Assembly of ZnO Nanoparticles on SiO2@α-Fe2O3 Nanocomposites for an Efficient Photo-Fenton Reaction
Abstract
1. Introduction
2. Results and Discussion
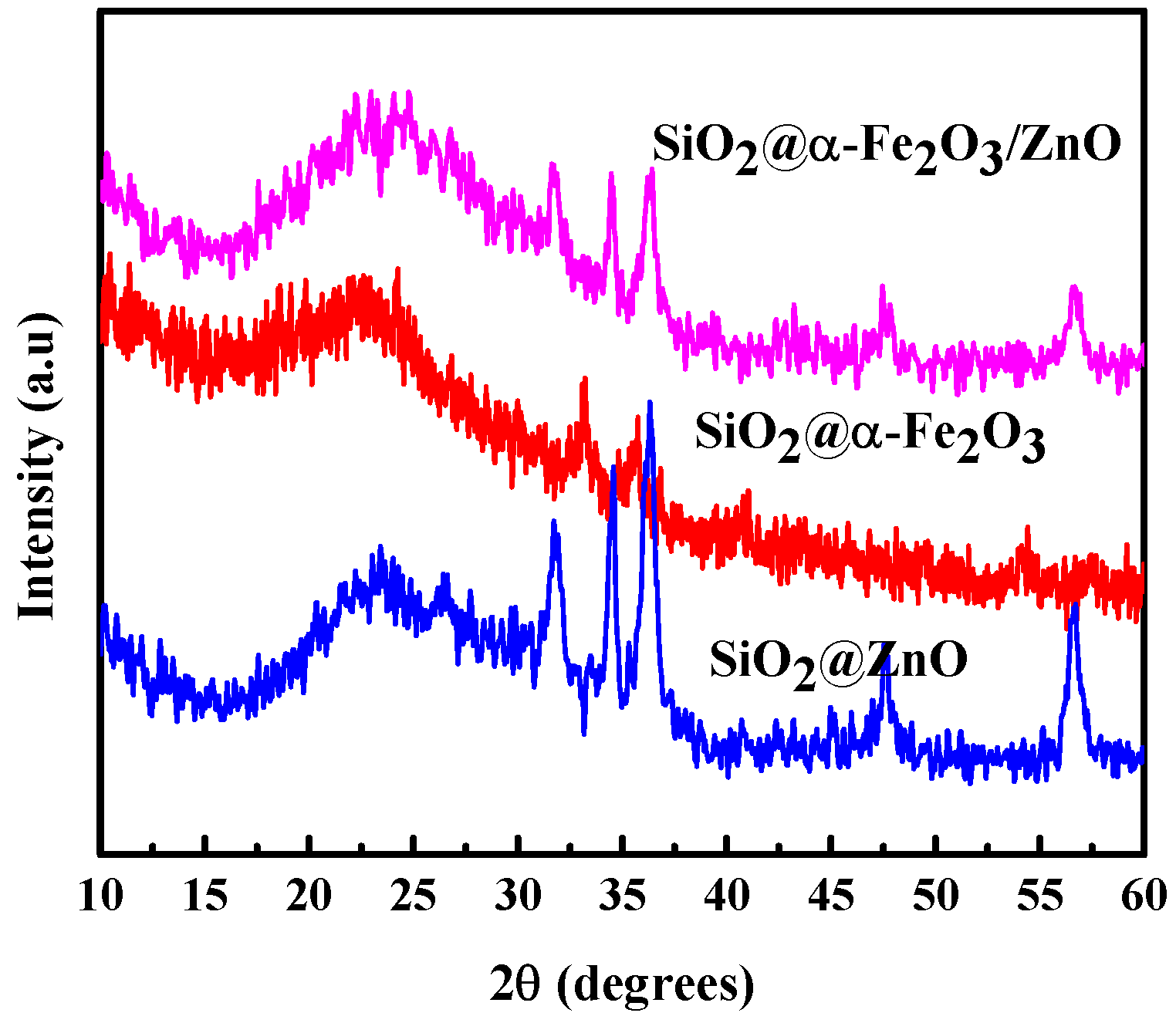
2.1. X-ray Diffraction (XRD)
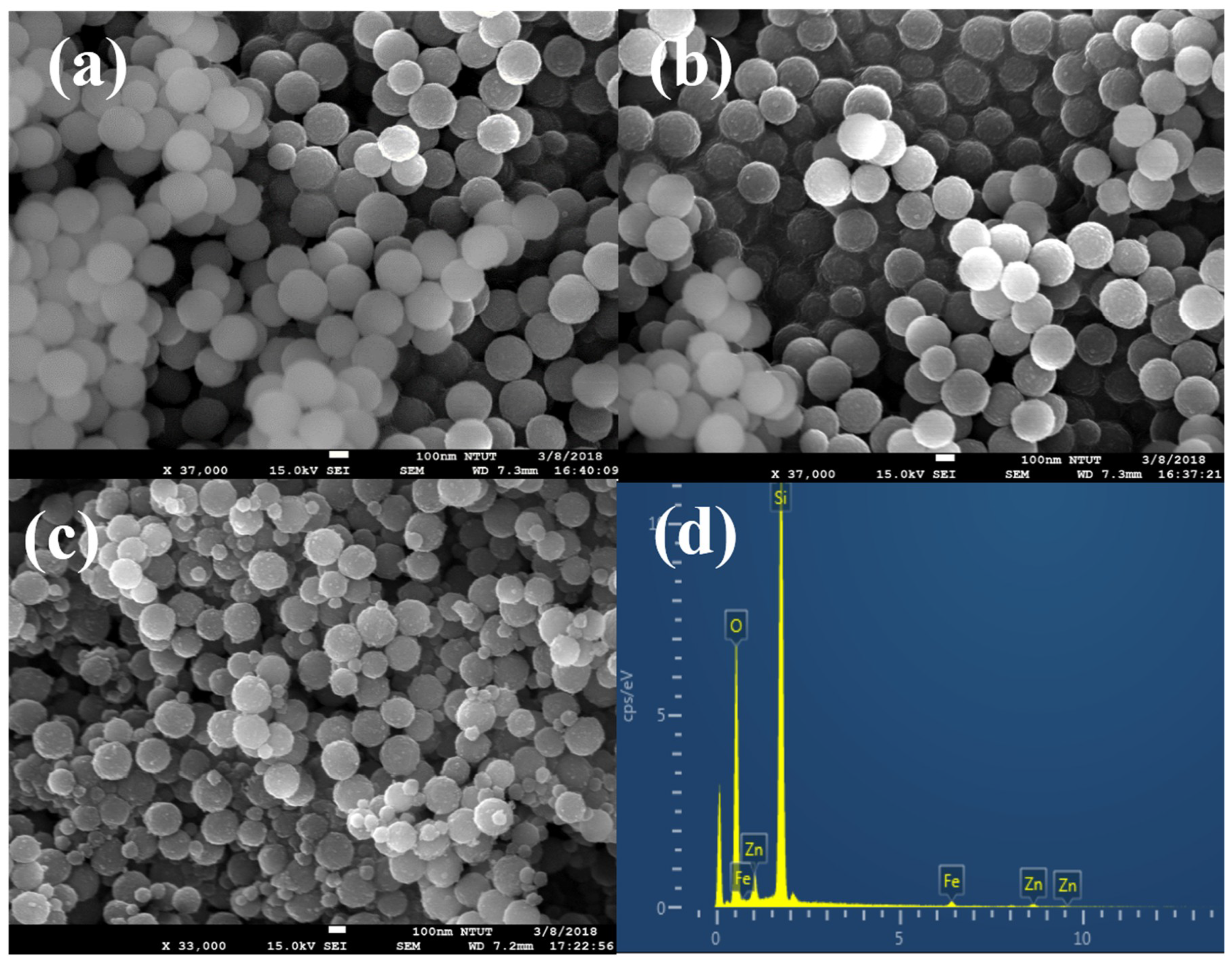
2.2. Structural Properties
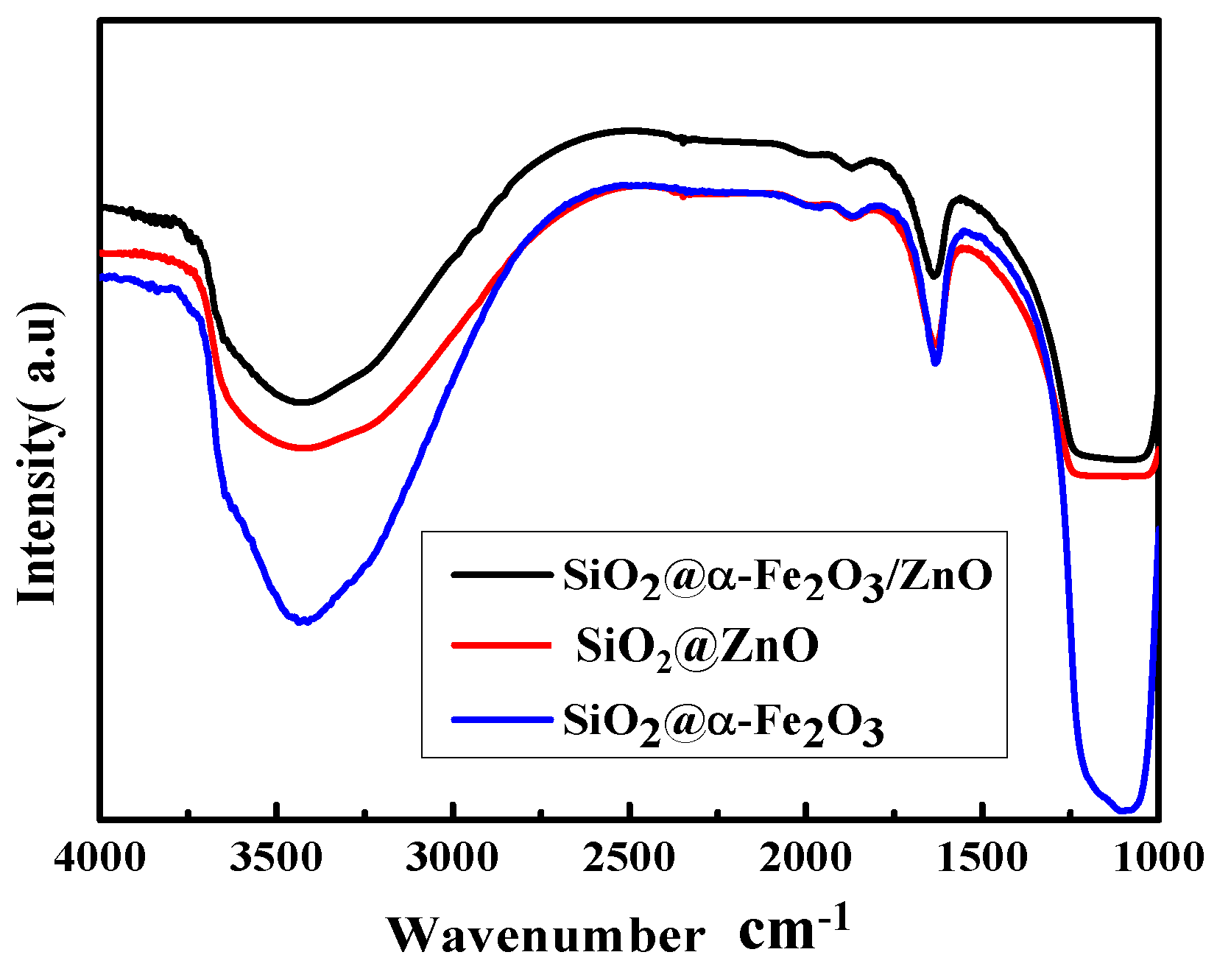
2.3. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
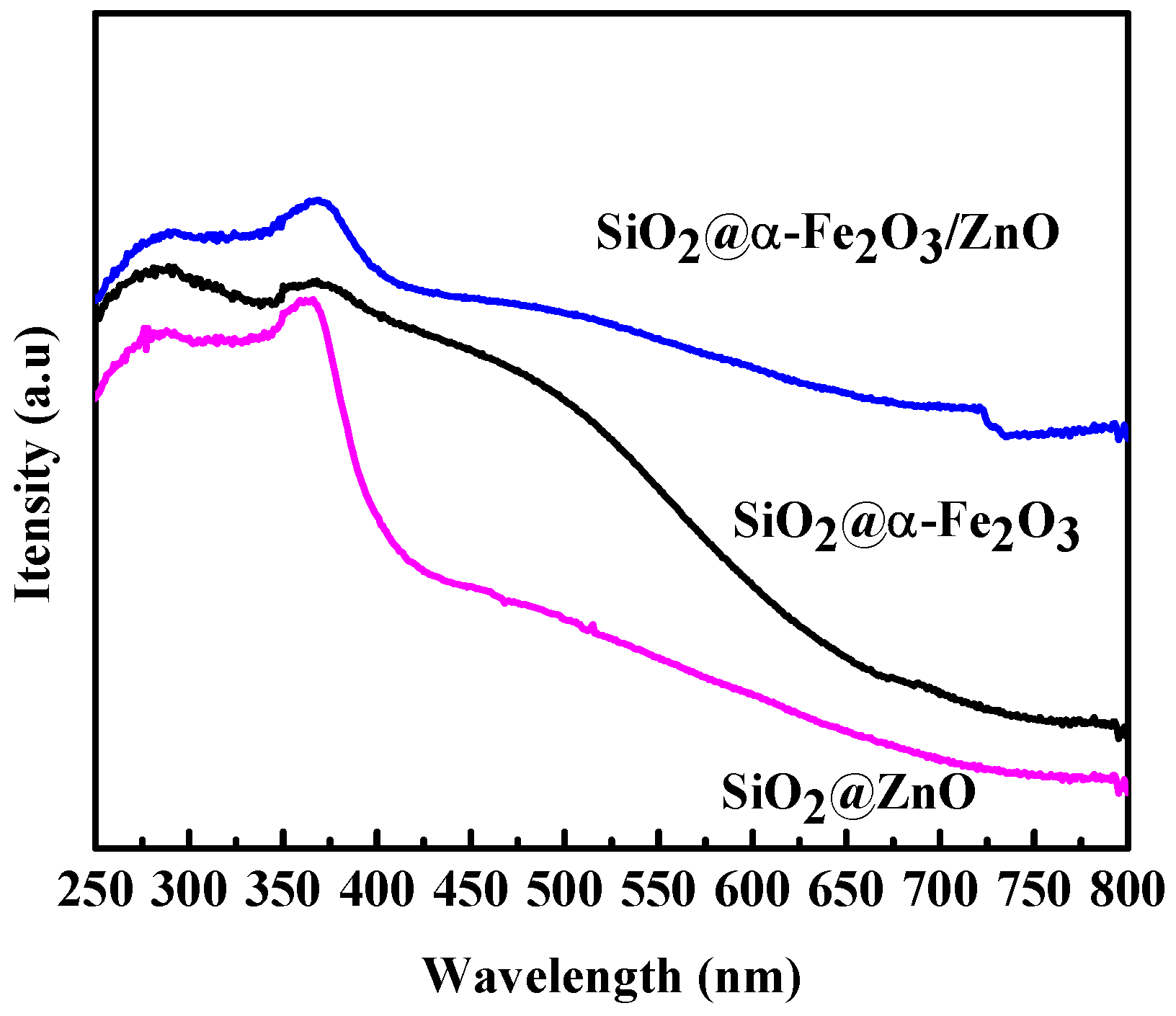
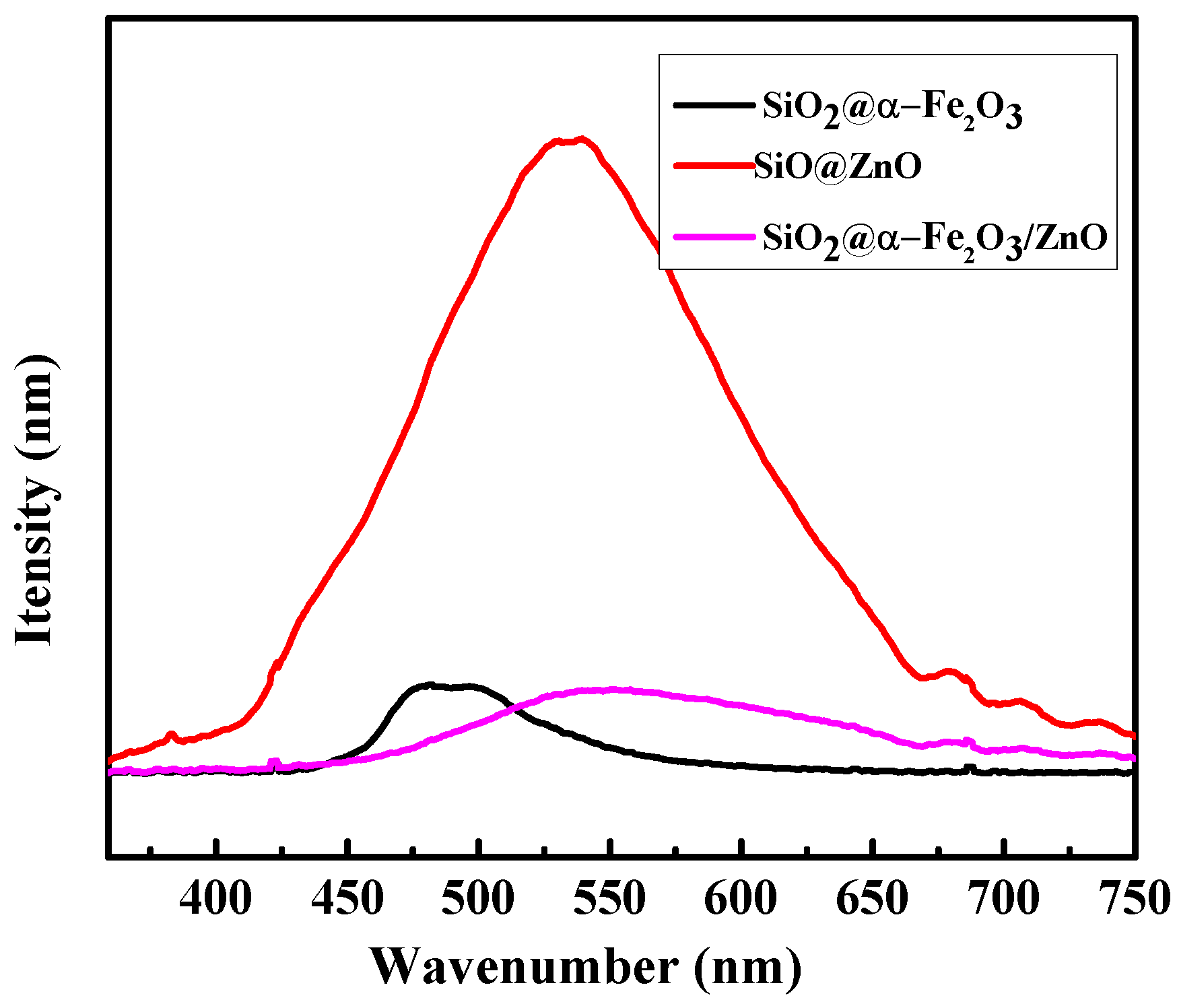
2.4. Optical Measurement
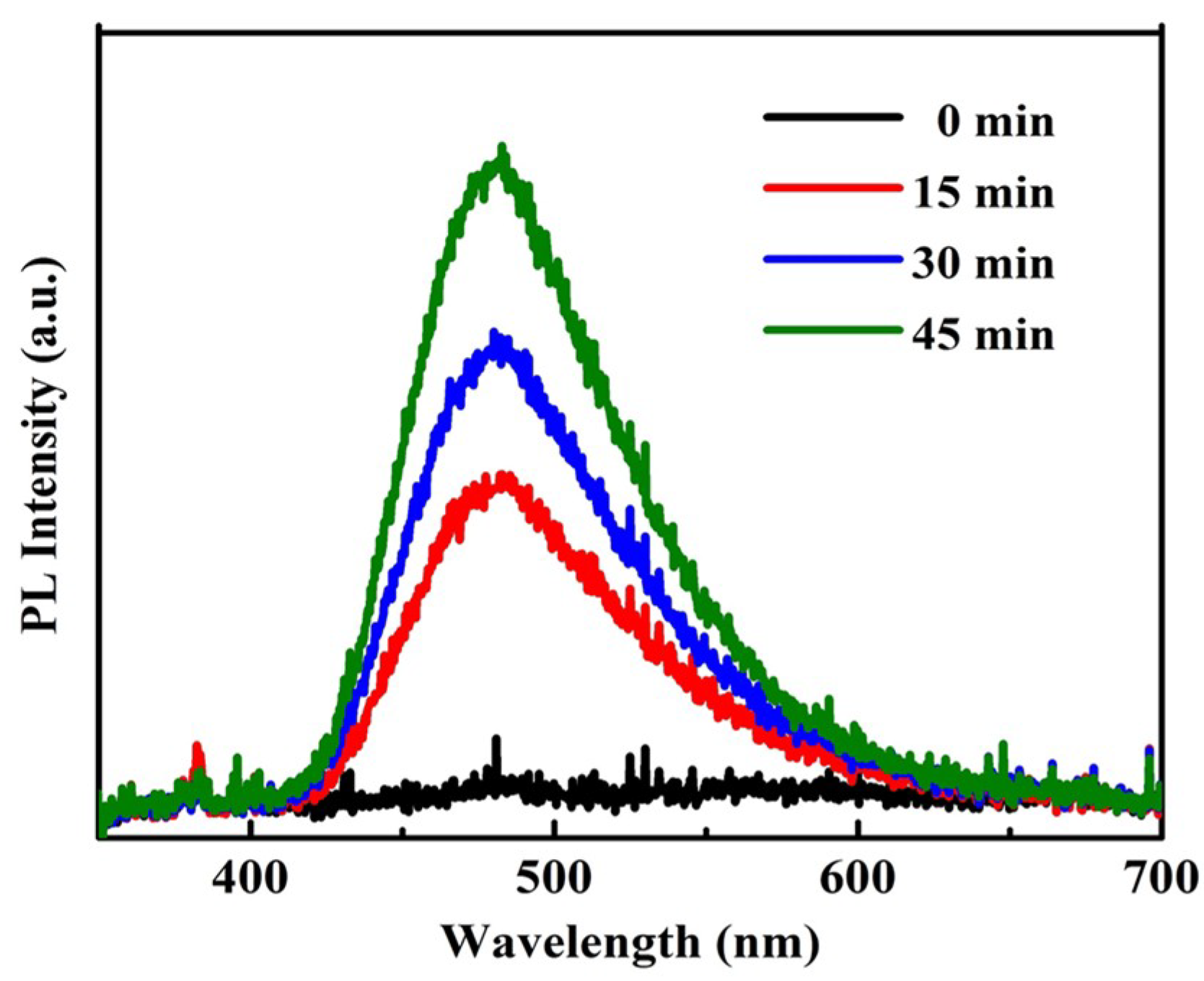
2.5. Photoluminescence (PL) Measurements
2.6. Brunauer-Emmett-Teller (BET)-N2 Adsorption-Desorption Analysis
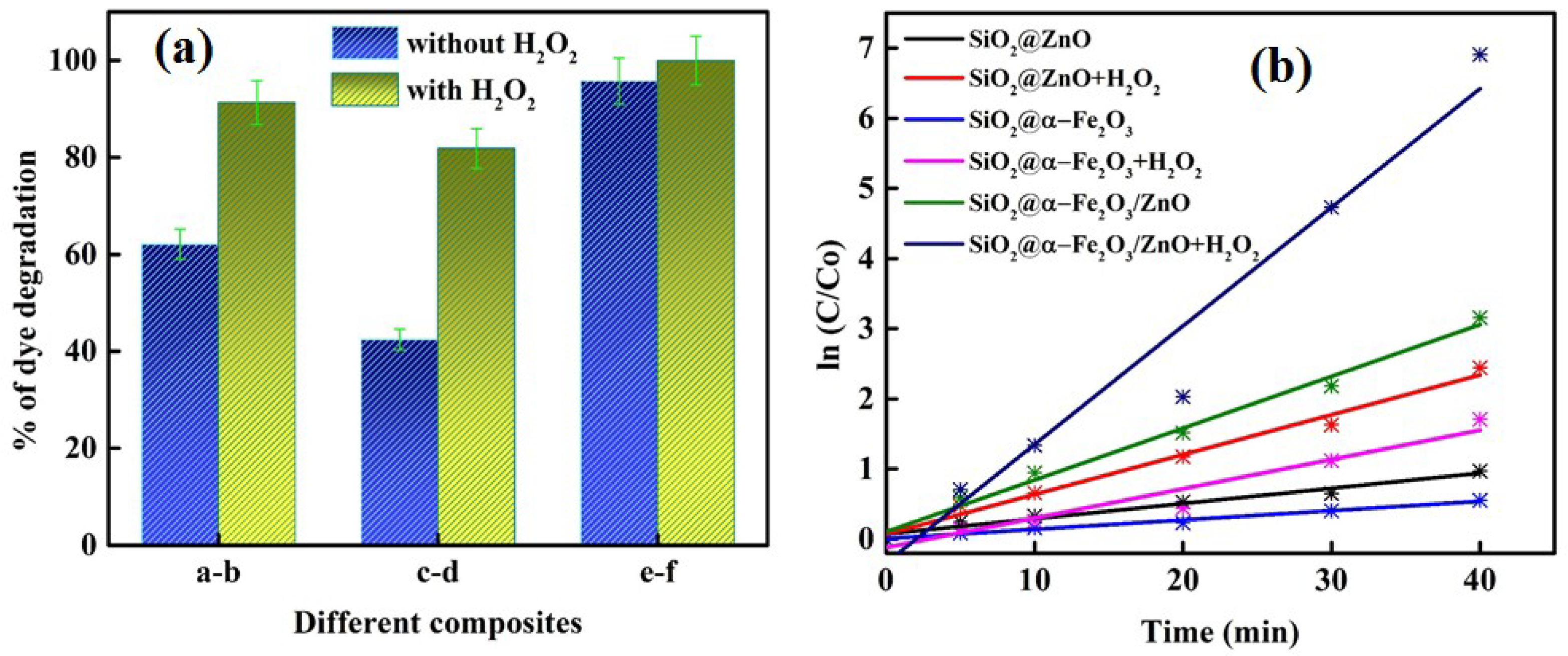
3. Photocatalytic Activity
4. Experimental Procedure
Photocatalytic Reaction
5. Characterization
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crespo, C.T. CunbO3 as a solar energy converter to fuel and electricity. Sol. Energy Mater. Sol. Cells 2018, 179, 305–311. [Google Scholar] [CrossRef]
- Qing, W.; Chen, K.; Wang, Y.; Liu, X.; Lu, M. Green synthesis of silver nanoparticles by waste tea extract and degradation of organic dye in the absence and presence of H2O2. Appl. Surf. Sci. 2017, 423, 1019–1024. [Google Scholar] [CrossRef]
