scandium–niobium vanadates for color tuning over the whole visible spectrum. Chem. Mater. 2016, 28, 2692–2703. [Google Scholar] [CrossRef]
- Liu, H.; Yuan, J.; Jiang, Z.; Shangguan, W.; Einaga, H.; Teroaka, Y. Novel photocatalyst of V–based solid solutions for overall water splitting. J. Mater. Chem. 2011, 21, 16535–16543. [Google Scholar] [CrossRef]
- Huang, X.Y.; Wang, J.X.; Yu, D.C.; Ye, S.; Zhang, Q.Y. Spectral conversion for solar cell efficiency enhancement using YVO4:Bi3+, Ln3+ (Ln = Dy, Er, Ho, Eu, Sm, and Yb) phosphors. J. Appl. Phys. 2011, 109, 113526. [Google Scholar] [CrossRef]
- Errandonea, D.; Garg, A.B. Recent progress on the characterization of the high-pressure behaviour of AVO4 orthovanadates. Prog. Mater. Sci. 2018, 97, 123–169. [Google Scholar] [CrossRef]
- International Centre for Diffraction Data (ICDD). PDF-4+ 2010 (Database); Kabekkodu, S., Ed.; International Centre for Diffraction Data: Newtown Square, PA, USA, 2010. [Google Scholar]
- Isasi, J.; Veiga, M.L.; Laureiro, Y.; Saez-Puche, R.; Pico, C. Synthesis, structural determination and magnetic behavior of YxGd1−xVO4 phases (x = 0.25, 0.50, 0.75). J. Alloys Compd. 1991, 177, 143–147. [Google Scholar] [CrossRef]
- Milligan, W.O.; Vernon, L.W. Crystal Structure of Heavy metal orthovanadates. J. Phys. Chem. 1952, 56, 145–148. [Google Scholar] [CrossRef]
- Chakoumakos, B.C.; Abraham, M.M.; Boatner, L.A. Crystal structure refinements of zircon-type MVO4 (M = Sc, Y, Ce, Pr, Nd, Tb, Ho, Er, Tm, Yb, Lu). J. Solid State Chem. 1994, 109, 197–202. [Google Scholar] [CrossRef]
