Optical and Structural Characterization of Cu-Doped Ga2O3 Nanostructures Synthesized via Hydrothermal Method
Abstract
1. Introduction
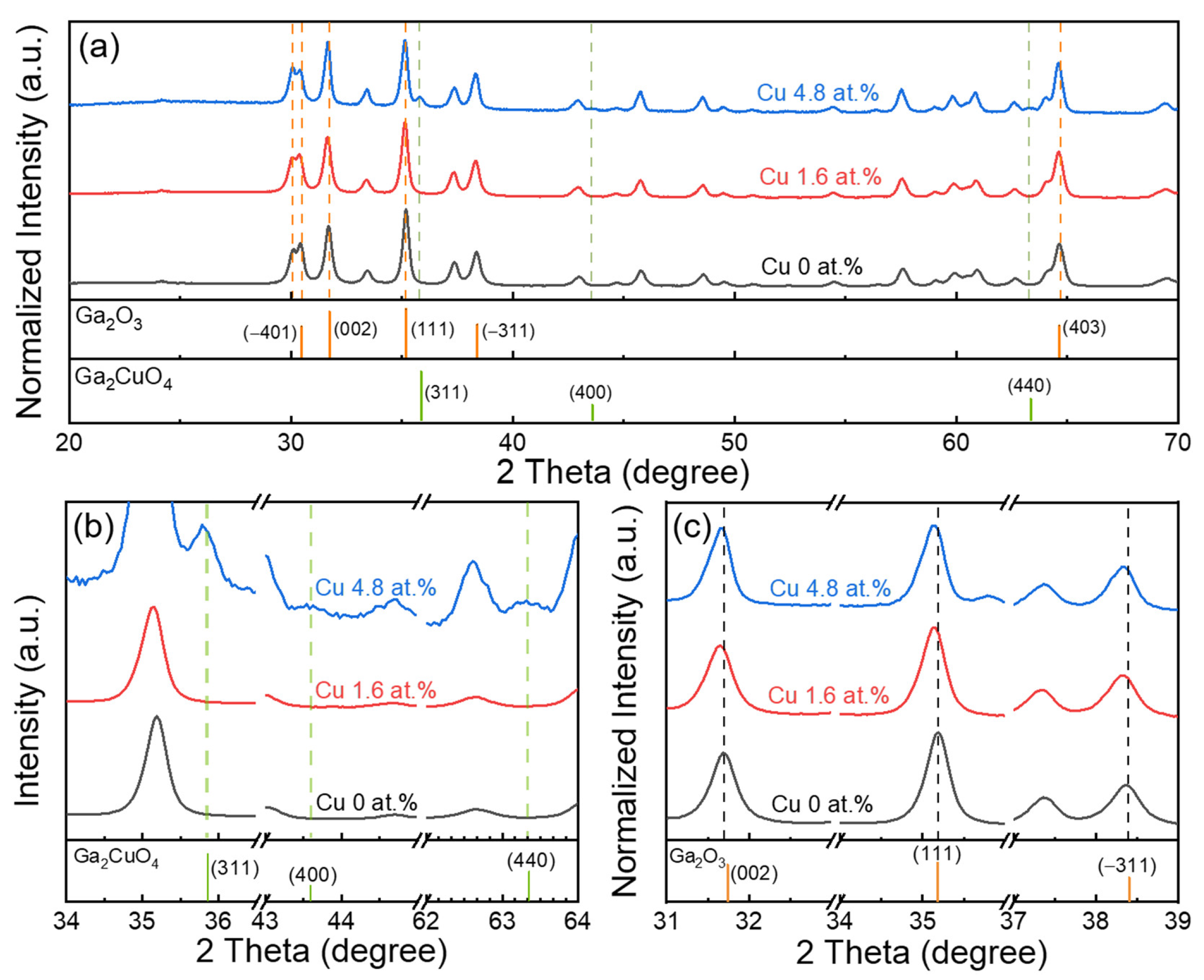
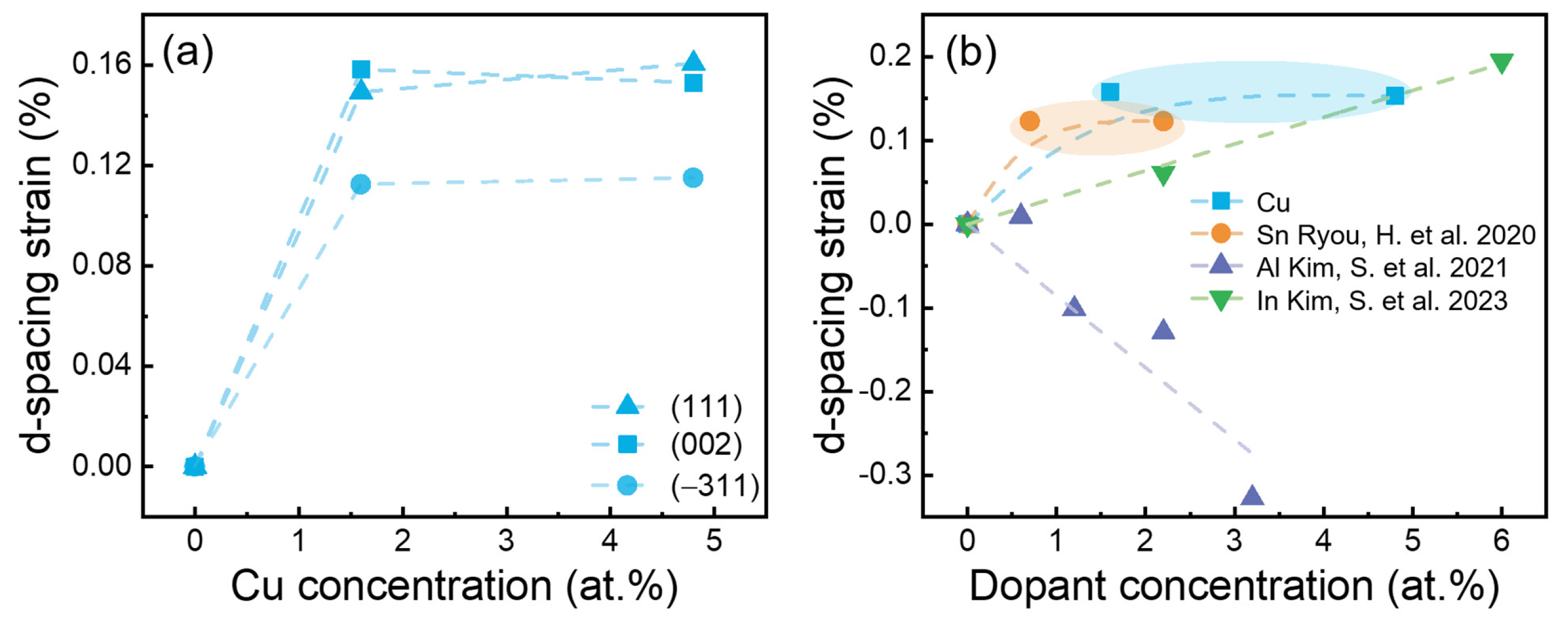
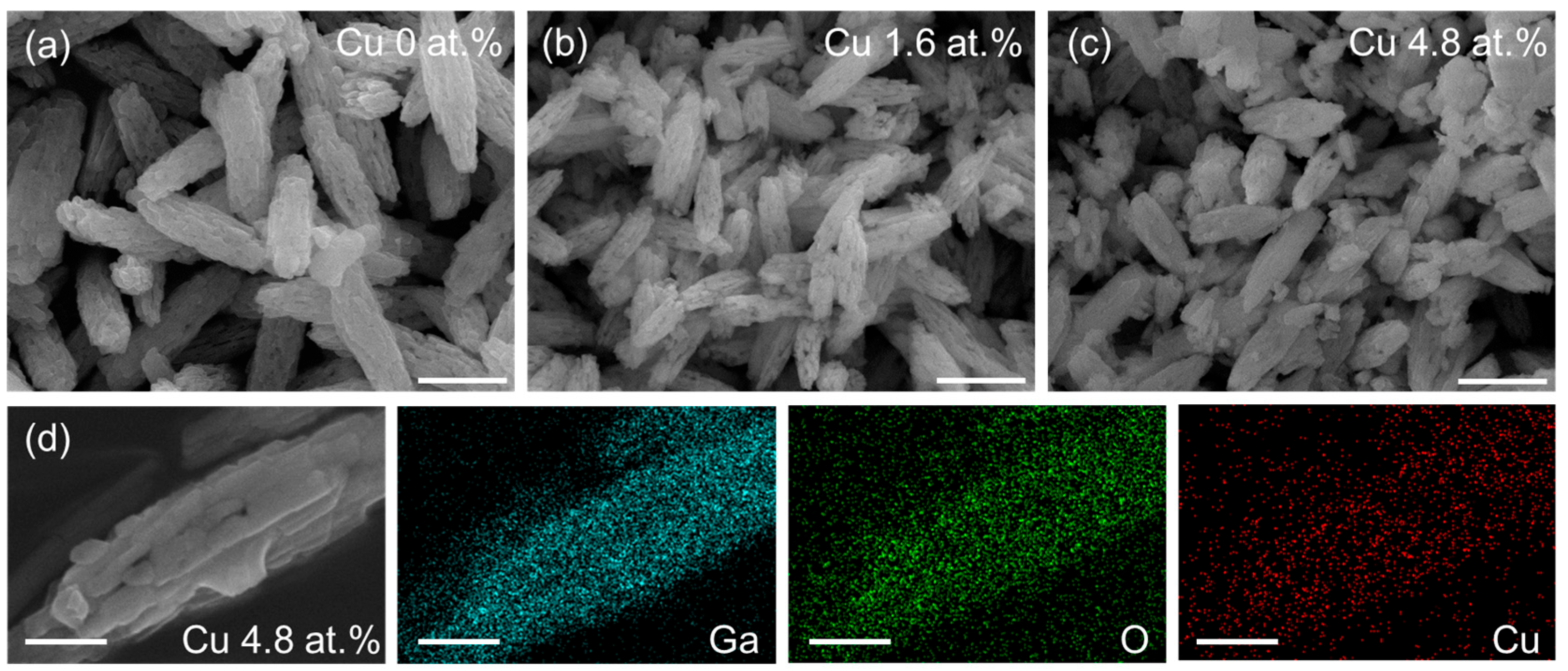
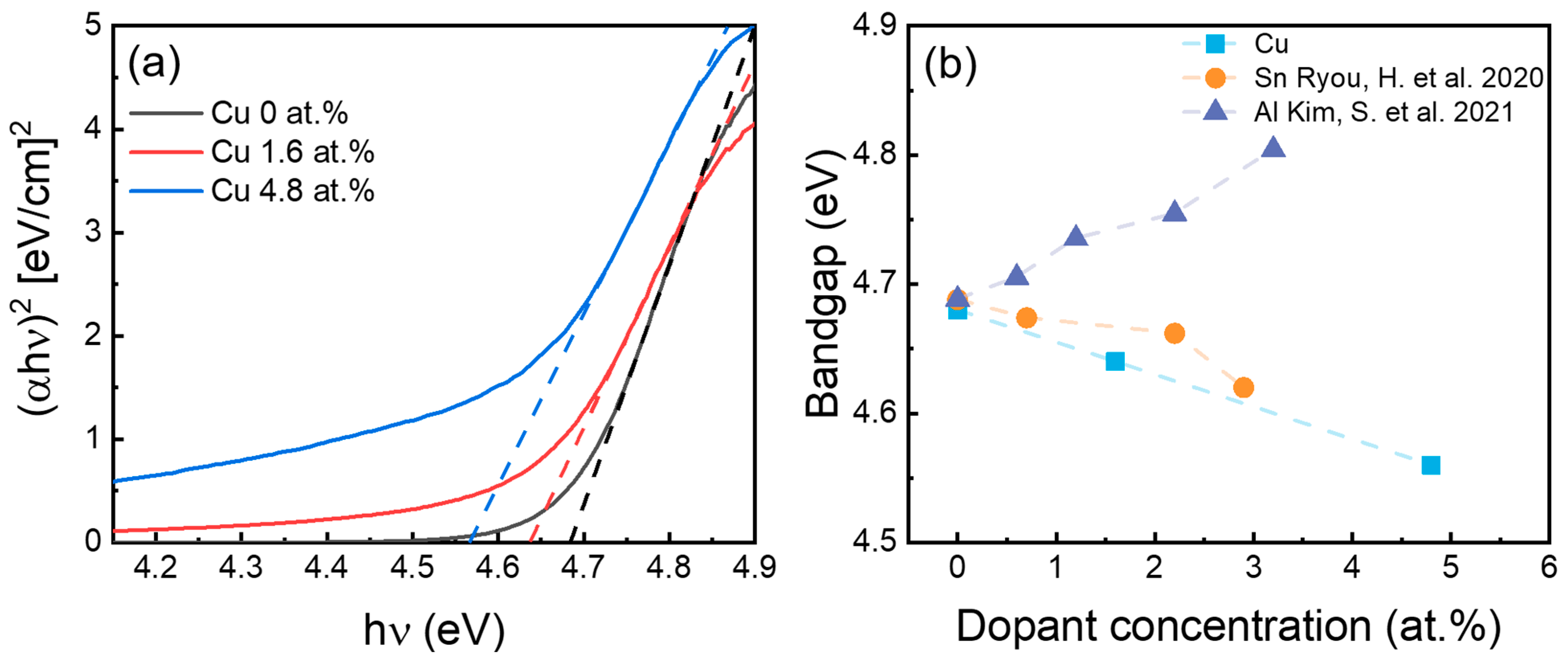
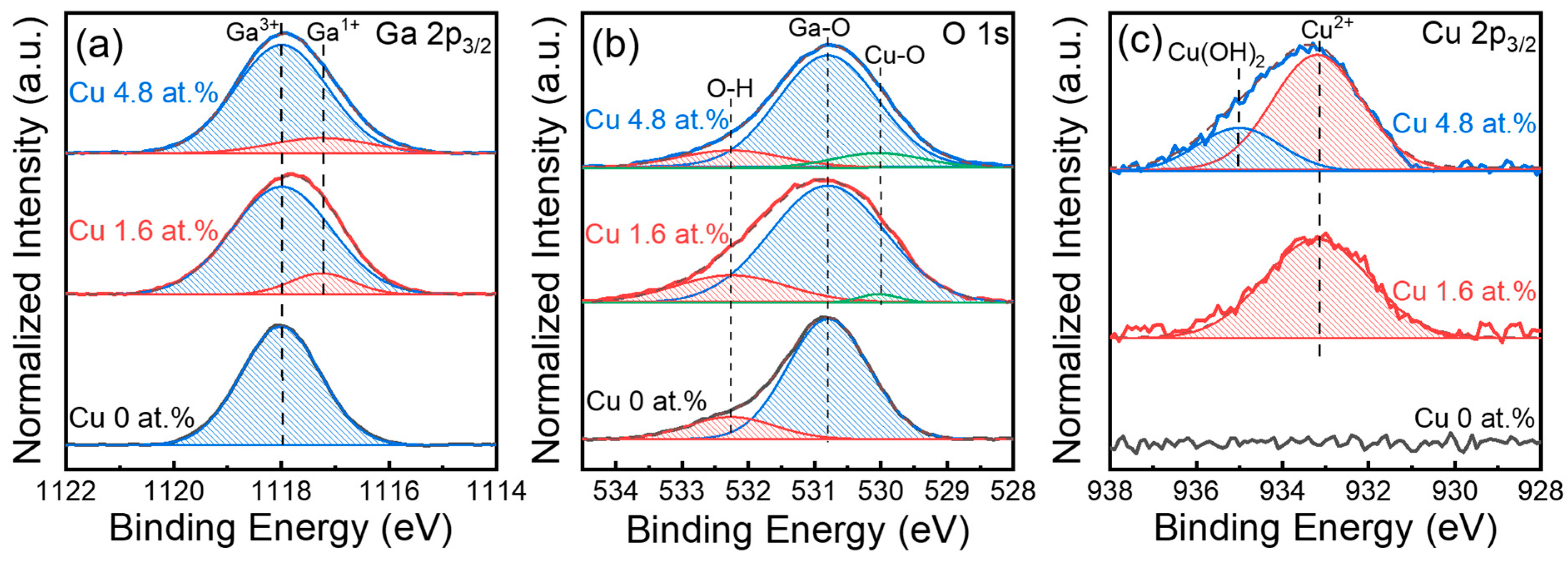
2. Results and Discussion
3. Materials and Methods
3.1. Hydrothermal Synthesis of Cu-Doped β-Ga2O3 Nanostructures
3.2. Characterization of Cu-Doped β-Ga2O3 Nanostructures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, D.; Guo, Q.; Chen, Z.; Wu, Z.; Li, P.; Tang, W. Review of Ga2O3-based optoelectronic devices. Mater. Today Phys. 2019, 11, 100157. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, J.; Qi, D.C.; Chen, L.; Zhang, K.H. Recent progress on the electronic structure, defect, and doping properties of Ga2O3. APL Mater. 2020, 8, 020906. [Google Scholar] [CrossRef]
- Tsao, J.Y.; Chowdhury, S.; Hollis, M.A.; Jena, D.; Johnson, N.M.; Jones, K.A.; Kaplar, R.J.; Rajan, S.; Van de Walle, C.G.; Bellotti, E.; et al. Ultrawide-bandgap semiconductors: Research opportunities and challenges. Adv. Electron. Mater. 2018, 4, 1600501. [Google Scholar] [CrossRef]
- Yoshioka, S.; Hayashi, H.; Kuwabara, A.; Oba, F.; Matsunaga, K.; Tanaka, I. Structures and energetics of Ga2O3 polymorphs. J. Phys. Condens. Matter 2007, 19, 346211. [Google Scholar] [CrossRef]
