Visible-Light Photocatalytic Degradation of Methylene Blue by Yb3+-Doped 3D Nanosheet Arrays BiOI Anchored on High-Chloride Fly Ash Composites
Abstract
1. Introduction
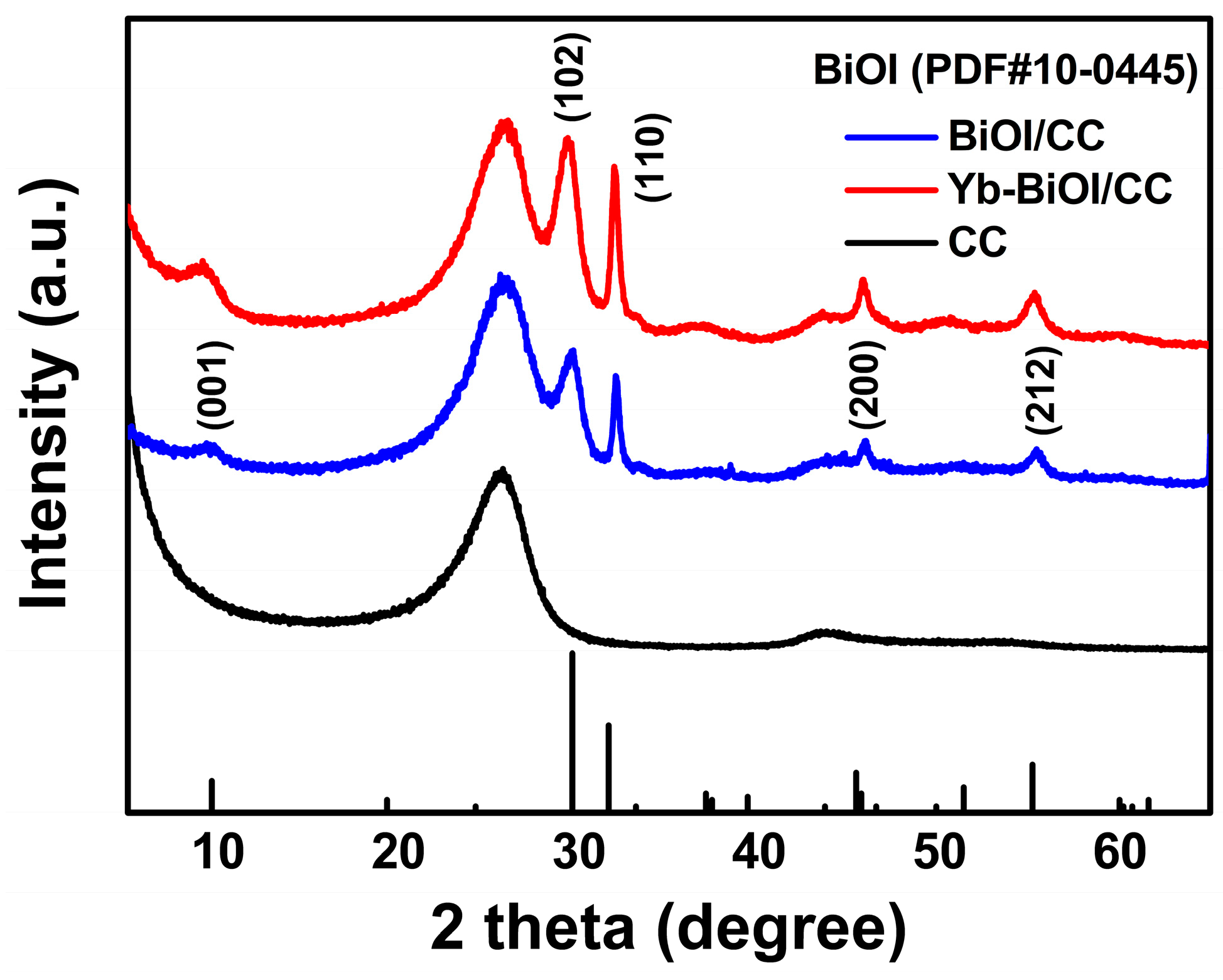
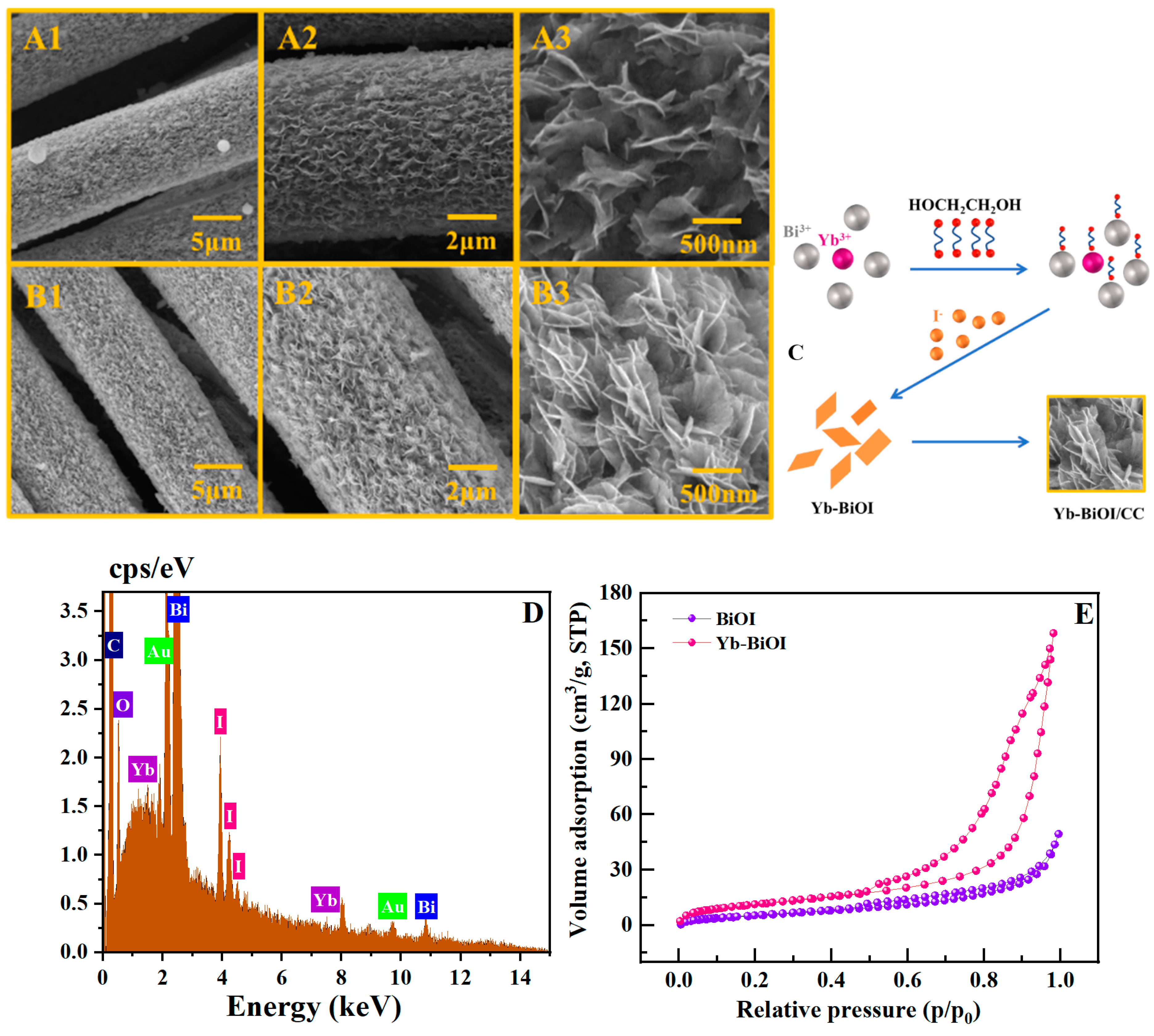
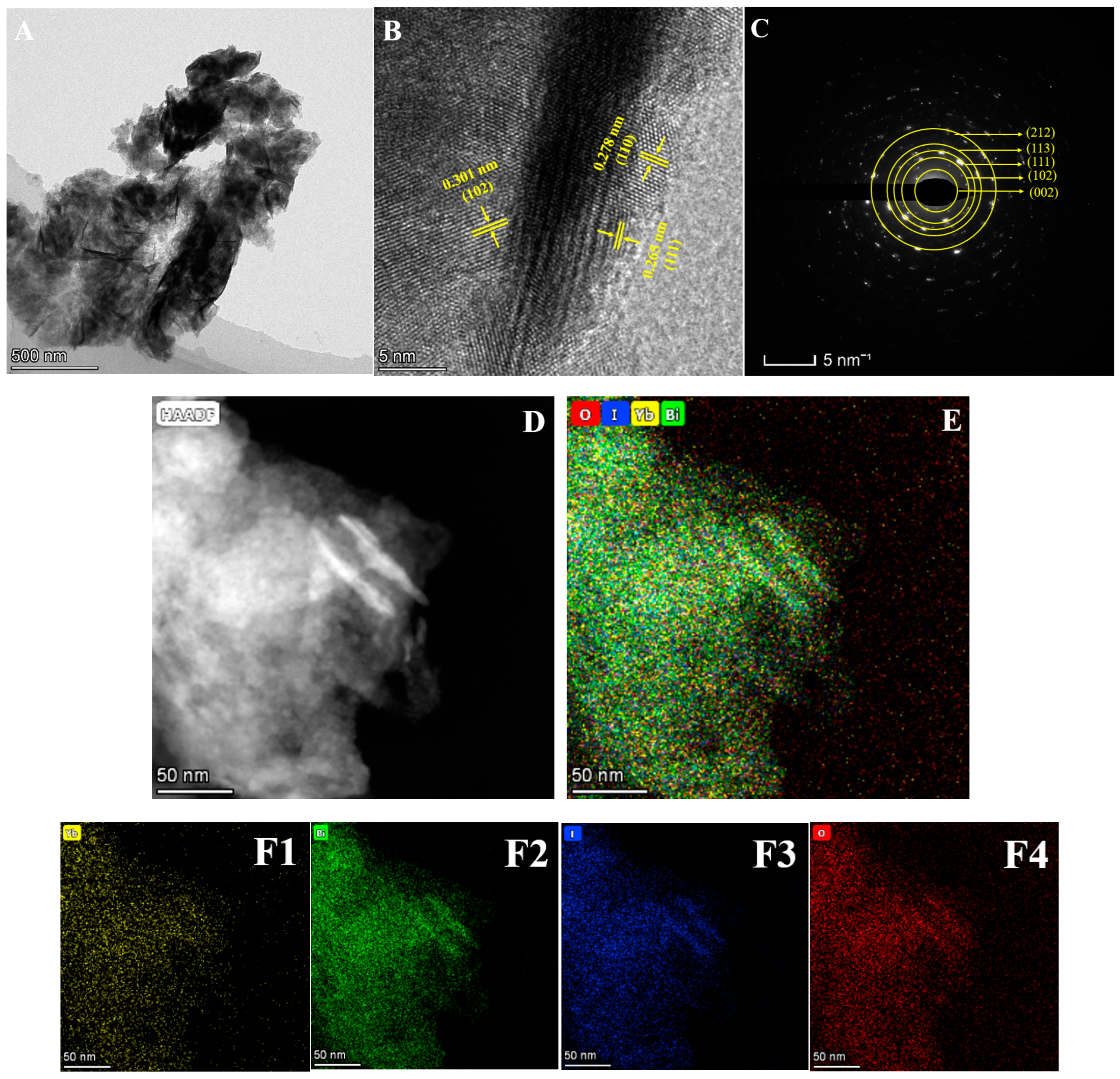
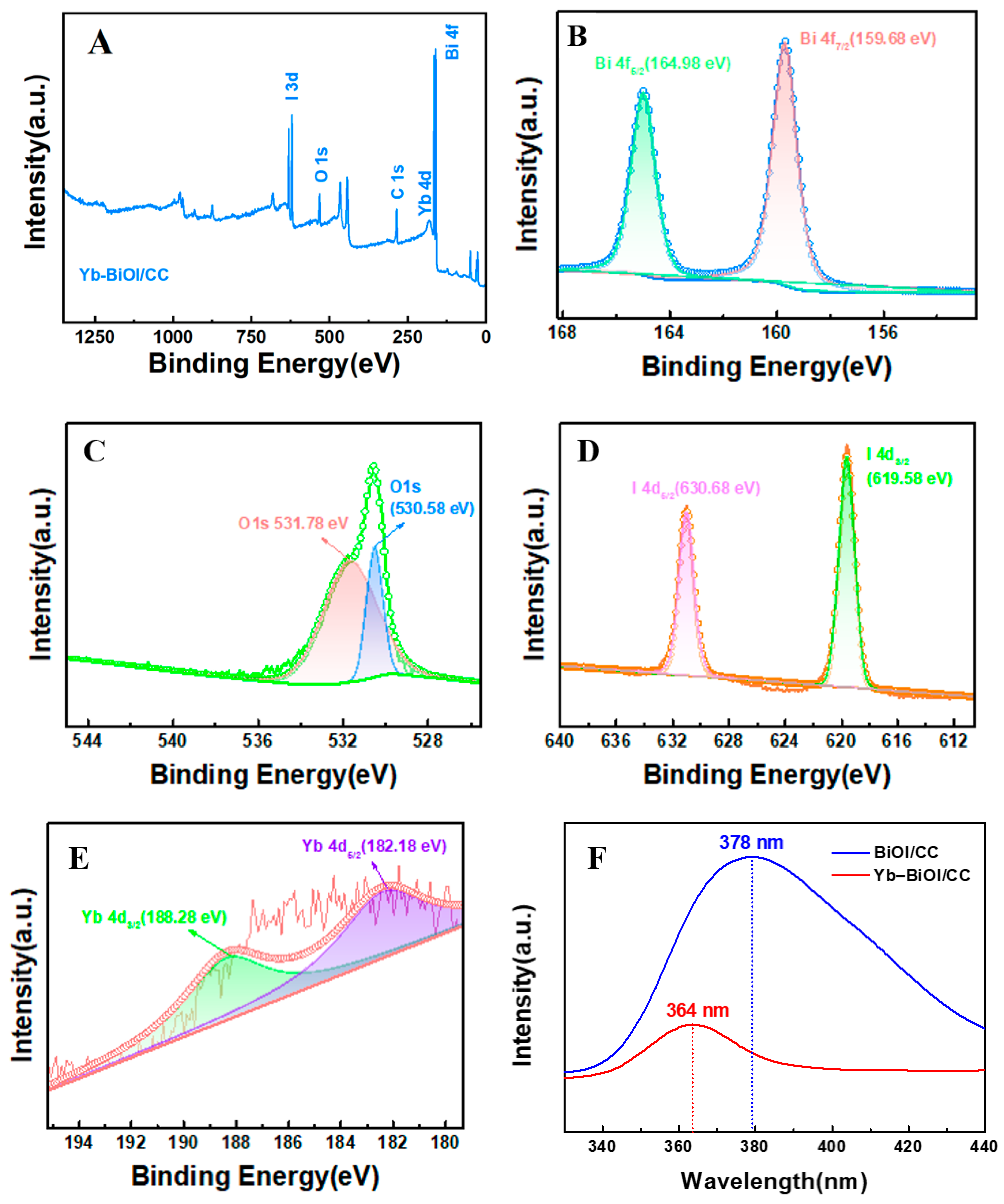
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Instrumentations and Characterizations
3.3. Pretreatment of Carbon Cloth and Preparation of Yb-BiOI/CC Photocatalytic Material
3.4. Preparation of Yb-BiOI/CC/FA
3.5. Photocatalytic Performance Testing
3.6. Photoelectrochemical Performance Measurement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Wasidi, A.S.; AlReshaidan, S. Enhanced Removal of Rhodamine b Dye from Aqueous Media via Adsorption on Facilely Synthesized Zinc Ferrite Nanoparticles. Inorganics 2024, 12, 191. [Google Scholar] [CrossRef]
- Al-Wasidi, A.S.; Shah, R.K.; Abdelrahman, E.A.; Mabrouk, E.-S.M. Facile Synthesis of CuFe2O4 Nanoparticles for Efficient Removal of Acid Blue 113 and Malachite Green Dyes from Aqueous Media. Inorganics 2024, 12, 143. [Google Scholar] [CrossRef]
- Pereira, M.F.G.; Nascimento, M.M.; Cardoso, P.H.N.; Oliveira, C.Y.B.; Tavares, G.F.; Araújo, E.S. Preparation, Microstructural Characterization and Photocatalysis Tests of V5+-Doped TiO2/WO3 Nanocomposites Supported on Electrospun Membranes. Inorganics 2022, 10, 143. [Google Scholar] [CrossRef]
- Varadi, A.; Leostean, C.; Stefan, M.; Popa, A.; Toloman, D.; Pruneanu, S.; Tripon, S.; Macavei, S. Fe3O4-ZnO:V Nanocomposites with Modulable Properties as Magnetic Recoverable Photocatalysts. Inorganics 2024, 12, 119. [Google Scholar] [CrossRef]