- Aldred, A.T. Cell Volumes of APO4, AVO4 and ANbO4 Compounds, where A = Sc, Y, La–Lu*. Acta Crystallogr. B 1984, 40, 569–574. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–757. [Google Scholar] [CrossRef]
- Rice, C.E.; Robinson, W.R. Lanthanum orthovanadate. Acta Crystallogr. B 1976, 32, 2232–2233. [Google Scholar] [CrossRef]
- Takeshita, S.; Ogata, H.; Isobe, T.; Sawayama, T.; Niikura, S. Effects of citrate additive on transparency and photostability properties of YVO4:Bi3+, Eu3+ nanophosphor. J. Electrochem. Soc. 2010, 157, J74. [Google Scholar] [CrossRef]
- Garces, N.Y.; Stevens, K.T.; Foundos, G.K.; Halliburton, L.E. Electron paramagnetic resonance and optical absorption study of V4+ centres in YVO4 crystals. J. Phys. Condens. Matter 2004, 16, 7095–7106. [Google Scholar] [CrossRef]
- Cooper, J.K.; Scott, S.B.; Ling, Y.; Yang, J.; Hao, S.; Li, Y.; Toma, F.M.; Stutzman, M.; Lakshmi, K.V.; Sharp, I.D. Role of Hydrogen in Defining the n-Type Character of BiVO4 Photoanodes. Chem. Mater. 2016, 28, 5761–5771. [Google Scholar] [CrossRef]
- Datta, R. Bismuth in yttrium vanadate and yttrium europium vanadate phosphors. J. Electrochem. Soc. 1967, 114, 1057–1063. [Google Scholar] [CrossRef]
- Cavalli, E.; Angiuli, F.; Mezzadri, F.; Trevisani, M.; Bettinelli, M.; Boutinaud, P.; Brik, M.G. Tunable luminescence of Bi3+-doped YPxV1−xO4 (0 ≤ x ≤1). J. Phys. Condens. Matter 2014, 26, 385503. [Google Scholar] [CrossRef] [PubMed]