- Pearton, S.J.; Yang, J.; Cary, P.H.; Ren, F.; Kim, J.; Tadjer, M.J.; Mastro, M.A. A review of Ga2O3 materials, processing, and devices. Appl. Phys. Rev. 2018, 5, 011301. [Google Scholar] [CrossRef]
- Biswas, M.; Nishinaka, H. Thermodynamically metastable α-, ε- (or κ-), and γ-Ga2O3: From material growth to device applications. APL Mater. 2022, 10, 060701. [Google Scholar] [CrossRef]
- Gao, S.; Li, W.; Dai, J.; Wang, Q.; Suo, Z. Effect of transition metals doping on electronic structure and optical properties of β-Ga2O3. Mater. Res. Express 2021, 8, 025904. [Google Scholar] [CrossRef]
- Kyrtsos, A.; Matsubara, M.; Bellotti, E. On the feasibility of p-type Ga2O3. Appl. Phys. Lett. 2018, 112, 032108. [Google Scholar] [CrossRef]
- Lyons, J.L. A survey of acceptor dopants for β-Ga2O3. Semicond. Sci. Technol. 2018, 33, 05LT02. [Google Scholar] [CrossRef]
- Suzuki, N.; Ohira, S.; Tanaka, M.; Sugawara, T.; Nakajima, K.; Shishido, T. Fabrication and characterization of transparent conductive Sn-doped β-Ga2O3 single crystal. Phys. Status Solidi C 2007, 4, 2310–2313. [Google Scholar] [CrossRef]
- Ryou, H.; Yoo, T.H.; Yoon, Y.; Lee, I.G.; Shin, M.; Cho, J.; Cho, B.J.; Hwang, W.S. Hydrothermal synthesis and photocatalytic property of Sn-doped β-Ga2O3 nanostructure. ECS J. Solid State Sci. Technol. 2020, 9, 045009. [Google Scholar] [CrossRef]