- Ruan, X.W.; Hu, H.; Che, G.B.; Zhou, P.J.; Liu, C.B.; Dong, H.J. Fabrication of Z-Scheme γ-Bi2MoO6/Bi12GeO20 Heterostructure for Visible-Light-Driven Photocatalytic Degradation of Organic Pollutants. Appl. Surf. Sci. 2020, 499, 143668. [Google Scholar] [CrossRef]
- Guan, G.M.; Shao, Y.X.; Xu, Z.L.; Liu, L.P.; Xu, W.Q.; Jiang, L.C. The Preparation and Photo-Electrocatalytic Properties of ZnO@Zn/TiO2 3D Array Composite. Ind. Water Treat. 2022, 42, 130–135. [Google Scholar]
- Chang, S.M.; Liu, W.S. Surface Doping Is More Beneficial than Bulk Doping to the Photocatalytic Activity of Vanadium-Doped TiO2. Appl. Catal. B Environ. 2011, 101, 333–342. [Google Scholar] [CrossRef]
- Mukherji, A.; Seger, B.; Lu, G.Q.; Wang, L.Z. Nitrogen Doped Sr2Ta2O7 Coupled with Graphene Sheets as Photocatalysts for Increased Photocatalytic Hydrogen Production. ACS Nano 2011, 5, 3483–3492. [Google Scholar] [CrossRef]
- Liu, D.H.; Huang, J.F.; Tao, X.W.; Wang, D. One-Step Synthesis of C-Bi2WO6 Crystallites with Improved Photocatalytic Activities under Visible Light Irradiation. RSC Adv. 2015, 5, 66464–66470. [Google Scholar] [CrossRef]
- Anwer, H.; Mahmood, A.; Lee, J.; Kim, K.H.; Park, J.W.; Yip, A.C.K. Photocatalysts for Degradation of Dyes in Industrial Effluents: Opportunities and Challenges. Nano Res. 2019, 12, 955–972. [Google Scholar] [CrossRef]
- Rafiq, A.; Ikram, M.; Ali, S.; Niaz, F.; Khan, M.; Khan, Q.; Maqbool, M. Photocatalytic Degradation of Dyes Using Semiconductor Photocatalysts to Clean Industrial Water Pollution. Ind. Eng. Chem. 2021, 97, 111–128. [Google Scholar] [CrossRef]
- Villabona-Leal, E.G.; López-Neira, J.P.; Pedraza-Avella, J.A.; Pérez, E.; Meza, O. Screening of Factors Influencing the Photocatalytic Activity of TiO2: Ln (Ln=La, Ce, Pr, Nd, Sm, Eu and Gd) in the Degradation of Dyes. Comput. Mater. Sci. 2015, 107, 48–53. [Google Scholar] [CrossRef]