- Xie, J.; Zhou, Z.; Lian, Y.; Hao, Y.; Li, P.; Wei, Y. Synthesis of α-Fe2O3/ZnO composites for photocatalytic degradation of pentachlorophenol under UV–vis light irradiation. Ceram. Int. 2015, 41, 2622–2625. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, J.; Liu, C.; Huo, P.; Wang, H. Construction of 3D porous g-C3N4/AgBr/rGO composite for excellent visible light photocatalytic activity. Appl. Surf. Sci. 2018, 458, 586–596. [Google Scholar] [CrossRef]
- Zhou, K.; Hu, X.Y.; Chen, B.Y.; Hsueh, C.C.; Zhang, Q.; Wang, J.; Lin, Y.J.; Chang, C.T. Synthesized TiO2/ZSM-5 composites used for the photocatalytic degradation of azo dye: Intermediates, reaction pathway, mechanism and bio-toxicity. Appl. Surf. Sci. 2016, 383, 300–309. [Google Scholar] [CrossRef]
- Mahadik, M.A.; An, G.W.; David, S.; Choi, S.H.; Cho, M.; Jang, J.S. Fabrication of A/R-TiO2 composite for enhanced photoelectrochemical performance: Solar hydrogen generation and dye degradation. Appl. Surf. Sci. 2017, 426, 833–843. [Google Scholar] [CrossRef]
- Wachs, I.E.; Routray, K. Catalysis science of bulk mixed oxides. ACS Catal. 2012, 2, 1235–1246. [Google Scholar] [CrossRef]
- Geng, Z.G.; Kong, X.D.; Chen, W.W.; Su, H.Y.; Liu, Y.; Cai, F.; Wang, G.X.; Zeng, J. Oxygen Vacancies in ZnO Nanosheets Enhance CO2 Electrochemical Reduction to CO. Angew. Chem. Int. Ed. 2018, 57, 6054–6059. [Google Scholar] [CrossRef] [PubMed]
- Boudjemaa, A.; Trari, M. Photo-catalytic hydrogen production over Fe2O3 based catalysts. Int. J. Hydrogen Energy 2010, 35, 7684–7689. [Google Scholar] [CrossRef]
