A Missing Member of the Anderson–Evans Family: Synthesis and Characterization of the Trimethylolmethane-Capped {MnMo6O24} Cluster
Abstract
1. Introduction
2. Results
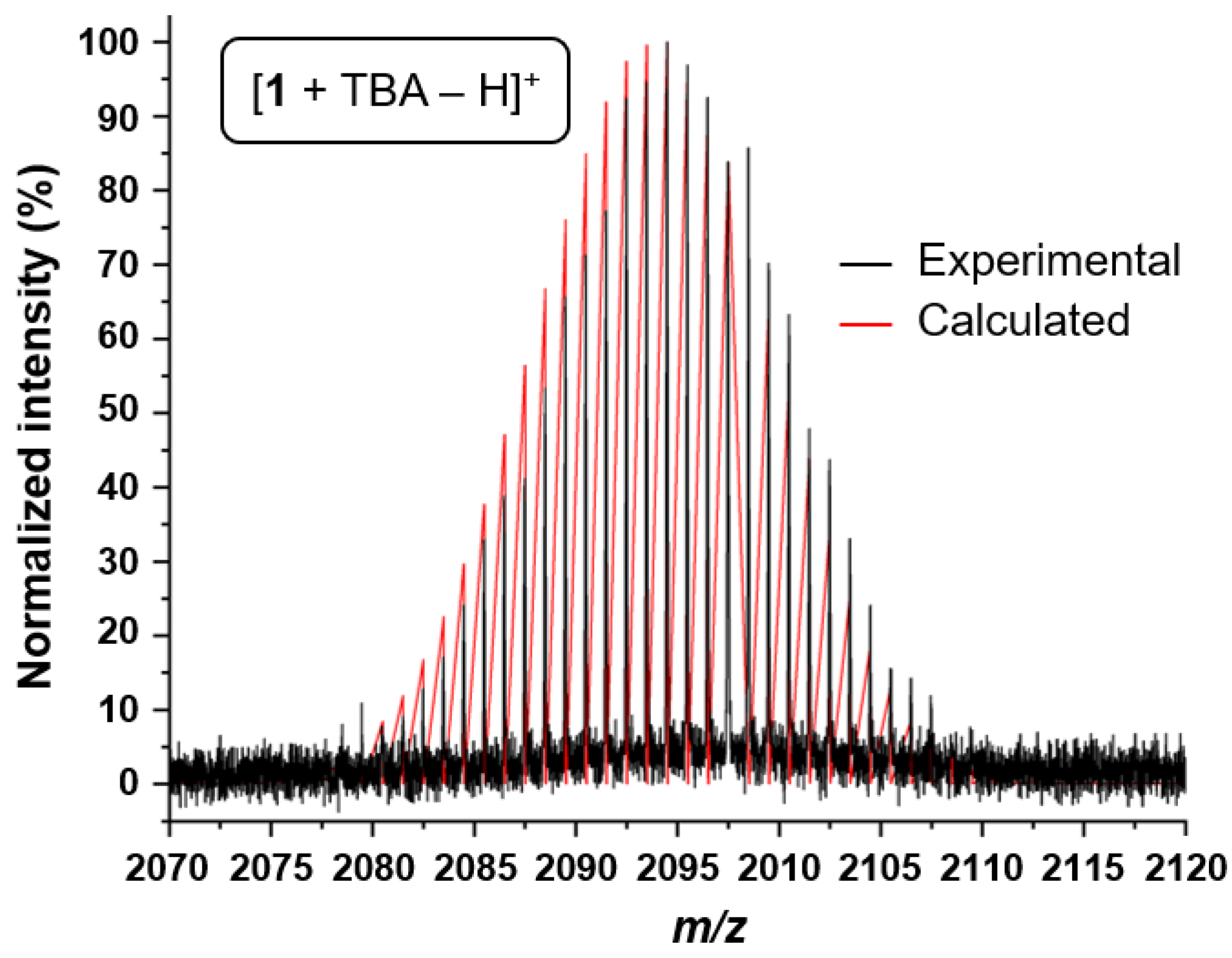
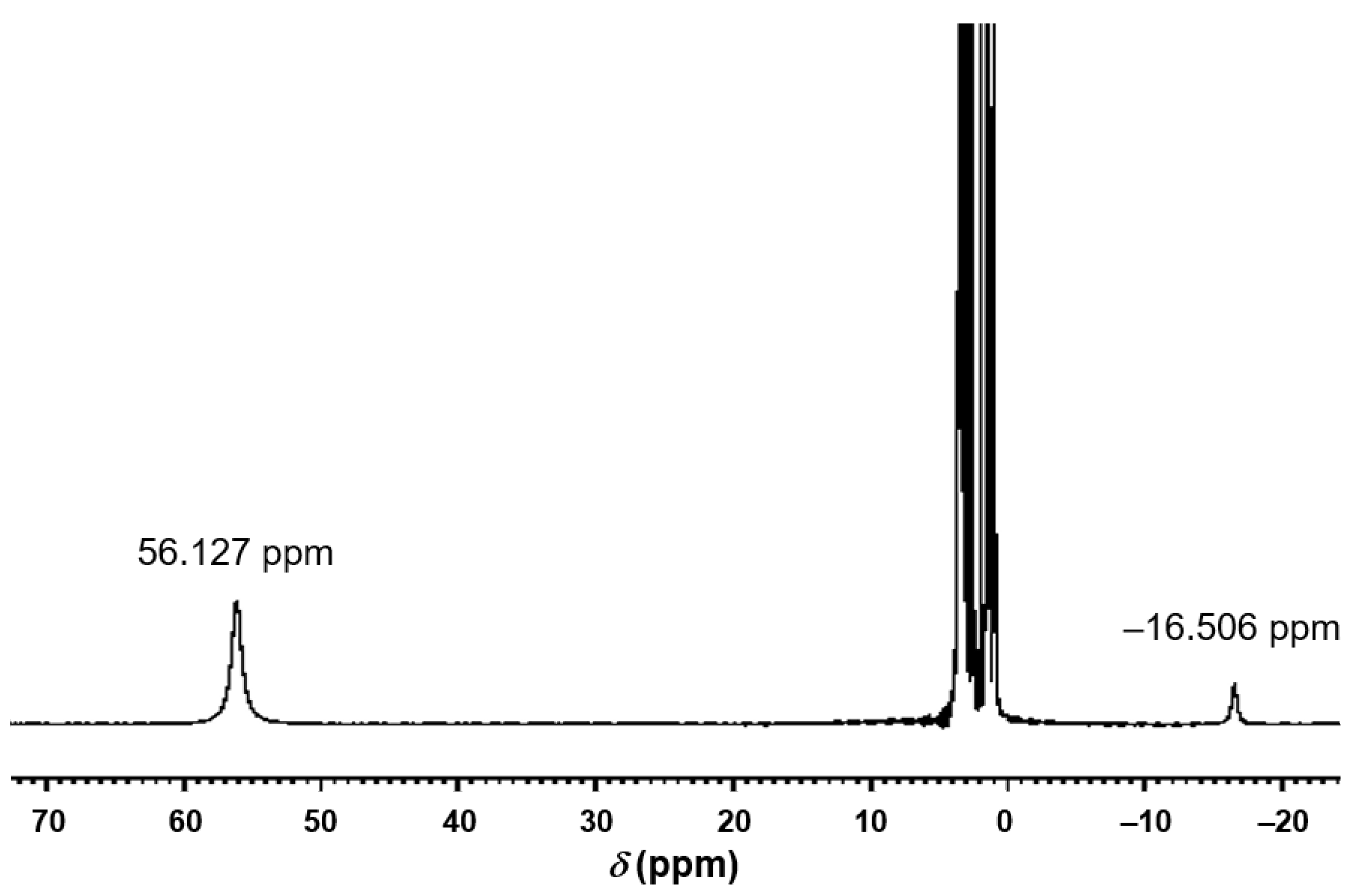
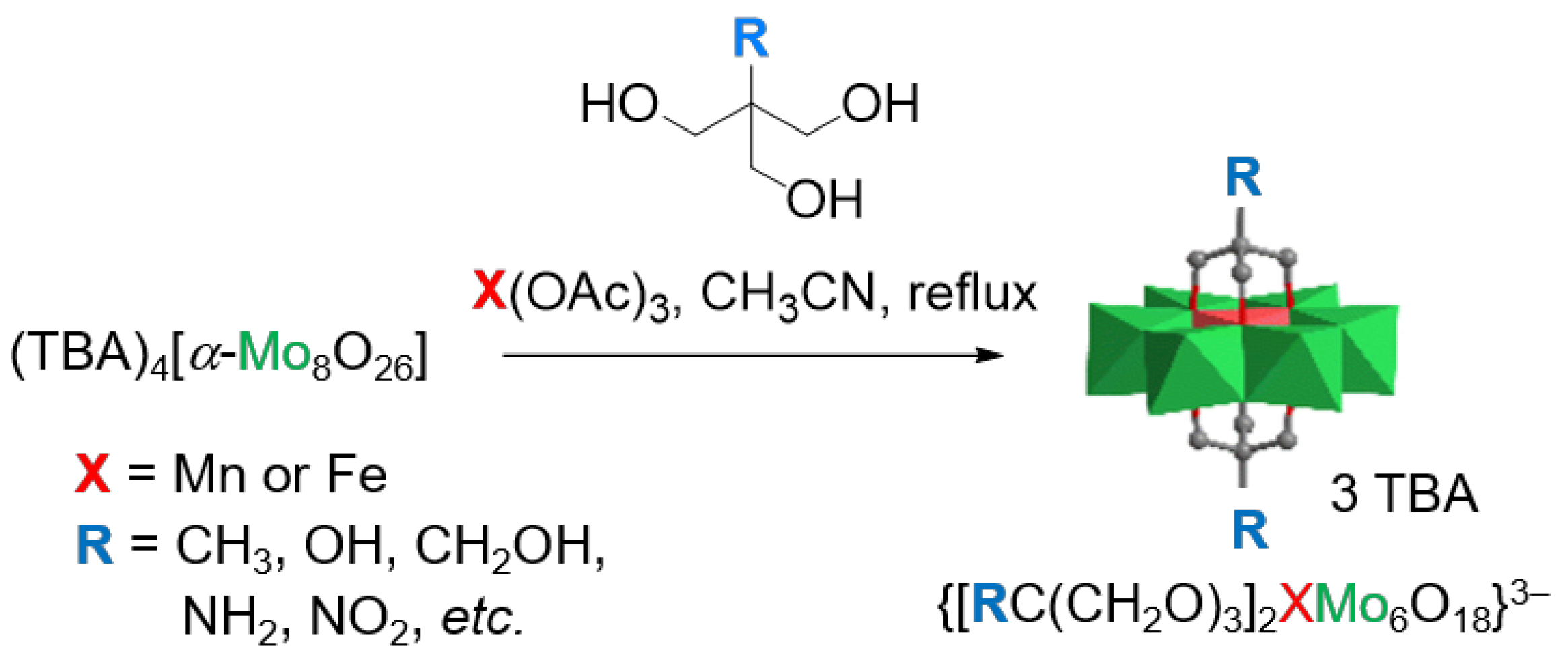
2.1. Synthesis and Characterization of 1
2.2. X-Ray Diffraction Analysis of 1
2.3. Computational Studies of 1
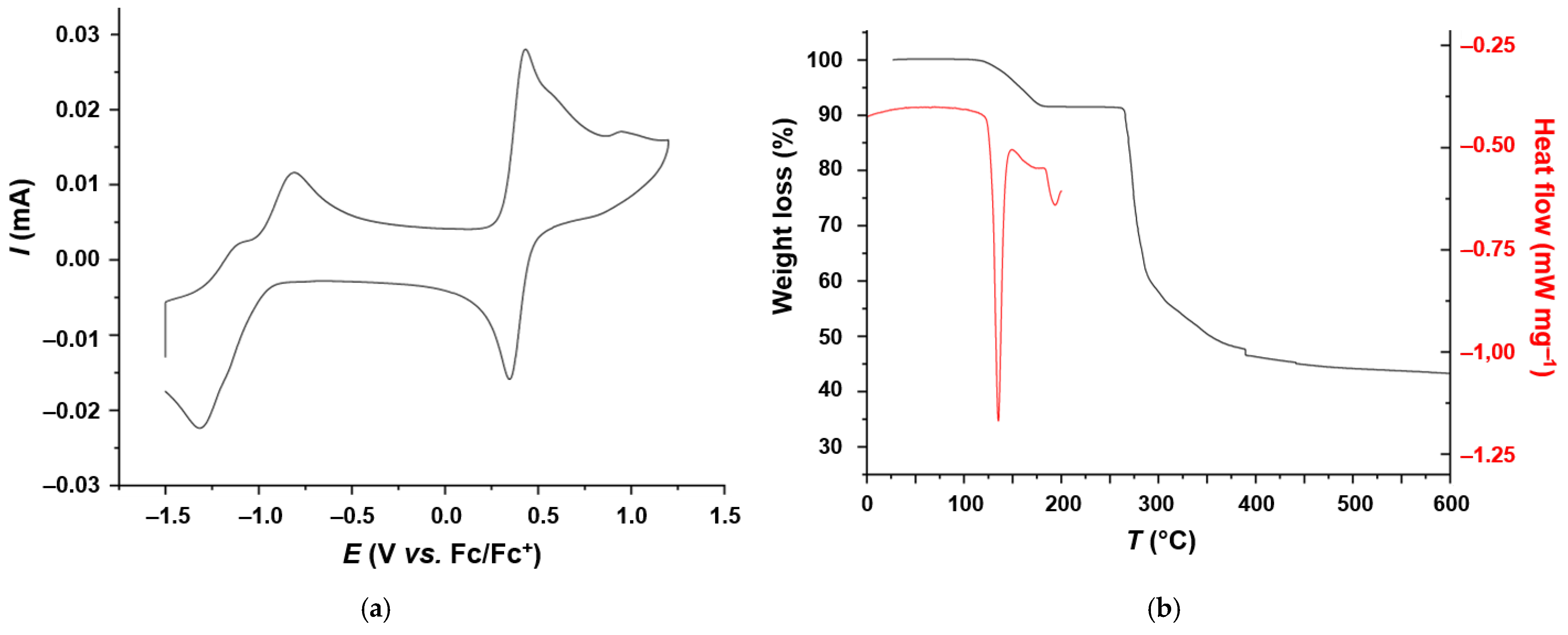
2.4. Electrochemical and Thermal Properties of 1
3. Discussion and Conclusions
4. Materials and Methods
4.1. General
4.2. Synthesis of 1
4.3. X-Ray Diffraction Analysis of 1
4.4. Computational Studies
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keggin, J.F. Structure of the molecule of 12-phosphotungstic acid. Nature 1933, 131, 908–909. [Google Scholar] [CrossRef]
- Dolbecq, A.; Dumas, E.; Mayer, C.R.; Mialane, P. Hybrid organic–inorganic oolyoxometalate compounds: From structural diversity to applications. Chem. Rev. 2010, 110, 6009–6048. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, B.; Yang, H.; Wan, Y.; Fang, H.; Bao, W.; Wang, W.; Wang, N.; Lu, Y. A review on polyoxometalates-based materials in addressing challenges faced by electrochemical energy storage systems. Chem. Eng. J. 2024, 483, 149143. [Google Scholar] [CrossRef]
- Woźniak Budych, M.J.; Staszak, K.; Bajek, A.; Pniewski, F.; Jastrząb, R.; Staszak, M.; Tylkowski, B.; Wieszczycka, K. The future of polyoxymetalates for biological and chemical apllications. Coord. Chem. Rev. 2023, 493, 215306. [Google Scholar] [CrossRef]