- Bhethanabotla, V.C.; Russell, D.R.; Kuhn, J.N. Assessment of Mechanisms for Enhanced Performance of Yb/Er/Titania Photocatalysts for Organic Degradation: Role of Rare Earth Elements in the Titania Phase. Appl. Catal. B Environ. 2017, 202, 156–164. [Google Scholar] [CrossRef]
- Adhikari, R.; Gyawali, G.; Cho, S.H.; Narro-García, R.; Sekino, T.; Lee, W.S. Er3+/Yb3+ Co-Doped Bismuth Molybdate Nanosheets Upconversion Photocatalyst with Enhanced Photocatalytic Activity. J. Solid State Chem. 2014, 209, 74–81. [Google Scholar] [CrossRef]
- Liu, T.; Tan, G.Q.; Zhao, C.C.; Xu, C.; Su, Y.N.; Wang, Y.; Ren, H.J.; Xia, A.; Shao, D.; Yan, S.M. Enhanced Photocatalytic Mechanism of the Nd-Er Co-Doped Tetragonal BiVO4 Photocatalysts. Appl. Catal. B Environ. 2017, 213, 87–96. [Google Scholar] [CrossRef]
- Hassan, M.S.; Amna, T.; Yang, O.-B.; Kim, H.-C.; Khil, M.-S. TiO2 Nanofibers Doped with Rare Earth Elements and Their Photocatalytic Activity. Ceram. Int. 2012, 38, 5925–5930. [Google Scholar] [CrossRef]
- Mehtab, A.; Ahmed, J.; Alshehri, S.M.; Mao, Y.B.; Ahmad, T. Rare Earth Doped Metal Oxide Nanoparticles for Photocatalysis: A Perspective. Nanotechnology 2022, 33, 142001. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, H.H.; Hong, S.C. Influence of Calcination Temperature on Ce/TiO2 Catalysis of Selective Catalytic Oxidation of NH3 to N2. Appl. Catal. A Gen. 2014, 470, 189–198. [Google Scholar] [CrossRef]
- Sin, J.C.; Lam, S.M.; Lee, K.T.; Mohamed, A.R. Preparation of Rare Earth-Doped ZnO Hierarchical Micro/Nanospheres and Their Enhanced Photocatalytic Activity under Visible Light Irradiation. Ceram. Int. 2014, 40, 5431–5440. [Google Scholar] [CrossRef]
- Li, H.; Hao, H.S.; Jin, S.S.; Guo, W.H.; Hu, X.F.; Hou, H.M.; Zhang, G.L.; Yan, S.; Gao, W.Y.; Liu, G.S. Synthesis of Yb3+/Ho3+ Co-Doped Bi2WO6 Upconversion Photocatalyst with Highly Improved Visible Light Photocatalytic Activity. Catal. Commun. 2017, 97, 60–64. [Google Scholar] [CrossRef]