- Zazubovich, S.; Krasnikov, A.; Zorenko, Y.; Gorbenko, V.; Babin, V.; Mihokova, E.; Nikl, M. Luminescence of Pb- and Bi-Related Centers in Aluminum Garnet, Perovskite, and Orthosilicate Single-Crystalline Films. In Nanocomposite, Ceram. Thin Film Scintill; Nikl, M., Ed.; Pan Stanford Publishing: Singapore, 2017; pp. 227–302. ISBN 978-981-4745-22-2. [Google Scholar]
- Krasnikov, A.; Luchechko, A.; Mihokova, E.; Nikl, M.; Syvorotka, I.I.; Zazubovich, S.; Zhydachevskii, Y. Origin of Bi3+-related luminescence in Gd3Ga5O12:Bi epitaxial films. J. Lumin. 2017, 190, 81–88. [Google Scholar] [CrossRef]
- Barendswaard, W.; Weber, R.T.; van der Waals, J.H. An EPR study of the luminescent triplet state of VO3−4 in YVO4 and YP0.96V0.04O4 single crystals at 1.2 K. J. Chem. Phys. 1987, 87, 3731. [Google Scholar] [CrossRef]
- Matos, M.; Rocha, L.; Nassar, E.; Verelst, M. Influence of Bi3+ ions on the excitation wavelength of the YVO4:Eu3+ matrix. Opt. Mater. 2016, 62, 12–18. [Google Scholar] [CrossRef]
- Boutinaud, P. Revisiting the spectroscopy of the Bi3+ ion in oxide compounds. Inorg. Chem. 2013, 52, 6028–6038. [Google Scholar] [CrossRef] [PubMed]
- Blasse, G.; Bril, A. Investigations on Bi3+-Activated Phosphors. J. Chem. Phys. 1968, 48, 217–222. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Aleksandrovsky, A.S.; Chimitova, O.D.; Diao, C.P.; Gavrilova, T.A.; Kesler, V.G.; Molokeev, M.S.; Krylov, A.S.; Bazarov, B.G.; Bazarova, J.G.; et al. Electronic structure of β-RbSm(MoO4)2 and chemical bonding in molybdates. Dalton Trans. 2015, 44, 1805. [Google Scholar] [CrossRef] [PubMed]