- Anyushin, A.V.; Kondinski, A.; Parac-Vogt, T.N. Hybrid polyoxometalates as post-functionalization platforms: From fundamentals to emerging applications. Chem. Soc. Rev. 2020, 49, 382–432. [Google Scholar] [CrossRef]
- Lu, M.; Wei, Y.; Xu, B.; Cheung, C.F.-C.; Peng, Z.; Powell, D.R. Hybrid molecular dumbbells: Bridging polyoxometalate clusters with an organic π-conjugated rod. Angew. Chem. Int. Ed. 2002, 41, 1566–1568. [Google Scholar] [CrossRef]
- Wang, L.-S.; Lu, Y.; Máille, G.M.Ó.; Anthony, S.P.; Nolan, D.; Draper, S.M. Sonogashira cross-coupling as a route to tunable hybrid organic–inorganic rods with a polyoxometalate backbone. Inorg. Chem. 2016, 55, 9497–9500. [Google Scholar] [CrossRef]
- Zhu, L.; Zhu, Y.; Meng, X.; Hao, J.; Li, Q.; Wei, Y.; Lin, Y. DCC-Assisted esterification of a polyoxometalate-functionalized phenol with carboxylic acids. Chem. Eur. J. 2008, 14, 10923–10927. [Google Scholar] [CrossRef]
- Bareyt, S.; Piligkos, S.; Hasenknopf, B.; Gouzerh, P.; Lacôte, E.; Thorimbert, S.; Malacria, M. Highly efficient peptide bond formation to functionalized Wells-Dawson-type polyoxotungstates. Angew. Chem. Int. Ed. 2003, 42, 3404–3406. [Google Scholar] [CrossRef]
- Marcoux, P.R.; Hasenknopf, B.; Vaissermann, J.; Gouzerh, P. Developing remote metal binding sites in heteropolymolybdates. Eur. J. Inorg. Chem. 2003, 2003, 2406–2412. [Google Scholar] [CrossRef]