- Uma, K.; Chen, S.W.; Arjun, N.; Pan, G.T.; Yang, T.C.K. The production of an efficient visible light photocatalyst for CO oxidation through the surface plasmonic effect of Ag nanoparticles on SiO2@α-Fe2O3 nanocomposites. RSC Adv. 2018, 8, 12547–12555. [Google Scholar] [CrossRef]
- Lv, R.; Wang, T.; Su, F.; Zhang, P.; Li, C.; Gong, J. Facile synthesis of zno nanopencil arrays for photoelectrochemical water splitting. Nano Energy 2014, 7, 143–150. [Google Scholar] [CrossRef]
- Tao, Q.; Chen, M.; He, H.; Komarneni, S. Hydrothermal transformation of mixed metal oxides and silicate anions to phyllosilicate under highly alkaline conditions. Appl. Clay Sci. 2018, 156, 224–230. [Google Scholar] [CrossRef]
- Voznyi, A.; Kosyak, V.; Opanasyuk, A.; Tirkusova, N.; Grase, L.; Medvids, A.; Mezinskis, G. Structural and electrical properties of SnS2 thin films. Mater. Chem. Phys. 2016, 173, 52–61. [Google Scholar] [CrossRef]
- Yan, K.L.; Shang, X.; Li, Z.; Dong, B.; Li, X.; Gao, W.K.; Chi, J.Q.; Chai, Y.M.; Liu, C.G. Ternary mixed metal Fe-doped NiCo2O4 nanowires as efficient electrocatalysts for oxygen evolution reaction. Appl. Surf. Sci. 2017, 416, 371–378. [Google Scholar] [CrossRef]
- Al-Owais, A.A. Synthesis and magnetic properties of hexagonally packed zno nanorods. Arab. J. Chem. 2013, 6, 229–234. [Google Scholar] [CrossRef]
- Millar, A.; Rahman, M.M.; Jiang, Z.T. Review of sol–gel derived mixed metal oxide thin film coatings with the addition of carbon materials for selective surface applications. J. Adv. Phys. 2014, 3, 179–193. [Google Scholar] [CrossRef]