- Atuchin, V.V.; Aleksandrovsky, A.S.; Molokeev, M.S.; Krylov, A.S.; Oreshonkov, A.S.; Zhou, D. Structural and spectroscopic properties of self-activated monoclinic molybdate BaSm2(MoO4)4. J. Alloys Compd. 2017, 729, 843–849. [Google Scholar] [CrossRef]
- Panchal, V.; Errandonea, D.; Segura, A.; Rodrguez-Hernandez, P.; Muoz, A.; Lopez-Moreno, S.; Bettinelli, M. The electronic structure of zircon-type orthovanadates: Effects of high-pressure and cation substitution. J. Appl. Phys. 2011, 110, 043723. [Google Scholar] [CrossRef]
- Garg, A.B.; Errandonea, D.; Rodrigues-Hernandez, P.; Munoz, A. ScVO4 under non-hydrostatic compression: A new metastable polymorph. J. Phys. Condens. Matter 2017, 29, 055401. [Google Scholar] [CrossRef] [PubMed]
- Dolgos, M.R.; Paraskos, A.M.; Stoltzfus, M.W.; Yarnell, S.C.; Woodward, P.M. The electronic structures of vanadate salts: Cation substitution as a tool for band gap manipulation. J. Solid State Chem. 2009, 182, 1964–1971. [Google Scholar] [CrossRef]
- Mahlik, S.; Amer, M.; Boutinaud, P. Energy level structure of Bi3+ in Zircon and Scheelite polymorphs of YVO4. J. Phys. Chem. C 2016, 120, 8261–8265. [Google Scholar] [CrossRef]
- Mullica, D.F.; Sappenfield, E.L.; Abraham, M.M.; Chakoumakos, B.C.; Boatner, L.A. Structural investigations of several LnVO4 compounds. Inorg. Chim. Acta 1996, 248, 85–88. [Google Scholar] [CrossRef]
- Oka, Y.; Yao, T.; Yamamoto, N.J. Hydrothermal synthesis of lanthanum vanadates: Synthesis and crystal structures of zircon-type LaVO4 and a new compound LaV3O9. Solid State Chem. 2000, 152, 486–491. [Google Scholar] [CrossRef]