- Schönweiz, S.; Rommel, S.A.; Kübel, J.; Micheel, M.; Dietzek, B.; Rau, S.; Streb, C. Covalent photosensitizer–polyoxometalate-catalyst dyads for visible-light-driven hydrogen evolution. Chem. Eur. J. 2016, 22, 12002–12005. [Google Scholar] [CrossRef] [PubMed]
- Cetindere, S.; Clausing, S.T.; Anjass, M.; Luo, Y.; Kupfer, S.; Dietzek, B.; Streb, C. Covalent linkage of BODIPY-photosensitizers to Anderson-type polyoxometalates using CLICK chemistry. Chem. Eur. J. 2021, 27, 17181–17187. [Google Scholar] [CrossRef] [PubMed]
- Santoni, M.-P.; Pal, A.K.; Hanan, G.S.; Proust, A.; Hasenknopf, B. Discrete covalent organic–inorganic hybrids: Terpyridine functionalized polyoxometalates obtained by a modular strategy and their metal complexation. Inorg. Chem. 2011, 50, 6737–6745. [Google Scholar] [CrossRef]
- Winter, A.; Endres, P.; Schröter, E.; Jäger, M.; Görls, H.; Neumann, C.; Turchanin, A.; Schubert, U.S. Towards covalent photosensitizer-polyoxometalate dyads-bipyridyl-functionalized polyoxometalates and their transition metal complexes. Molecules 2019, 24, 4446. [Google Scholar] [CrossRef]
- Qin, Z.; Li, Q.; Huang, Y.; Zhang, J.; Li, G.; Wei, Y. Recent advances in controllable alkoxylation chemistry of Anderson-type polyoxometalates from synthetic strategies perspective. Chin. Sci. Bull. 2018, 63, 3263. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Li, G.; Wei, Y. Recent advances in alkoxylation chemistry of polyoxometalates: From synthetic strategies, structural overviews to functional applications. Coord. Chem. Rev. 2019, 378, 395–414. [Google Scholar] [CrossRef]
- Blazevic, A.; Rompel, A. The Anderson–Evans polyoxometalate: From inorganic building blocks via hybrid organic–inorganic structures to tomorrows “Bio-POM”. Coord. Chem. Rev. 2016, 307, 42–64. [Google Scholar] [CrossRef]
- Wu, P.; Wang, Y.; Huang, B.; Xiao, Z. Anderson-type polyoxometalates: From structures to functions. Nanoscale 2021, 13, 7119–7133. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, H.; Li, B.; Hou, G.; Yin, Z.; Wu, L.; Yam, V.W.W. Smart self-assemblies based on a surfactant-encapsulated photoresponsive polyoxometalate complex. Angew. Chem. Int. Ed. 2010, 49, 9233–9236. [Google Scholar] [CrossRef]
- Yan, Y.; Li, B.; He, Q.; He, Z.; Ai, H.; Wang, H.; Yin, Z.; Wu, L. Synthesis and redox-responsive self-assembly of ferrocene grafted Anderson-type polyoxometalate hybrid complexes. Soft Matter 2012, 8, 1593–1600. [Google Scholar] [CrossRef]
- Li, X.-X.; Wang, Y.-X.; Wang, R.-H.; Cui, C.-Y.; Tian, C.-B.; Yang, G.-Y. Designed assembly of heterometallic cluster organic frameworks based on Anderson-type polyoxometalate clusters. Angew. Chem. Int. Ed. 2016, 55, 6462–6466. [Google Scholar] [CrossRef]
- Hagen, T. Formation of Enantiomer-Uniform Tris(hydroxymethyl)methane Derivatives. Ph.D. Thesis, Dortmund University, Dortmund, Germany, 2000. [Google Scholar]