- Kim, S.; Ryou, H.; Lee, I.G.; Shin, M.; Cho, B.J.; Hwang, W.S. Impact of Al doping on a hydrothermally synthesized β-Ga2O3 nanostructure for photocatalysis applications. RSC Adv. 2021, 11, 7338–7346. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Y.; Dong, L.; Jia, R. First-principles calculations of electronic and optical properties of aluminum-doped β-Ga2O3 with intrinsic defects. Results Phys. 2017, 7, 1582–1589. [Google Scholar] [CrossRef]
- Kim, S.; Ryou, H.; Moon, J.; Lee, I.G.; Hwang, W.S. Codoping of Al and In atoms in β-Ga2O3 semiconductors. J. Alloys Compd. 2023, 931, 167502. [Google Scholar] [CrossRef]
- Kim, S.; Ryou, H.; Kim, H.W.; Hwang, W.S. Enhanced photocatalytic properties of Ge-doped Ga2O3 nanostructures synthesized via hydrothermal method. Semicond. Sci. Technol. 2025, 40, 035003. [Google Scholar] [CrossRef]
- Saleh, M.; Bhattacharyya, A.; Varley, J.B.; Swain, S.; Jesenovec, J.; Krishnamoorthy, S.; Lynn, K. Electrical and optical properties of Zr doped β-Ga2O3 single crystals. Appl. Phys. Express 2019, 12, 085502. [Google Scholar] [CrossRef]
- Yoo, T.H.; Ryou, H.; Lee, I.G.; Cho, B.J.; Hwang, W.S. Enhanced photocatalytic activity of electrospun β-Ga2O3 nanofibers via in-situ Si doping using tetraethyl orthosilicate. Catalysts 2019, 9, 1005. [Google Scholar] [CrossRef]