- Xue, S.S.; He, H.B.; Fan, Q.Z.; Yu, C.L.; Yang, K.; Huang, W.Y.; Zhou, Y.; Xie, Y. La/Ce-Codoped Bi2O3 Composite Photocatalysts with High Photocatalytic Performance in Removal of High Concentration Dye. J. Environ. Sci. 2017, 60, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Zagaynov, I.V.; Liberman, E.Y.; Prikhodko, O.P.; Kon’kova, T.V. Catalytic Activity of CeO2@TiO2 for Environmental Protection. New J. Chem. 2024, 48, 2842. [Google Scholar] [CrossRef]
- Ma, Z.W.; Li, C. Research on Fly Ash Cenospheres-Supported Photocatalyst for the Degradation of Dye Wastewater. Ind. Water Treat. 2015, 35, 65–68. [Google Scholar]
- He, M.Q.; Li, W.B.; Xia, J.X.; Xu, L.; Di, J.; Xu, H.; Yin, S.; Li, H.M.; Li, M.N. The Enhanced Visible Light Photocatalytic Activity of Yttrium-Doped BiOBr Synthesized via a Reactable Ionic Liquid. Appl. Surf. Sci. 2015, 331, 170–178. [Google Scholar] [CrossRef]
- Huo, Y.N.; Zhang, J.; Miao, M.; Jin, Y. Solvothermal Synthesis of Flower-Like BiOBr Microspheres with Highly Visible-Light Photocatalytic Performances. Appl. Catal. B Environ. 2012, 111–112, 334–341. [Google Scholar] [CrossRef]
- Jiang, L.C.; Gao, X.Y.; Chen, S.L. Oxygen-Deficient WO3/TiO2/CC Nanorod Arrays for Visible-Light Photocatalytic Degradation of Methylene Blue. Catalysts 2021, 11, 1349. [Google Scholar] [CrossRef]
- Chowdhury, A.; Balu, S.; Yang, T.C.-K. Construction of α-Fe2O3-NPs@AgVO3-NRs Z-scheme heterojunction: An efficient photo(electro)catalyst for Cr(VI) reduction and oxygen evolution reactions under visible-light. J. Environ. Chem. Eng. 2023, 11, 109769. [Google Scholar] [CrossRef]