- Cheng, M.; Xiao, Z.; Yu, L.; Lin, X.; Wang, Y.; Wu, P. Direct syntheses of nanocages and frameworks based on Anderson-type polyoxometalates via one-pot reactions. Inorg. Chem. 2019, 58, 11988–11992. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Lu, S.; Xiang, Y. Polyoxometalate-based electrolyte materials in redox flow batteries: Current trends and emerging opportunities. Mater. Rep. Energy 2022, 2, 100094. [Google Scholar] [CrossRef]
- Sun, P.; Gu, Y.; You, W.; Tan, Z. Studies on an iron heteropolyacid redox flow battery. J. Electrochem. 1997, 3, 71. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, Q.; Feng, Y.; Lv, H. Recent advances in polyoxometalate-based catalysts for light-driven hydrogen evolution. Dalton Trans. 2024, 53, 18083–18088. [Google Scholar] [CrossRef]
- Zeb, Z.; Huang, Y.; Chen, L.; Zhou, W.; Liao, M.; Jiang, Y.; Li, H.; Wang, L.; Wang, L.; Wang, H.; et al. Comprehensive overview of polyoxometalates for electrocatalytic hydrogen evolution reaction. Coord. Chem. Rev. 2023, 482, 215058. [Google Scholar] [CrossRef]
- Streb, C. New trends in polyoxometalate photoredox chemistry: From photosensitisation to water oxidation catalysis. Dalton Trans. 2012, 41, 1651–1659. [Google Scholar] [CrossRef]
- Zhang, M.; Li, H.; Zhang, J.; Lv, H.; Yang, G.-Y. Research advances of light-driven hydrogen evolution using polyoxometalate-based catalysts. Chin. J. Catal. 2021, 42, 855–871. [Google Scholar] [CrossRef]