- Zheng, T.; Wang, Q.; Dang, J.; He, W.; Wang, L.; Zheng, S. Effects of Si concentration on electronic structure and optical gap of Si-doped β-Ga2O3. Comput. Mater. Sci. 2020, 174, 109505. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, J.; Li, Q.; Qu, C.; Zhang, L.; Xie, W. Optical and structural properties of Cu-doped β-Ga2O3 films. Mater. Sci. Eng. B 2011, 176, 846–849. [Google Scholar] [CrossRef]
- Yan, H.; Guo, Y.; Song, Q.; Chen, Y. First-Principles Study on Electronic Structure and Optical Properties of Cu-Doped β-Ga2O3. Phys. B Condens. Matter 2014, 434, 181–184. [Google Scholar] [CrossRef]
- Gong, G.; Gou, L. Microstructure and Properties of Cu-Doped β-Ga2O3 Rod Prepared with Liquid Metallic Gallium. J. Eur. Ceram. Soc. 2025, 45, 116916. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, F.; Duan, J.; Meng, H.; Zhang, W.; Luo, W.; Wu, Y.; Wang, Y.; Zhang, Y. Band engineering of ReS2/V-In2S3 S-scheme heterojunction for high-efficiency visible-light-driven hydrogen evolution. Chem. Eng. J. 2025, 514, 163380. [Google Scholar] [CrossRef]
- Babu, A.; Rao, N.M. Effect of Copper Substitution on the Structural, Optical, and Magnetic Properties of β-Ga2O3 Powders. Appl. Phys. A 2025, 131, 172. [Google Scholar] [CrossRef]
- Kamimura, T.; Sasaki, K.; Wong, M.H.; Krishnamurthy, D.; Kuramata, A.; Masui, T.; Yamakoshi, S.; Higashiwaki, M. Band Alignment and Electrical Properties of Al2O3/β-Ga2O3 Heterojunctions. Appl. Phys. Lett. 2014, 104, 192104. [Google Scholar] [CrossRef]