- Dai, W.W.; Zhao, Z.Y. Electronic Structure and Optical Properties of BiOI as a Photocatalyst Driven by Visible Light. Catalysts 2016, 6, 133–148. [Google Scholar] [CrossRef]
- Mishra, N.S.; Saravanan, P. Z-scheme promoted heterojunction photocatalyst (Ag@AgVO3/rGO/CeVO4) with improved interfacial charge transfer for efficient removal of aqueous organics irradiated under LED light. Chemosphere 2023, 310, 136896. [Google Scholar] [CrossRef]
- Balu, S.; Venkatesvaran, H.; Wang, C.-C.; Juan, J.C.; Yang, T.C.-K. Synthesis of Sulfonic Acid-Functionalized g-C3N4/BiOI Bifunctional Heterojunction for Enhanced Photocatalytic Removal of Tartrazine and PEC Oxygen Evolution Reaction. Inorganics 2024, 12, 243. [Google Scholar] [CrossRef]
- Wang, W.D.; Huang, F.Q.; Lin, X.P.; Yang, J.H. Visible-light-responsive photocatalysts xBiOBr–(1-x)BiOI. Catal. Commun. 2008, 9, 8–12. [Google Scholar] [CrossRef]
- Wang, H.; Yong, D.Y.; Chen, S.C.; Jiang, S.L.; Zhang, X.D.; Shao, W.; Zhang, Q.; Yan, W.S.; Pan, B.C.; Xie, Y. Oxygen-vacancy-mediated exciton dissociation in BiOBr for boosting charge-carrier-involved molecular oxygen activation. J. Am. Chem. Soc. 2018, 140, 1760–1766. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y. XPS studies of the intermediate valence state of Yb in (YbS)1.25CrS2. J. Electron. Spectrosc. Relat. Phenom. 2008, 165, 1–4. [Google Scholar] [CrossRef]
- Tricoire, M.; Sroka, W.; Rajeshkumar, T.; Scopelliti, R.; Sienkiewicz, A.; Maron, L.; Mazzanti, M. Multielectron Redox Chemistry of Ytterbium Complexes Reaching the +1 and Zero Formal Oxidation States. J. Am. Chem. Soc. 2025, 147, 1162–1171. [Google Scholar] [CrossRef]
- Ullah, R.; Dutta, J. Photocatalytic Degradation of Organic Dyes With Manganese-Doped ZnO Nanoparticles. J. Hazard. Mater. 2008, 156, 194–200. [Google Scholar] [CrossRef]