- Perdomo, C.; Pérez, A.; Molina, R.; Moreno, S. Storage capacity and oxygen mobility in mixed oxides from transition metals promoted by cerium. Appl. Surf. Sci. 2016, 383, 42–48. [Google Scholar] [CrossRef]
- Balu, S.; Uma, K.; Pan, G.T.; Yang, T.; Ramaraj, S. Degradation of methylene blue dye in the presence of visible light using SiO2@α-Fe2O3 nanocomposites deposited on SnS2 flowers. Materials 2018, 11, 1030. [Google Scholar] [CrossRef] [PubMed]
- Bindu, P.; Thomas, S. Estimation of lattice strain in ZnO nanoparticles: X-ray peak profile analysis. J. Theor. Appl. Phys. 2014, 8, 123–134. [Google Scholar] [CrossRef]
- Uma, K.; Arjun, N.; Pan, G.T.; Yang, T.C.K. The photodeposition of surface plasmon Ag metal on SiO2@α-Fe2O3 nanocomposites sphere for enhancement of the photo-fenton behavior. Appl. Surf. Sci. 2017, 425, 377–383. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Liu, R.; Zhao, X.; Qu, J. The mechanism of antimony(III) removal and its reactions on the surfaces of Fe–Mn binary oxide. J. Colloid Interface Sci. 2011, 363, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Segets, D.; Gradl, J.; Taylor, R.K.; Vassilev, V.; Peukert, W. Analysis of optical absorbance spectra for the determination of ZnO nanoparticle size distribution, solubility, and surface energy. ACS Nano 2009, 3, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhou, Y.; Liu, Y.; Zhang, G.; Zhang, X. Photoluminescence spectra of nano-structured ZnO thin films. J. Lumin. 