- Oruç, Ç.; Altındal, A. Structural and Dielectric Properties of CuO Nanoparticles. Ceram. Int. 2017, 43, 10708–10714. [Google Scholar] [CrossRef]
- Akgul, F.A.; Gumus, C.; Ali, O.E.; Farha, A.H.; Akgul, G.; Ufuktepe, Y.; Liu, Z. Structural and Electronic Properties of SnO2. J. Alloys Compd. 2013, 579, 50–56. [Google Scholar] [CrossRef]
- Chikoidze, E.; von Bardeleben, H.J.; Akaiwa, K.; Shigematsu, E.; Kaneko, K.; Fujita, S.; Dumont, Y. Electrical, Optical, and Magnetic Properties of Sn Doped α-Ga2O3 Thin Films. J. Appl. Phys. 2016, 120, 025109. [Google Scholar] [CrossRef]
- Suri, R.; Lichtenwalner, D.J.; Misra, V. Interfacial Self Cleaning during Atomic Layer Deposition and Annealing of HfO2 Films on Native (100)-GaAs Substrates. Appl. Phys. Lett. 2010, 96, 112905. [Google Scholar] [CrossRef]
- Yang, L.X.; Zhao, X.; Xu, S.; Lu, Y.Y.; Chang, H.; Liu, J. Oxide Transformation and Break-Up of Liquid Metal in Boiling Solutions. Sci. China Technol. Sci. 2020, 63, 289–296. [Google Scholar] [CrossRef]
- Chen, R.X.; Zhu, S.L.; Mao, J.; Cui, Z.D.; Yang, X.J.; Liang, Y.Q.; Li, Z.Y. Synthesis of CuO/Co3O4 Coaxial Heterostructures for Efficient and Recycling Photodegradation. Int. J. Photoenergy 2015, 1, 183468. [Google Scholar]
- Shao, P.R.; Deng, S.Z.; Chen, J.; Chen, J.; Xu, N.S. Study of Field Emission, Electrical Transport, and Their Correlation of Individual Single CuO Nanowires. J. Appl. Phys. 2011, 109, 023710. [Google Scholar] [CrossRef]