- Shen, W.Z.; Li, Z.J.; Wang, H.; Liu, Y.H.; Guo, Q.J.; Zhang, Y.L. Photocatalytic Degradation for Methylene Blue Using Zinc Oxide Prepared By Codeposition and Sol–Gel Methods. J. Hazard. Mater. 2008, 152, 172–175. [Google Scholar] [CrossRef]
- Pawinrat, P.; Mekasuwandumrong, O.; Panpranot, J. Synthesis of Au–ZnO and Pt–ZnO Nanocomposites By One-Step Flame Spray Pyrolysis and Its Application for Photocatalytic Degradation of Dyes. Catal. Commun. 2009, 10, 1380–1385. [Google Scholar] [CrossRef]
- Muruganandhm, M.; Chen, I.S.; Wu, J.J. Effect of Temperature on the Formation of Macroporous ZnO Bundles and Its Application In Photocatalysis. J. Hazard. Mater. 2009, 172, 700–706. [Google Scholar] [CrossRef]
- Yan, H.W.; Hou, J.B.; Fu, Z.P.; Yang, B.F.; Yang, P.H.; Liu, K.P.; Wen, M.W.; Chen, Y.J.; Fu, S.Q.; Li, F.Q. Growth and Photocatalytic Properties of One-Dimensional ZnO Nanostructures Prepared By Thermal Evaporation. Mater. Res. Bull. 2009, 44, 1954–1958. [Google Scholar] [CrossRef]
- Uddin, M.T.; Nicolas, Y.; Qlivier, C.; Toupance, T.; Servant, L.; Muller, M.M.; Kleebe, H.J.; Ziegler, J.; Jaegermann, W. Nanostructured SnO2-ZnO Heterojunction Photocatalysts Showing Enhanced Photocatalytic Activity for the Degradation of Organic Dyes. Inorg. Chem. 2012, 51, 7764–7773. [Google Scholar] [CrossRef]
- Soltani, N.; Saion, E.; Yunus, W.M.M.; Erfani, M.; Navasery, M.; Bahmanrokh, G.; Rezaee, K. Enhancement of Visible Light Photocatalytic Activity of ZnS and CdS Nanoparticles Based on Organic and Inorganic Coating. Appl. Surf. Sci. 2014, 290, 440–447. [Google Scholar] [CrossRef]
- Peng, Y.; Yu, P.P.; Zhou, H.Y.; Xu, A.W. Synthesis of BiOI/Bi4O5I2/Bi2O2CO3 p–n–p Heterojunctions with Superior Photocatalyticactivities. New J. Chem. 2015, 39, 8321–8328. [Google Scholar] [CrossRef]