- Akselrud, L.; Grin, Yu. WinCSD: Software package for crystallographic calculations (Version 4). J. Appl. Crystallogr. 2014, 47, 803–805. [Google Scholar] [CrossRef]
- Zhydachevskyy, Y.; Syvorotka, I.I.; Tsiumra, V.; Baran, M.; Lipińska, L.; Wierzbicka, A.; Suchocki, A. Quantum efficiency of the down-conversion process in Bi3+–Yb3+ and Ce3+–Yb3+ co-doped garnets. Sol. Energy Mater. Sol. Cells 2018, 185, 240–251. [Google Scholar] [CrossRef]
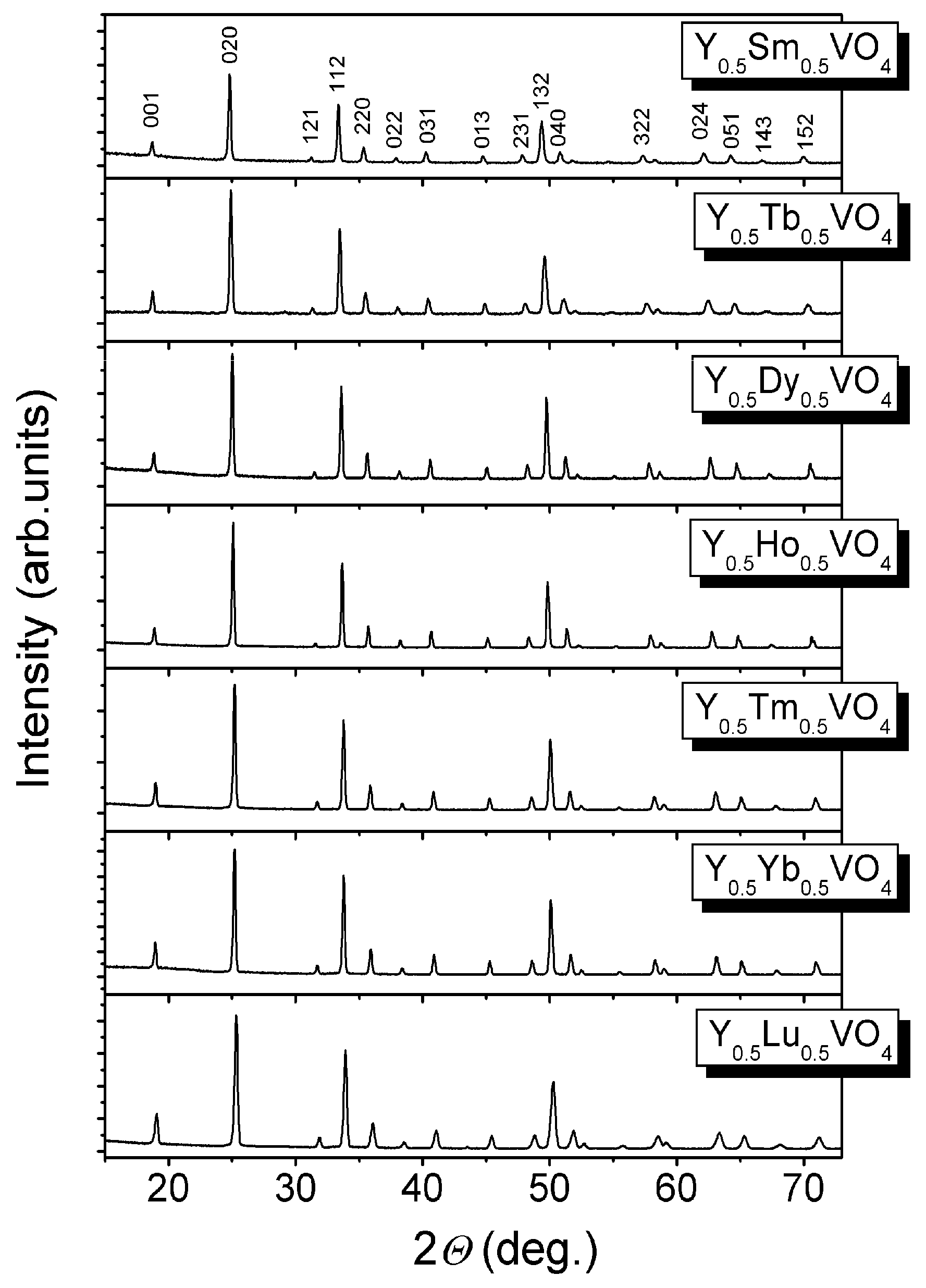
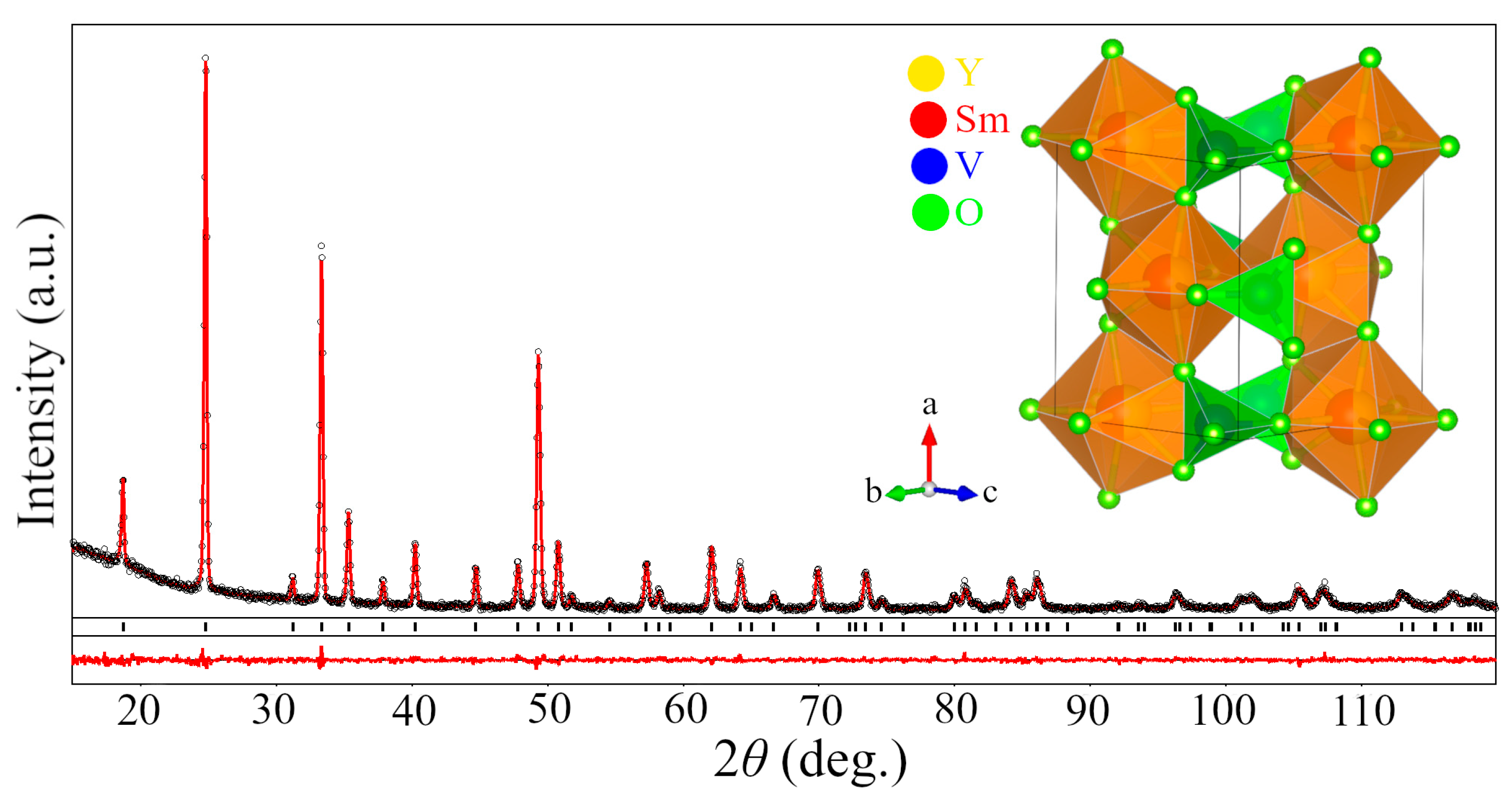
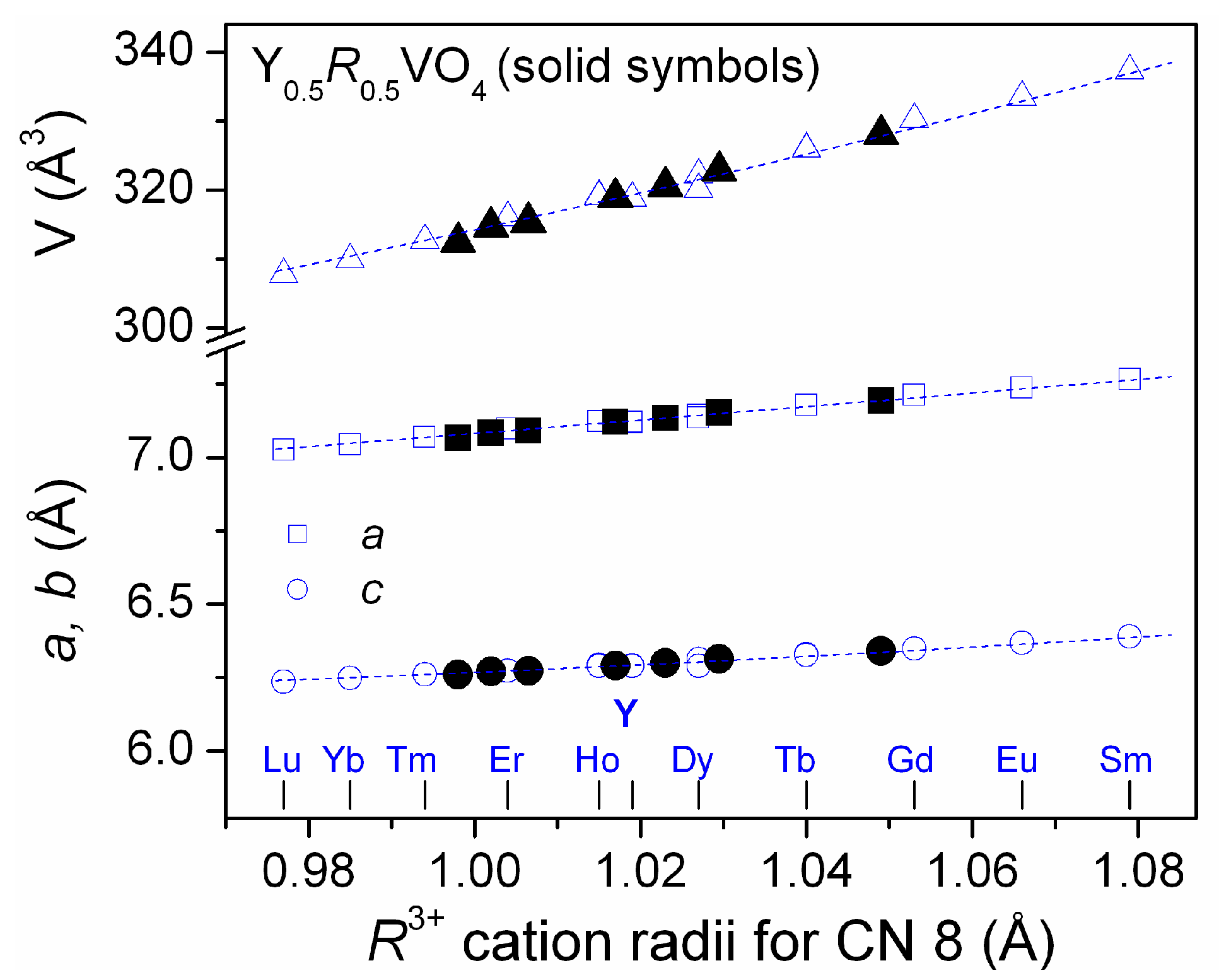
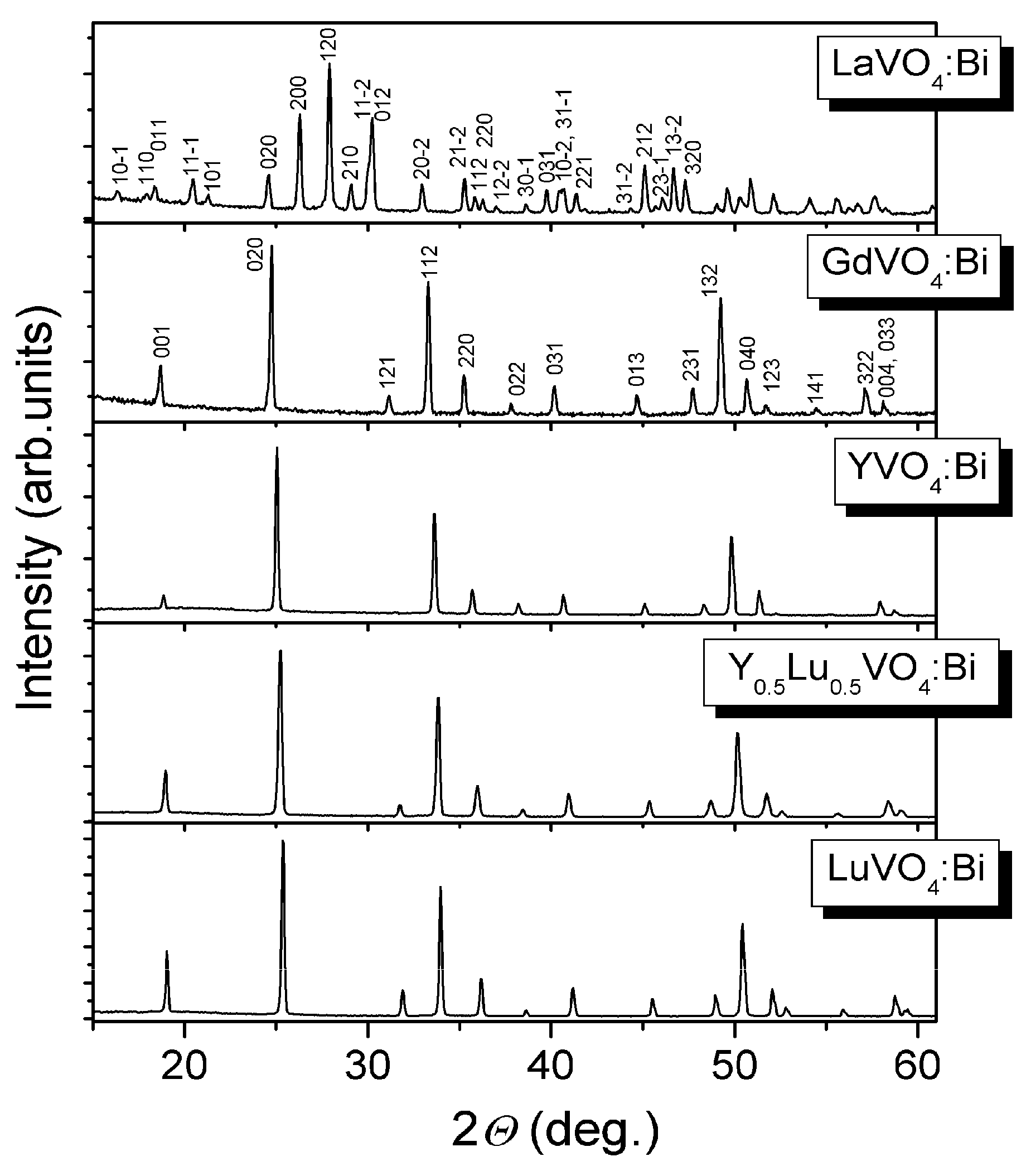
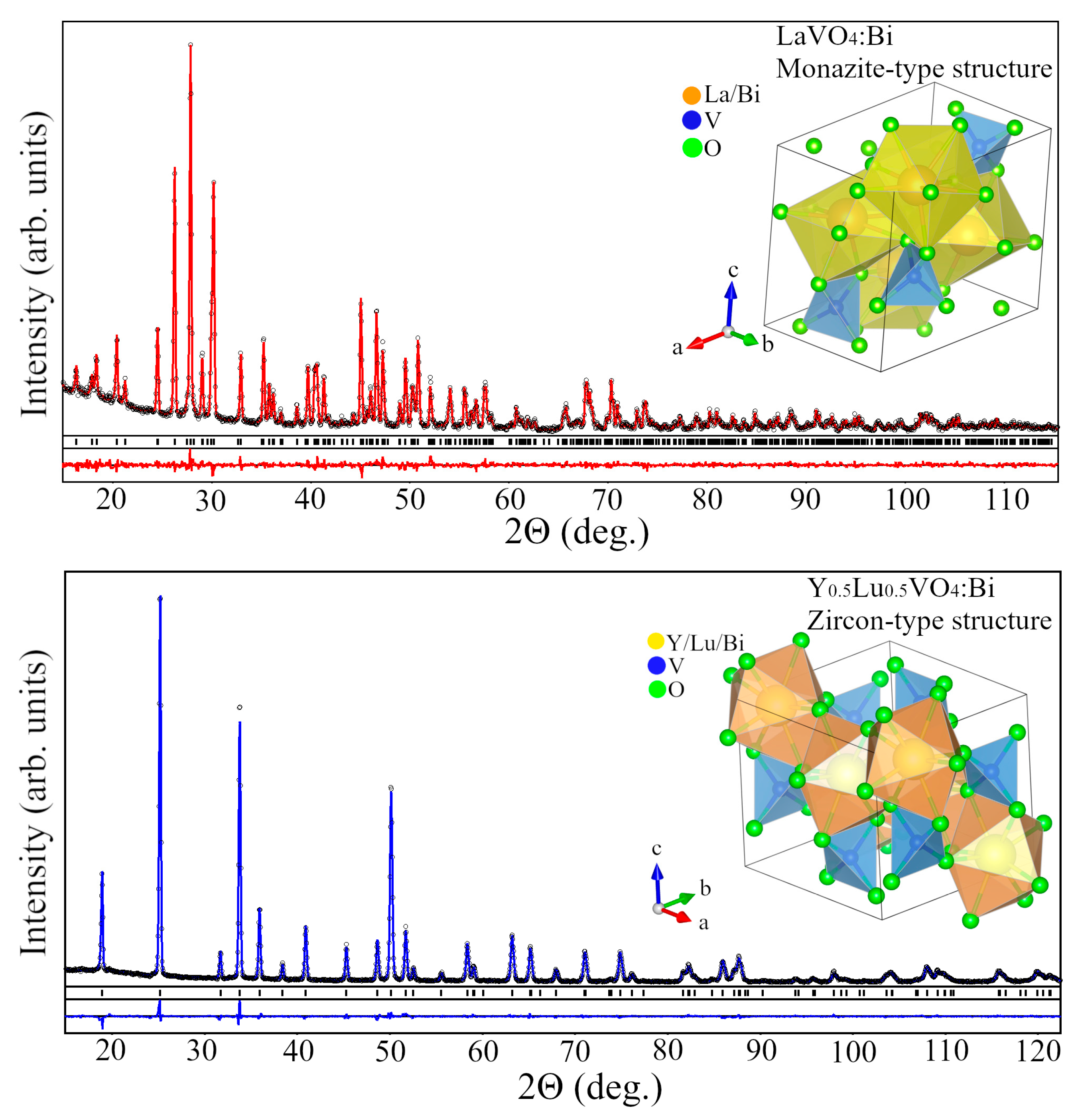
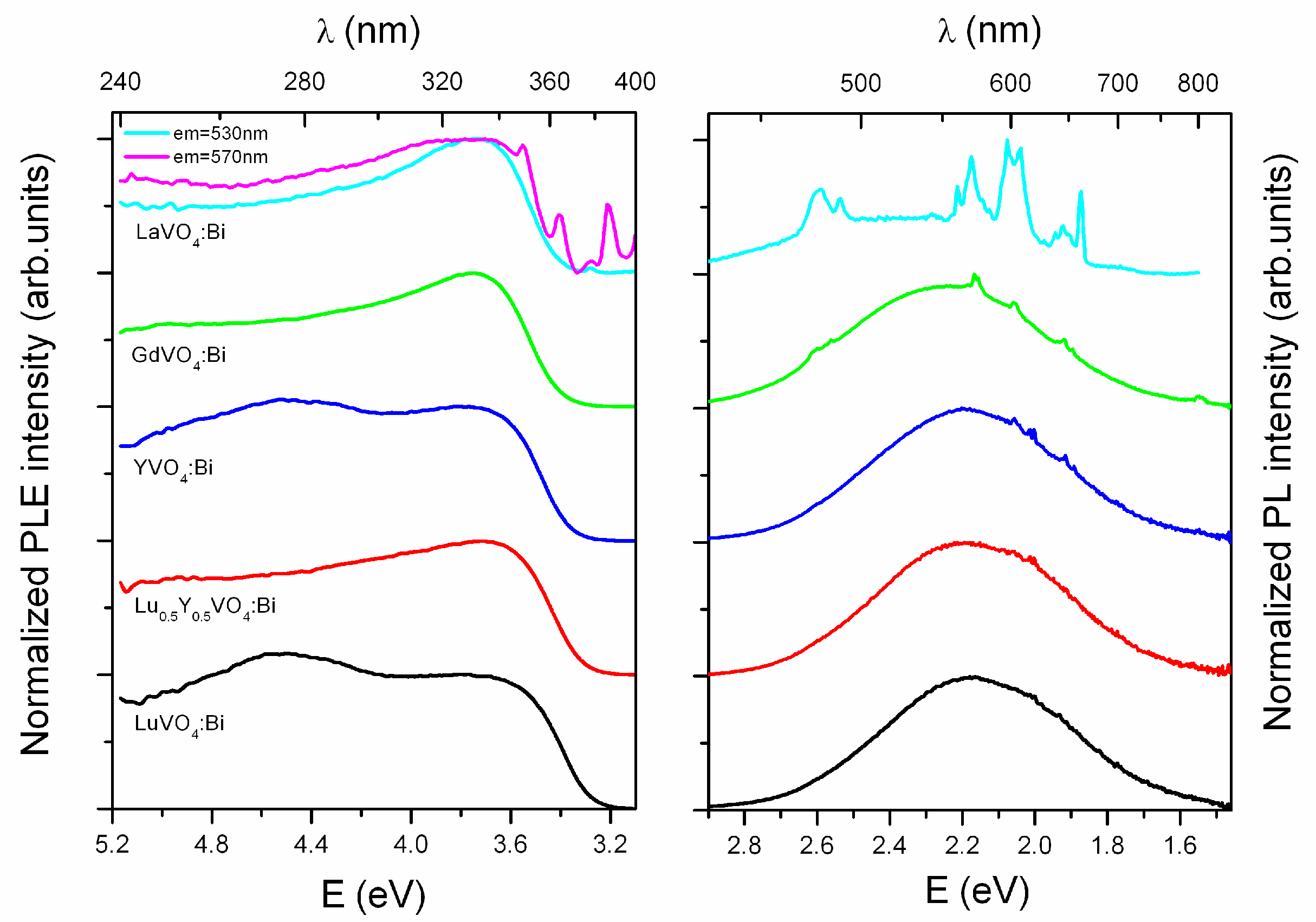
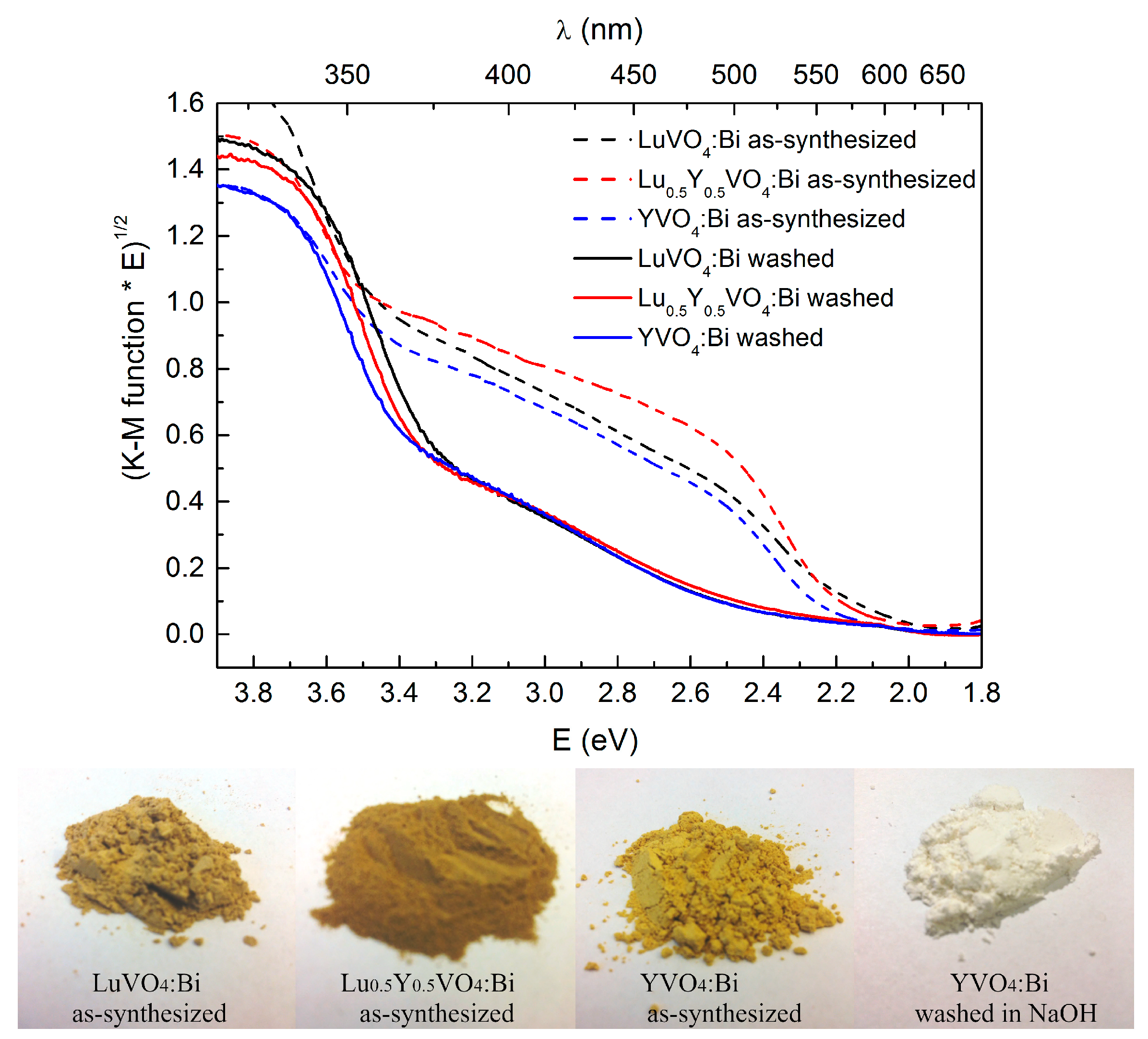
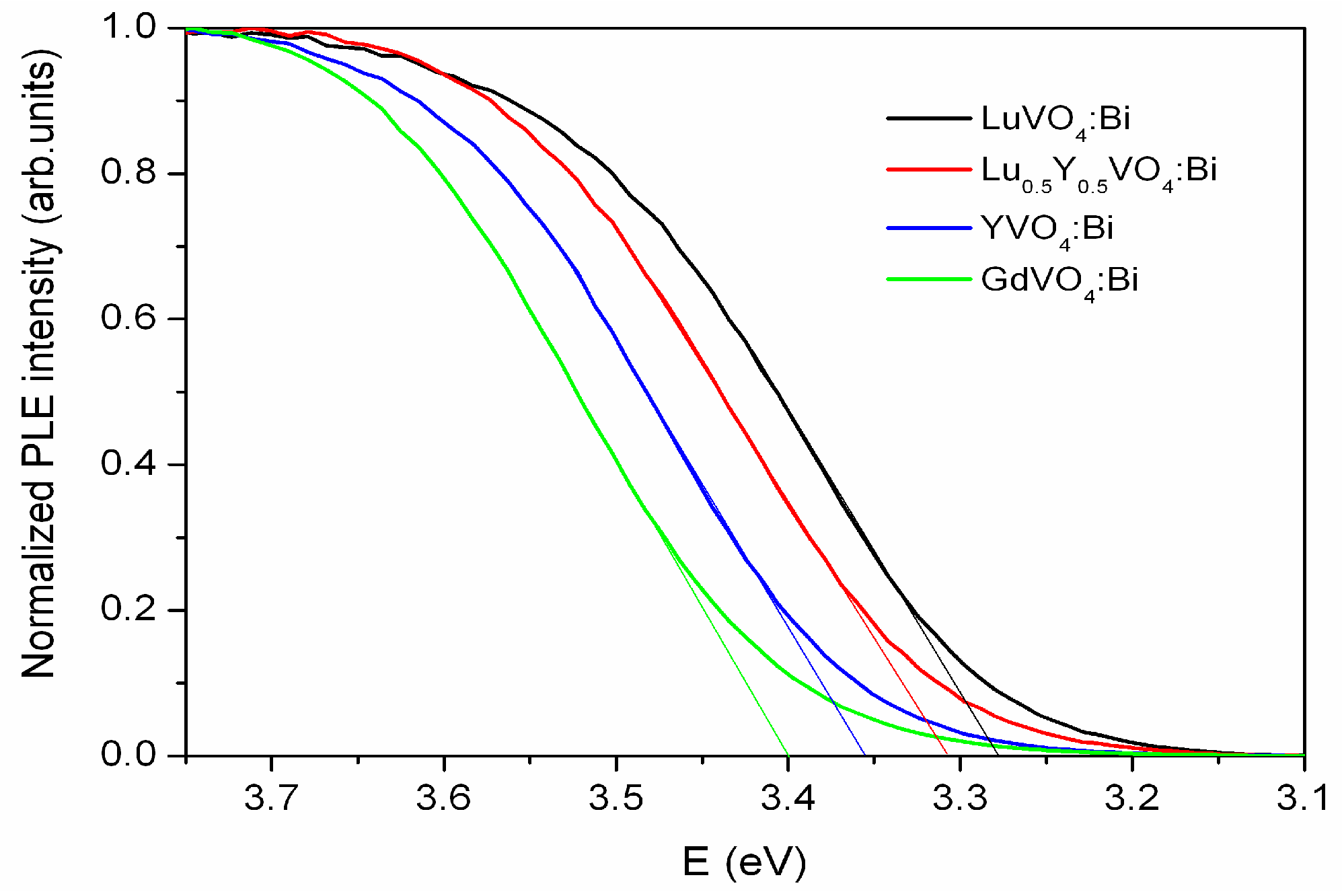
| Parameters, Residuals | R in Y0.5R0.5VO4 | ||||||
|---|---|---|---|---|---|---|---|
| Sm | Tb | Dy | Ho | Tm | Yb | Lu | |
| a, Å | 7.1933(4) | 7.1509(3) | 7.1337(2) | 7.1206(1) | 7.0907(2) | 7.0815(1) | 7.0655(2) |
| c, Å | 6.3398(4) | 6.3126(3) | 6.2991(2) | 6.2901(1) | 6.2724(2) | 6.2684(2) | 6.2588(2) |
| V, Å3 | 328.05(5) | 322.79(5) | 320.56(2) | 318.92(2) | 315.36(2) | 314.35(2) | 312.45(3) |
| Biso(Y/R), Å2 | 0.59(4) | 0.62(3) | 0.78(3) | 1.17(3) | 0.88(2) | 0.84(2) | 0.67(2) |
| Biso(V), Å2 | 0.71(12) | 0.45(7) | 0.64(6) | 0.48(5) | 0.80(4) | 0.65(4) | 0.52(4) |
| y/b(O) | 0.4302(13) | 0.4278(7) | 0.4313(6) | 0.4308(5) | 0.4312(4) | 0.4320(5) | 0.4321(4) |
| z/c(O) | 0.2033(13) | 0.2071(7) | 0.2048(7) | 0.2054(5) | 0.2040(4) | 0.2062(5) | 0.2034(4) |
| Biso(O), Å2 | 1.1(3) | 1.42(15) | 1.55(14) | 1.29(10) | 1.48(8) | 1.38(9) | 1.51(9) |