- Ikegami, S.; Yagasaki, A. Efficient syntheses of [(n-C4H9)4N]4[α-Mo8O26] and [(n-C4H9)4N]2[Mo2O7]. Materials 2009, 2, 869–875. [Google Scholar] [CrossRef]
- Hasenknopf, B.; Delmont, R.; Herson, P.; Gouzerh, P. Anderson-type heteropolymolybdates containing tris(alkoxo) ligands: Synthesis and structural characterization. Eur. J. Inorg. Chem. 2002, 2002, 1081–1087. [Google Scholar] [CrossRef]
- Boulicault, J.E.; Alves, S.; Cole, R.B. Negative ion MALDI mass Spectrometry of polyoxometalates (POMs): Mechanism of singly charged anion formation and chemical properties evaluation. J. Am. Soc. Mass Spectrom. 2016, 27, 1301–1313. [Google Scholar] [CrossRef]
- Gumerova, N.I.; Rompel, A. Polyoxometalates in solution: Speciation under spotlight. Chem. Soc. Rev. 2020, 49, 7568–7601. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-G.; Hutin, M.; Busche, C.; Bell, N.L.; Long, D.-L.; Cronin, L. Elucidating the paramagnetic interactions of an inorganic–organic hybrid radical-functionalized Mn-Anderson cluster. Dalton Trans. 2021, 50, 2350–2353. [Google Scholar] [CrossRef]
- Song, Y.-F.; Long, D.-L.; Cronin, L. Hybrid polyoxometalate clusters with appended aromatic platforms. CrystEngComm 2010, 12, 109–115. [Google Scholar] [CrossRef]