- Singh, S.; Khare, N. Reduced Graphene Oxide Coupled CdS/CoFe2O4 Ternary Nanohybrid with Enhanced Photocatalytic Activity and Stability: A Potential Role of Reduced Graphene Oxide as A Visible Light Responsive Photosensitizer. RSC Adv. 2015, 5, 96562. [Google Scholar] [CrossRef]
- Zhu, C.S.; Zhang, L.; Jiang, B.; Zheng, J.T.; Hu, P.; Li, S.J.; Wu, M.B.; Wu, W.T. Fabrication of Z-Scheme Ag3PO4/MoS2 Composites with Enhanced Photocatalytic Activity and Stability for Organic Pollutant Degradation. Appl. Surf. Sci. 2016, 377, 99–108. [Google Scholar] [CrossRef]
- Ayu, D.G.; Gea, S.; Andriayani; Junita, D.; Telaumbanua, J.; Piliang, A.F.R.; Harahap, M.; Yen, Z.H.; Goei, R.; Tok, A.I.Y. Photocatalytic Degradation of Methylene Blue Using N-Doped ZnO/Carbon Dot (N-ZnO/CD) Nanocomposites Derived from Organic Soybean. ACS Omega 2023, 8, 14965–14984. [Google Scholar] [CrossRef]
- Mohammed, W.; Matalkeh, M.; Soubaihi, R.M.A.; Elzatahry, A. Visible Light Photocatalytic Degradation of Methylene Blue Dye and Pharmaceutical Wastes over Ternary NiO/Ag/TiO2 Heterojunction. ACS Omega 2023, 8, 40063–40077. [Google Scholar] [CrossRef]
- An, H.; Lin, B.; Xue, C.; Yan, X.Q.; Dai, Y.Z.; Wei, J.J.; Yang, G.D. Formation of BiOI/g-C3N4 Nanosheet Composites with High Visible-Light-Driven Photocatalytic Activity. Chin. J. Catal. 2018, 39, 654–663. [Google Scholar] [CrossRef]

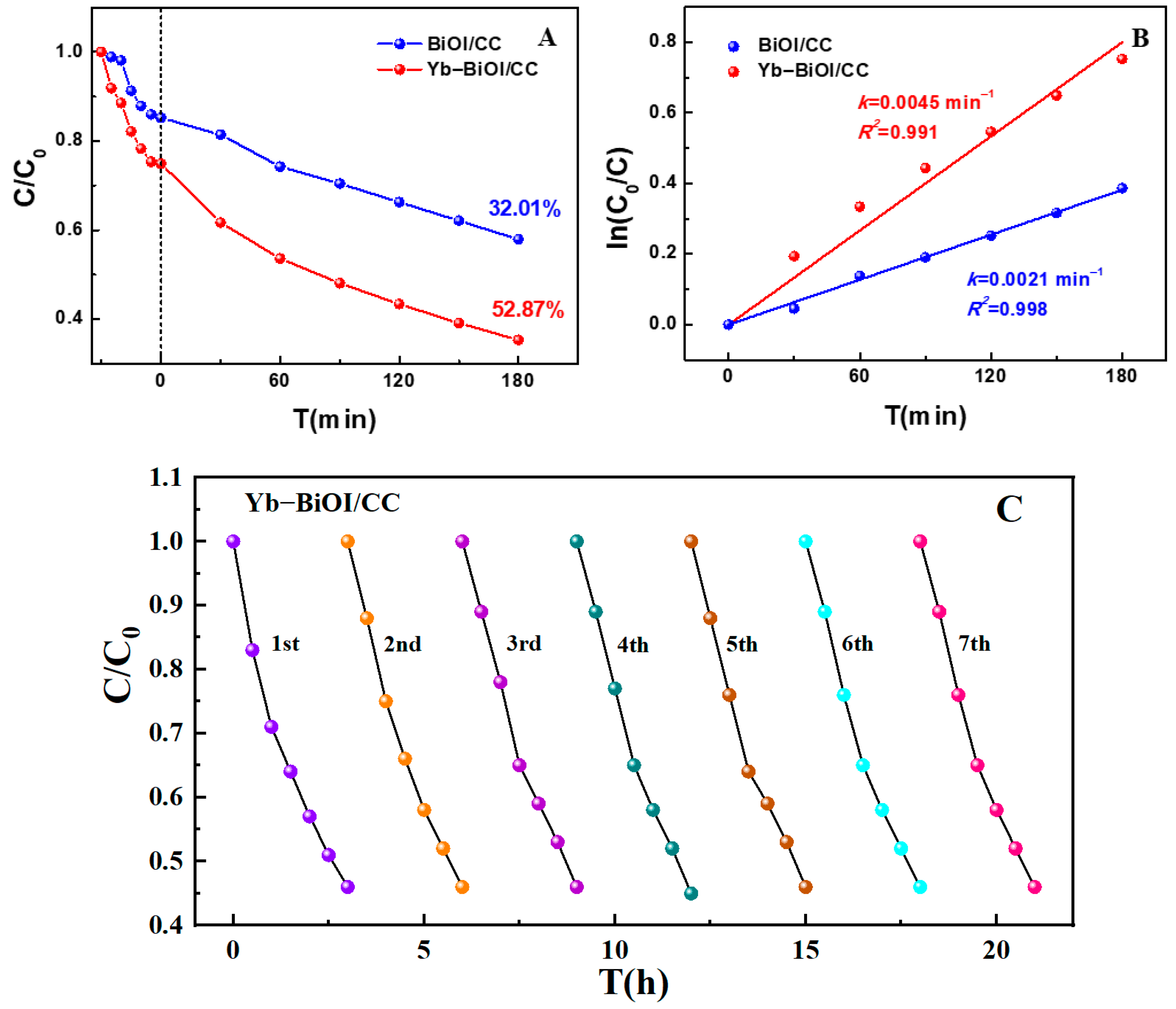
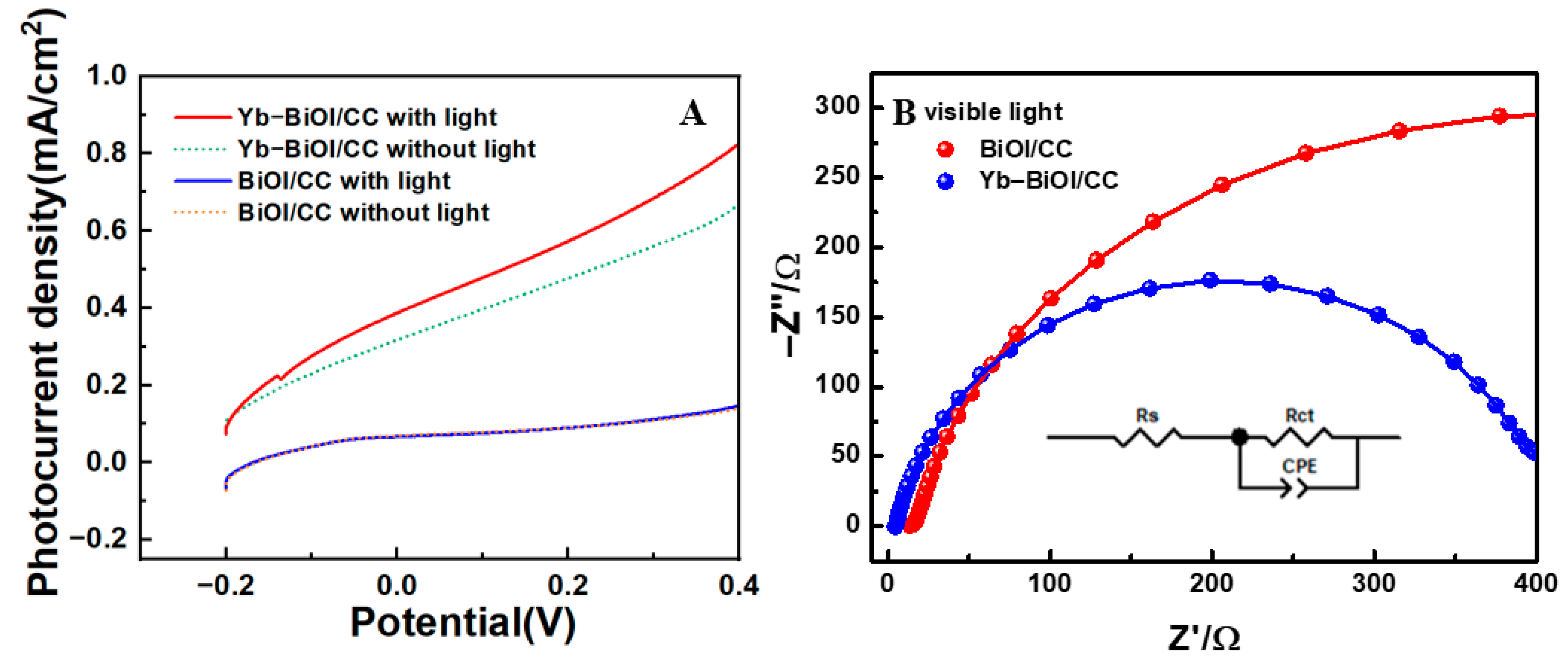
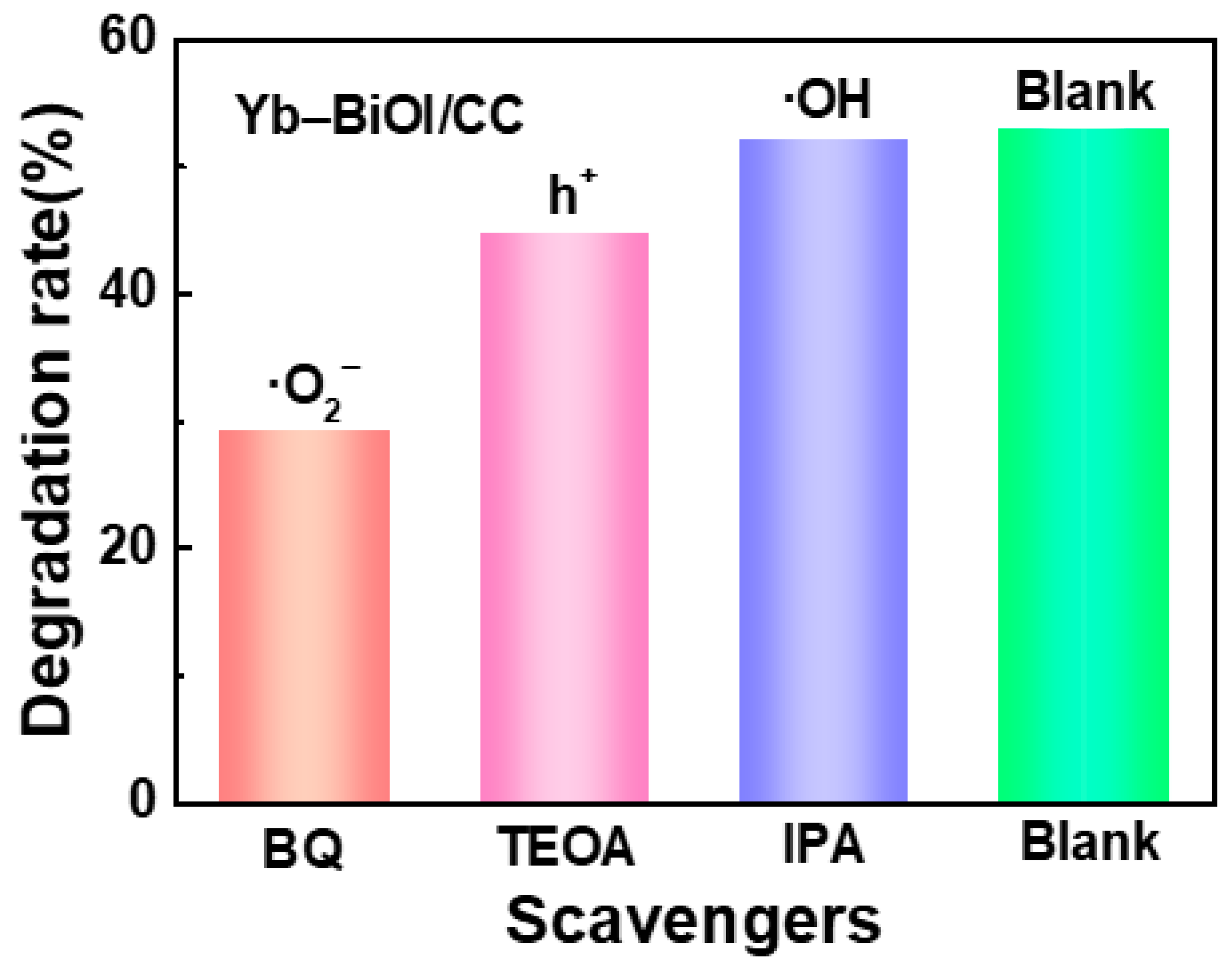

| Order | Photocatalyst | Dyes | Light | Time (min) | Degradation Rate | References |
|---|---|---|---|---|---|---|
| 1 | Mn-doped ZnO | MB | Visible | 60 | 100% | [35] |
| 2 | ZnO-Si | MB | Visible | 60 | 90% | [36] |
| 3 | ZnO | MB | UV | 180 | 96% | [37] |
| 4 | ZnO | MB | UV | 60 | 39.7% | [38] |