2006, 119–120, 233–236. [Google Scholar] [CrossRef]
- Quintana, M.; Edvinsson, T.; Hagfeldt, A.; Boschloo, G. Comparison of dye-sensitized ZnO and TiO2 solar cells: Studies of charge transport and carrier lifetime. J. Phys. Chem. C 2007, 111, 1035–1041. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, L.; Li, M.; Hao, Y.; Lian, Y.; Li, Z.; Wei, Y. A-Fe2O3 modified ZnO flower-like microstructures with enhanced photocatalytic performance for pentachlorophenol degradation. Ceram. Int. 2015, 41, 9420–9425. [Google Scholar] [CrossRef]
- Saravanan, R.; Shankar, H.; Prakash, T.; Narayanan, V.; Stephen, A. ZnO/CdO composite nanorods for photocatalytic degradation of methylene blue under visible light. Mater. Chem. Phys. 2011, 125, 277–280. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, H.; Lv, Z.; Zhan, S.; Yang, J.; Peng, X.; Ren, Y.; Wu, X. Simulated-sunlight-activated photocatalysis of methylene blue using cerium-doped SiO2/TiO2 nanostructured fibers. J. Environ. Sci. 2012, 24, 1867–1875. [Google Scholar] [CrossRef]
- Senapati, K.K.; Borgohain, C.; Sarma, K.C.; Phukan, P. Photocatalytic degradation of methylene blue in water using CoFe2O4–Cr2O3–SiO2 fluorescent magnetic nanocomposite. J. Mol. Catal. A Chem. 2011, 346, 111–116. [Google Scholar] [CrossRef]