- Makeswaran, N.; Battu, A.K.; Swadipta, R.; Manciu, F.S.; Ramana, C.V. Spectroscopic Characterization of the Electronic Structure, Chemical Bonding, and Band Gap in Thermally Annealed Polycrystalline Ga2O3 Thin Films. ECS J. Solid State Sci. Technol. 2019, 8, Q3249. [Google Scholar] [CrossRef]
- Dhaka, R.; Yadav, A.; Pal, P.; Prakash, C.; Gupta, G.; Dixit, A.; Dutta, S.; Shukla, A.K. Growth of β-Ga2O3-ZnO Nanowires Heterostructure on Si Substrate for UV-Visible Multi-Band Photodetector Applications. 2024. Available online: https://ssrn.com/abstract=4988085 (accessed on 4 April 2025).
- Tang, C.; Dong, H. The Effects of Cu2+ Adsorption on Surface Dissolution of Albite. Colloids Surf. A Physicochem. Eng. Asp. 2022, 644, 128832. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, B.; Zhang, W.; Yu, M.; Cui, L.; Cao, X.; Liu, J. Super-light Cu@Ni nanowires/graphene oxide composites for significantly enhanced microwave absorption performance. Sci. Rep. 2017, 7, 1584. [Google Scholar] [CrossRef] [PubMed]
- Tumuluri, A.; Naidu, K.L.; Raju, K.J. Band gap determination using Tauc’s plot for LiNbO3 thin films. Int. J. ChemTech Res. 2014, 6, 3353–3356. [Google Scholar]
- George, P.; Chowdhury, P. Complex Dielectric Transformation of UV-Vis Diffuse Reflectance Spectra for Estimating Optical Band-Gap Energies and Materials Classification. Analyst 2019, 144, 3005–3012. [Google Scholar] [CrossRef]