- Tian, A.-x.; Ying, J.; Peng, J.; Sha, J.-q.; Pang, H.-j.; Zhang, P.-p.; Chen, Y.; Zhu, M.; Su, Z.-m. Tuning the dimensionality of the coordination polymer based on polyoxometalate by changing the spacer length of ligands. Cryst. Growth Des. 2008, 8, 3717–3724. [Google Scholar] [CrossRef]
- Kupfer, S. Quantum chemical data: A missing member of the Anderson-Evans family: Synthesis and characterization of the trimethylolmethane-capped {MnMo6O24} cluster. Zenodo 2025. [Google Scholar] [CrossRef]
- Schönweiz, S.; Heiland, M.; Anjass, M.; Jacob, T.; Rau, S.; Streb, C. Experimental and theoretical investigation of the light-driven hydrogen evolution by polyoxometalate–photosensitizer dyads. Chem. Eur. J. 2017, 23, 15370–15376. [Google Scholar] [CrossRef]
- Novotny, J.; Komorovsky, S.; Marek, R. Paramagnetic effects in NMR spectroscopy of transition-metal complexes: Principles and chemical concepts. Acc. Chem. Res. 2024, 57, 1467–1477. [Google Scholar] [CrossRef]
- Ravera, E.; Gigli, L.; Fiorucci, L.; Luchinat, C.; Parigi, G. The evolution of paramagnetic NMR as a tool in structural biology. Phys. Chem. Chem. Phys. 2022, 24, 17397–17416. [Google Scholar] [CrossRef]
- Han, M.; Sun, W.; Hu, W.; Liu, Y.; Chen, J.; Zhang, C.; Li, J. Emerging polyoxometalate clusters-based redox flow batteries: Performance metrics, application prospects, and development strategies. Energy Storage Mater. 2024, 71, 103576. [Google Scholar] [CrossRef]
- NMRium: NMR Spectra Processing for Everybody. Available online: www.nmrium.org (accessed on 15 June 2025).