- Jang, Y.J.; Simer, C.; Ohm, T. Comparison of zinc oxide nanoparticles and its nano-crystalline particles on the photocatalytic degradation of methylene blue. Mater. Res. Bull. 2006, 41, 67–77. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, H.; Tong, Z.; Song, Z.; Chen, N. A facile synthesis of Fe3O4@SiO2@ZnO with superior photocatalytic performance of 4-nitrophenol. J. Environ. Chem. Eng. 2017, 5, 2207–2213. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, S.; Xiao, X.; Zhou, J.; Ren, F.; Sun, L.; Jiang, C. Controllable synthesis, magnetic properties, and enhanced photocatalytic activity of spindlelike mesoporous α-Fe2O3/ZnO core–shell heterostructures. ACS Appl. Mater. Interfaces 2012, 4, 3602–3609. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.V.; Huang, N.; Yusoff, N.; Lim, H. High performance magnetically separable graphene/zinc oxide nanocomposite. Mater. Lett. 2013, 93, 411–414. [Google Scholar] [CrossRef]
- Muthukrishnaraj, A.; Vadivel, S.; Kamalakannan, V.; Balasubramanian, N. α-Fe2O3/reduced graphene oxide nanorod as efficient photocatalyst for methylene blue degradation. Mater. Res. Innov. 2015, 19, 258–264. [Google Scholar] [CrossRef]