| RI | 0.030 | 0.029 | 0.021 | 0.023 | 0.022 | 0.029 | 0.023 |
| RP | 0.124 | 0.129 | 0.095 | 0.080 | 0.082 | 0.073 | 0.085 |
| D, nm | 108 | 202 | 92 | 158 | 106 | 129 | 93 |
| <ε>, % | 0.121 | 0.185 | 0.032 | 0.039 | 0.090 | 0.065 | 0.207 |
| R | 0.014 | 0.045 | 0.003 | 0.042 | 0.003 | 0.006 | 0.044 |
| Sm | Tb | Dy | Ho | Tm | Yb | Lu | |
|---|---|---|---|---|---|---|---|
| VO4 tetrahedra | |||||||
| V–O × 4 | 1.693(9) | 1.656(10) | 1.680(7) | 1.673(8) | 1.673(6) | 1.665(8) | 1.675(8) |
| O···O × 4 | 2.846(11) | 2.781(6) | 2.816(9) | 2.811(10) | 2.811(8) | 2.789(10) | 2.813(10) |
| O···O × 2 | 2.593(13) | 2.543(7) | 2.590(10) | 2.567(12) | 2.568(9) | 2.571(12) | 2.571(12) |
| O–V–O × 4 | 114.41(3) | 114.22(2) | 113.93(3) | 114.30(3) | 114.27(3) | 113.81(3) | 114.26(3) |
| O–V–O × 2 | 99.99(3) | 100.34(2) | 100.88(3) | 100.20(3) | 100.25(3) | 101.11(3) | 100.27(3) |
| RO8 polyhedra | |||||||
| R–O × 4 | 2.353(9) | 2.361(11) | 2.328(7) | 2.331(8) | 2.315(7) | 2.312(8) | 2.300(8) |
| R–O × 4 | 2.453(9) | 2.452(10) | 2.450(7) | 2.437(8) | 2.430(6) | 2.442(8) | 2.425(8) |
| O···O × 2 | 2.593(13) | 2.543(7) | 2.590(10) | 2.567(12) | 2.568(9) | 2.571(12) | 2.571(12) |
| O···O × 4 | 2.767(11) | 2.810 (6) | 2.763(10) | 2.757(11) | 2.739(9) | 2.762(11) | 2.723(11) |
| O···O × 8 | 3.080(10) | 3.069(5) | 3.053(8) | 3.050(9) | 3.037(7) | 3.032(9) | 3.025(9) |
| O···O × 4 | 3.401(9) | 3.419(5) | 3.368(8) | 3.371(8) | 3.348(7) | 3.348(9) | 3.326(9) |
| O–R–O × 2 | 63.83(2) | 62.49(13) | 63.81(2) | 63.55(2) | 63.80(2) | 63.52(2) | 64.03(2) |