| 5 | Au-ZnO | MB | UV | 60 | 71% | [39] |
| 6 | SnO2-ZnO | MB | UV | 20 | 88% | [40] |
| 7 | CdS/ZnS | MB | Visible | 360 | 75% | [41] |
| 8 | BiOI/Bi4O5I2/Bi2O2CO3 | MB | Solar light | 45 | 97% | [42] |
| 9 | GO/CdS/CoFe2O4 | MB | Visible | 120 | 82% | [43] |
| 10 | Ag3PO4/MoS2 | MB | Visible | 60 | 98.2% | [44] |
| 11 | WO3/TiO2/CC | MB | Visible | 180 | 68% | [26] |
| 12 | N-ZnO/CDs | MB | UV | 60 | 58.2% | [45] |
| 13 | NiO/Ag/TiO2 | MB | Visible | 60 | 93.15% | [46] |
| 14 | Yb3+-BiOI/CC | MB | Visible | 180 | 52.87% | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, S.; Zhao, D.; Luo, R.; Liu, X.; Yang, J.; Xie, L.; Gao, X.; Jiang, L. Visible-Light Photocatalytic Degradation of Methylene Blue by Yb3+-Doped 3D Nanosheet Arrays BiOI Anchored on High-Chloride Fly Ash Composites. Inorganics 2025, 13, 147. https://doi.org/10.3390/inorganics13050147
Qiu S, Zhao D, Luo R, Liu X, Yang J, Xie L, Gao X, Jiang L. Visible-Light Photocatalytic Degradation of Methylene Blue by Yb3+-Doped 3D Nanosheet Arrays BiOI Anchored on High-Chloride Fly Ash Composites. Inorganics. 2025; 13(5):147. https://doi.org/10.3390/inorganics13050147
Chicago/Turabian StyleQiu, Shuxian, Danhua Zhao, Runtong Luo, Xiaohong Liu, Jianping Yang, Lijun Xie, Xingyuan Gao, and Liaochuan Jiang. 2025. "Visible-Light Photocatalytic Degradation of Methylene Blue by Yb3+-Doped 3D Nanosheet Arrays BiOI Anchored on High-Chloride Fly Ash Composites" Inorganics 13, no. 5: 147. https://doi.org/10.3390/inorganics13050147
APA StyleQiu, S., Zhao, D., Luo, R., Liu, X., Yang, J., Xie, L., Gao, X., & Jiang, L. (2025). Visible-Light Photocatalytic Degradation of Methylene Blue by Yb3+-Doped 3D Nanosheet Arrays BiOI Anchored on High-Chloride Fly Ash Composites. Inorganics, 13(5), 147. https://doi.org/10.3390/inorganics13050147