- Yan, Y.; Guan, H.; Liu, S.; Jiang, R. Ag3PO4/Fe2O3 composite photocatalysts with an n–n heterojunction semiconductor structure under visible-light irradiation. Ceram. Int. 2014, 40, 9095–9100. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, L.; Hu, Y.; Guo, C.; Zhang, F.; Lou, X.W.D. A magnetically separable photocatalyst based on nest-like γ-Fe2O3/ZnO double-shelled hollow structures with enhanced photocatalytic activity. Nanoscale 2012, 4, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.; Mkhalid, I.; Baeissa, E.; Al-Rayyani, M. Photocatalytic degradation of methylene blue by Fe/ZnO/SiO2 nanoparticles under visiblelight. J. Nanotechnol. 2012, 2012. [Google Scholar] [CrossRef]

| Samples | BET Surface Area | Pore Volume |
|---|---|---|
| SiO2 | 8 m2/g | 0.032 cm2/g |
| SiO2@α-Fe2O3 | 21 m2/g | 0.041 cm2/g |
| SiO2@ZnO | 33 m2/g | 0.045 cm2/g |
| SiO2@α-Fe2O3/ZnO | 41 m2/g | 0.051 cm2/g |
| Samples | r0 | k′ (min−1) | R2 | T (min) | % Deg |
|---|---|---|---|---|---|
| SiO2@ZnO | 0.0067 | 0.0216 | 0.9843 | 40 | 62 |
| SiO2@ZnO + H2O2 | 0.0177 | 0.0567 | 0.9932 | 40 | 91 |
| SiO2@α-Fe2O3 | 0.0041 | 0.0132 | 0.9939 | 40 | 42 |
| SiO2@α-Fe2O3 + H2O2 | 0.0130 | 0.0418 | 0.9727 | 40 | 82 |
| SiO2@α-Fe2O3/ZnO | 0.0230 | 0.0738 | 0.9948 | 40 | 96 |
| SiO2@α-Fe2O3/ZnO + H2O2 | 0.0527 | 0.1691 | 0.9797 | 25 | 100 |
| Catalyst | Dye | Catalyst Loading | Time (min) | Reference |
|---|---|---|---|---|
| ZnO/CdO | MB | *R.a.—500 mL | 360 | [26] |
| Ce-doped SiO2/TiO2 | MB | 100 mg—1000 mL | 120 | [27] |
| CoFe2O4–Cr2O3–SiO2 | MB | 20 mg—50 mL | 120 | [28] |
| ZnO | MB | 1500 mg—650 mL | 120 | [29] |