- Patiny, L.; Musallam, H.; Bolaños, A.; Zasso, M.; Wist, J.; Karayilan, M.; Ziegler, E.; Liermann, J.C.; Schlörer, N.E. NMRium: Teaching nuclear magnetic resonance spectra interpretation in an online platform. Beilstein J. Org. Chem. 2024, 20, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Hooft, R.W.W. COLLECT—Data Collection Software. Nonius B.V.: Delft, The Netherlands, 1998. [Google Scholar]
- Otwinowski, Z.; Minor, W. Processing of X-Ray Diffraction Data Collected in Oscillation Mode. In Methods in Enzymology, Vol. 276, Macromolecular Crystallography (Part A); Carter, C.W., Sweet, R.M., Eds.; Academic Press: London, UK, 1997; pp. 307–326. [Google Scholar]
- Sheldrick, G.M. SADABS—Version 2.10. University of Göttingen: Göttingen, Germany, 2002. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spek, A. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Cryst. Sect. C 2015, 71, 9–18. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 (rev. C.02); Gaussian: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. Part III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Mennucci, B.; Cappelli, C.; Guido, C.A.; Cammi, R.; Tomasi, J. Structures and Properties of Electronically Excited Chromophores in Solution from the Polarizable Continuum Model Coupled to the Time-Dependent Density Functional Theory. J. Phys. Chem. A 2009, 113, 3009–3020. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]


| δH (ppm) | T1 (ms) 1 | δC (ppm) | |
|---|---|---|---|
| TBA cation | 3.16, 1.57, 1.31, 0.94 | n/a | 58.0, 23.6, 19.8, 14.2 |
| HC[CH2 O−]3 | −56.13 | 0.99 | n/a 2 |
| HC[CH2 O−]3 | −16.51 | 2.01 | n/a 2 |
| 1 | |
|---|---|
| Formula | 3[C16H36N]+, [C8H14MnMo6O24]−, 4/3(C4H9NO)[+ solvent] |
| Formula weight | 1968.30 g mol−1 a |
| Color | red-orange prisms |
| Crystal size | 0.102 × 0.092 × 0.088 mm3 |
| Crystal system | triclinic |
| Space group | P ī |
| Unit-cell dimensions | a = 20.6350(3), b = 25.0808(3), c = 25.7043(3) Å, α = 87.564(1), β = 80.831(1), γ = 73.226(1)° |
| Unit-cell volume | 12,574.2(3) Å3 |
| Temperature | −140(2) °C |
| Wavelength | 0.071 nm |
| Z | 6 |
| Calculated density | 1.560 g cm−3 |
| Absorption coefficient | 10.84 cm−1 a |
| Absorption method | multi-scan |
| Absorption corr. transmin/max | 0.7044/0.7456 |
| F(000) | 6072 |
| Measured data | 92,744 reflections in h(−26/26), k(−32/32), l(−33/32) |
| Range | 1.483° ≤ Θ ≤ 27.472° |
| Completeness | 96.7% |
| Independent data/Rint | 55,280/0.0369 |
| Data with I > 2σ(I) | 37,801 |
| Parameters | 2715 |
| Restraints | 30 |
| wR2 (all data, on F2) b | 0.1341 |
| R1 (I > 2σ(I)) b | 0.0679 |
| s c | 1059 |
| Res. dens./e∙Å−3 | 2.764/−1.892 e Å−3 |
| CCDC deposition number | 2226003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winter, A.; Endres, P.; Singh, N.; Schlörer, N.E.; Görls, H.; Kupfer, S.; Schubert, U.S. A Missing Member of the Anderson–Evans Family: Synthesis and Characterization of the Trimethylolmethane-Capped {MnMo6O24} Cluster. Inorganics 2025, 13, 254. https://doi.org/10.3390/inorganics13080254
Winter A, Endres P, Singh N, Schlörer NE, Görls H, Kupfer S, Schubert US. A Missing Member of the Anderson–Evans Family: Synthesis and Characterization of the Trimethylolmethane-Capped {MnMo6O24} Cluster. Inorganics. 2025; 13(8):254. https://doi.org/10.3390/inorganics13080254
Chicago/Turabian StyleWinter, Andreas, Patrick Endres, Nishi Singh, Nils E. Schlörer, Helmar Görls, Stephan Kupfer, and Ulrich S. Schubert. 2025. "A Missing Member of the Anderson–Evans Family: Synthesis and Characterization of the Trimethylolmethane-Capped {MnMo6O24} Cluster" Inorganics 13, no. 8: 254. https://doi.org/10.3390/inorganics13080254
APA StyleWinter, A., Endres, P., Singh, N., Schlörer, N. E., Görls, H., Kupfer, S., & Schubert, U. S. (2025). A Missing Member of the Anderson–Evans Family: Synthesis and Characterization of the Trimethylolmethane-Capped {MnMo6O24} Cluster. Inorganics, 13(8), 254. https://doi.org/10.3390/inorganics13080254