| O–R–O × 4 | 70.27(2) | 71.42(13) | 70.63(2) | 70.60(2) | 70.46(2) | 70.97(2) | 70.31(2) |
| O–R–O × 8 | 79.68(2) | 79.20(13) | 79.38(2) | 79.50(2) | 79.53(2) | 79.20(2) | 79.57(2) |
| O–R–O × 4 | 92.55(3) | 92.75(13) | 92.70(2) | 92.63(2) | 92.62(2) | 92.78(2) | 92.61(2) |
| Lattice Parameters, Residuals | Atoms, Sites | x/a | y/b | z/c | Biso/eq, Å2 |
|---|---|---|---|---|---|
| LaVO4:Bi, space group P21/n (D = 145 nm; <ε> = 0.048%) | |||||
| a = 7.0396(2) Å b = 7.2777(3) Å c = 6.7212(2) Å β = 104.856(2)° | La/Bi, 4e | 0.2768(3) | 0.1574(4) | 0.1037(4) | 0.97(3) |
| V, 4e | 0.3013(9) | 0.1643(11) | 0.6170(10) | 0.82(11) | |
| O1, 4e | 0.253(3) | 0.008(3) | 0.431(3) | 0.5(5) | |
| O2, 4e | 0.384(3) | 0.344(4) | 0.496(3) | 1.1(4) | |
| O3, 4e | 0.478(3) | 0.099(3) | 0.826(3) | 1.4(5) | |
| O4, 4e | 0.118(3) | 0.218(3) | 0.729(3) | 2.2(6) | |
| RI = 0.031; Rp = 0.122 | |||||
| GdVO4:Bi, space group I41/amd (D = 138 nm; <ε> = 0.020%) | |||||
| a = 7.2118(4) Å c = 6.3484(4) Å | Gd/Bi, 4a | 0 | 3/4 | 1/8 | 0.95(9) |
| V, 4b | 0 | 1/4 | 3/8 | 1.0(3) | |
| O1, 16h | 0 | 0.433(2) | 0.206(2) | 0.5(4) | |
| RI = 0.029; Rp = 0.167 | |||||
| YVO4:Bi, space group I41/amd (D = 207 nm; <ε> = 0.032%) | |||||
| a = 7.1188(1) Å c = 6.2902(2) Å | Y/Bi, 4a | 0 | 3/4 | 1/8 | 1.20(4) |
| V, 4b | 0 | 1/4 | 3/8 | 0.47(7) | |
| O1, 16h | 0 | 0.4319(7) | 0.2096(7) | 1.60(13) | |
| RI = 0.035; Rp = 0.076 | |||||
| LuVO4:Bi, space group I41/amd (D = 251 nm; <ε> = 0.037%) | |||||
| a = 7.0269(1) Å c = 6.2341(1) Å | Lu/Bi, 4a | 0 | 3/4 | 1/8 | 1.47(2) |