| Fe3O4@SiO2@ZnO | 4NP | 400 mg—30 mL | 120 | [30] |
| α-Fe2O3/ZnO | RhB | 20 mg—40 mL | 105 | [31] |
| G-Fe2O3/ZnO | MB | 10 mg—50 mL | 90 | [32] |
| (RGO)/α-Fe2O3 | MB | 100 mg—100 mL | 90 | [33] |
| Ag3PO4/Fe2O3 | MO | 200 mg—25 mL | 60 | [34] |
| γ-Fe2O3/ZnO | MB | 50 mg—100 mL | 50 | [35] |
| Fe/ZnO/SiO2 | MB | 75 mg—300 mL | 30 | [36] |
| SiO2@α-Fe2O3/ZnO | MB | 50 mg—100 mL | 40 | This work |
| SiO2@α-Fe2O3/ZnO+H2O2 | MB | 50 mg—100 mL | 25 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uma, K.; Balu, S.; Pan, G.-T.; Yang, T.C.-K. Assembly of ZnO Nanoparticles on SiO2@α-Fe2O3 Nanocomposites for an Efficient Photo-Fenton Reaction. Inorganics 2018, 6, 90. https://doi.org/10.3390/inorganics6030090
Uma K, Balu S, Pan G-T, Yang TC-K. Assembly of ZnO Nanoparticles on SiO2@α-Fe2O3 Nanocomposites for an Efficient Photo-Fenton Reaction. Inorganics. 2018; 6(3):90. https://doi.org/10.3390/inorganics6030090
Chicago/Turabian StyleUma, Kasimayan, Sridharan Balu, Guan-Ting Pan, and Thomas C.-K. Yang. 2018. "Assembly of ZnO Nanoparticles on SiO2@α-Fe2O3 Nanocomposites for an Efficient Photo-Fenton Reaction" Inorganics 6, no. 3: 90. https://doi.org/10.3390/inorganics6030090
APA StyleUma, K., Balu, S., Pan, G.-T., & Yang, T. C.-K. (2018). Assembly of ZnO Nanoparticles on SiO2@α-Fe2O3 Nanocomposites for an Efficient Photo-Fenton Reaction. Inorganics, 6(3), 90. https://doi.org/10.3390/inorganics6030090