| Cu Doping | Plane | ||
|---|---|---|---|
| (002) | (111) | (–311) | |
| 0 at.% | 31.687 | 31.648 | 31.648 |
| 1.6 at.% | 35.193 | 35.154 | 35.114 |
| 4.8 at.% | 38.344 | 38.305 | 38.344 |
| Cu Doping | Atomic Ratio from XPS Quantification | ||
|---|---|---|---|
| Cu 2p | Ga 2p | O 1s | |
| 0 at.% | 0% | 29.7% | 70.3% |
| 1.6 at.% | 1.6% | 26.3% | 72.1% |
| 4.8 at.% | 4.8% | 50% | 45.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Ryou, H.; Lee, J.; Kim, S.; Hwang, W.S. Optical and Structural Characterization of Cu-Doped Ga2O3 Nanostructures Synthesized via Hydrothermal Method. Inorganics 2025, 13, 231. https://doi.org/10.3390/inorganics13070231
Kim J, Ryou H, Lee J, Kim S, Hwang WS. Optical and Structural Characterization of Cu-Doped Ga2O3 Nanostructures Synthesized via Hydrothermal Method. Inorganics. 2025; 13(7):231. https://doi.org/10.3390/inorganics13070231
Chicago/Turabian StyleKim, Jiwoo, Heejoong Ryou, Janghun Lee, Sunjae Kim, and Wan Sik Hwang. 2025. "Optical and Structural Characterization of Cu-Doped Ga2O3 Nanostructures Synthesized via Hydrothermal Method" Inorganics 13, no. 7: 231. https://doi.org/10.3390/inorganics13070231
APA StyleKim, J., Ryou, H., Lee, J., Kim, S., & Hwang, W. S. (2025). Optical and Structural Characterization of Cu-Doped Ga2O3 Nanostructures Synthesized via Hydrothermal Method. Inorganics, 13(7), 231. https://doi.org/10.3390/inorganics13070231