| V, 4b | 0 | 1/4 | 3/8 | 0.40(6) | |
| O1, 16h | 0 | 0.4252(8) | 0.2117(9) | 1.63(15) | |
| RI = 0.035; Rp = 0.069 | |||||
| (Y,Lu)VO4:Bi, space group I41/amd (D = 98 nm; <ε> = 0.095%) | |||||
| a = 7.0690(2) Å c = 6.2595(3) Å | Y/Lu/Bi, 4a | 0 | 3/4 | 1/8 | 1.41(3) |
| V, 4b | 0 | 1/4 | 3/8 | 0.38(6) | |
| O1, 16h | 0 | 0.4286(7) | 0.2035(7) | 1.69(14) | |
| RI = 0.031; Rp = 0.077 | |||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasylechko, L.; Tupys, A.; Hreb, V.; Tsiumra, V.; Lutsiuk, I.; Zhydachevskyy, Y. New Mixed Y0.5R0.5VO4 and RVO4:Bi Materials: Synthesis, Crystal Structure and Some Luminescence Properties. Inorganics 2018, 6, 94. https://doi.org/10.3390/inorganics6030094
Vasylechko L, Tupys A, Hreb V, Tsiumra V, Lutsiuk I, Zhydachevskyy Y. New Mixed Y0.5R0.5VO4 and RVO4:Bi Materials: Synthesis, Crystal Structure and Some Luminescence Properties. Inorganics. 2018; 6(3):94. https://doi.org/10.3390/inorganics6030094
Chicago/Turabian StyleVasylechko, Leonid, Andrii Tupys, Vasyl Hreb, Volodymyr Tsiumra, Iryna Lutsiuk, and Yaroslav Zhydachevskyy. 2018. "New Mixed Y0.5R0.5VO4 and RVO4:Bi Materials: Synthesis, Crystal Structure and Some Luminescence Properties" Inorganics 6, no. 3: 94. https://doi.org/10.3390/inorganics6030094
APA StyleVasylechko, L., Tupys, A., Hreb, V., Tsiumra, V., Lutsiuk, I., & Zhydachevskyy, Y. (2018). New Mixed Y0.5R0.5VO4 and RVO4:Bi Materials: Synthesis, Crystal Structure and Some Luminescence Properties. Inorganics, 6(3), 94. https://doi.org/10.3390/inorganics6030094





