Microstructure and Electrochemical Properties of Pure and Vanadium-Doped Li4Ti5O12 Nanoflakes for High Performance Supercapacitors
Abstract
1. Introduction
2. Results and Discussion
2.1. Studies of Pristine Li4Ti5O12Nanoflakes
2.1.1. XRD Analysis
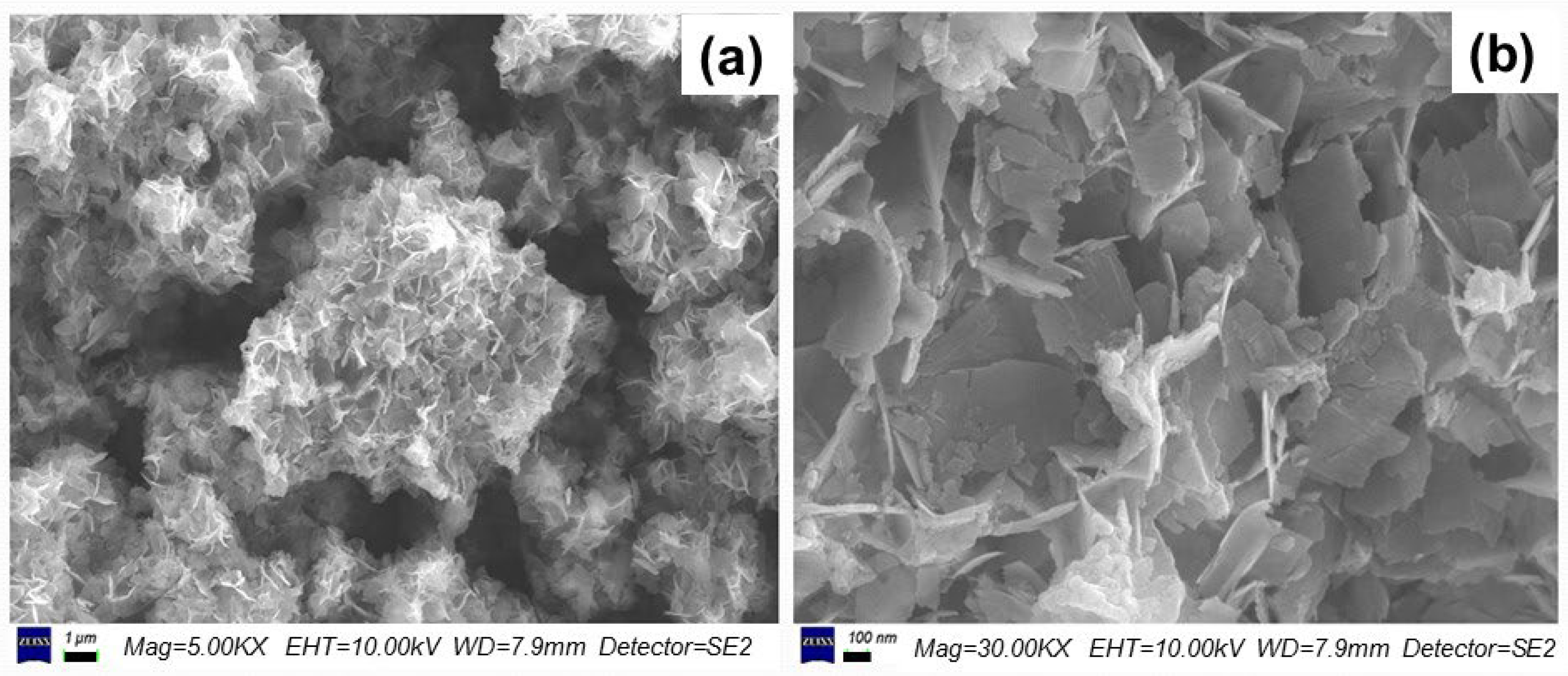
2.1.2. Surface Morphology Analysis
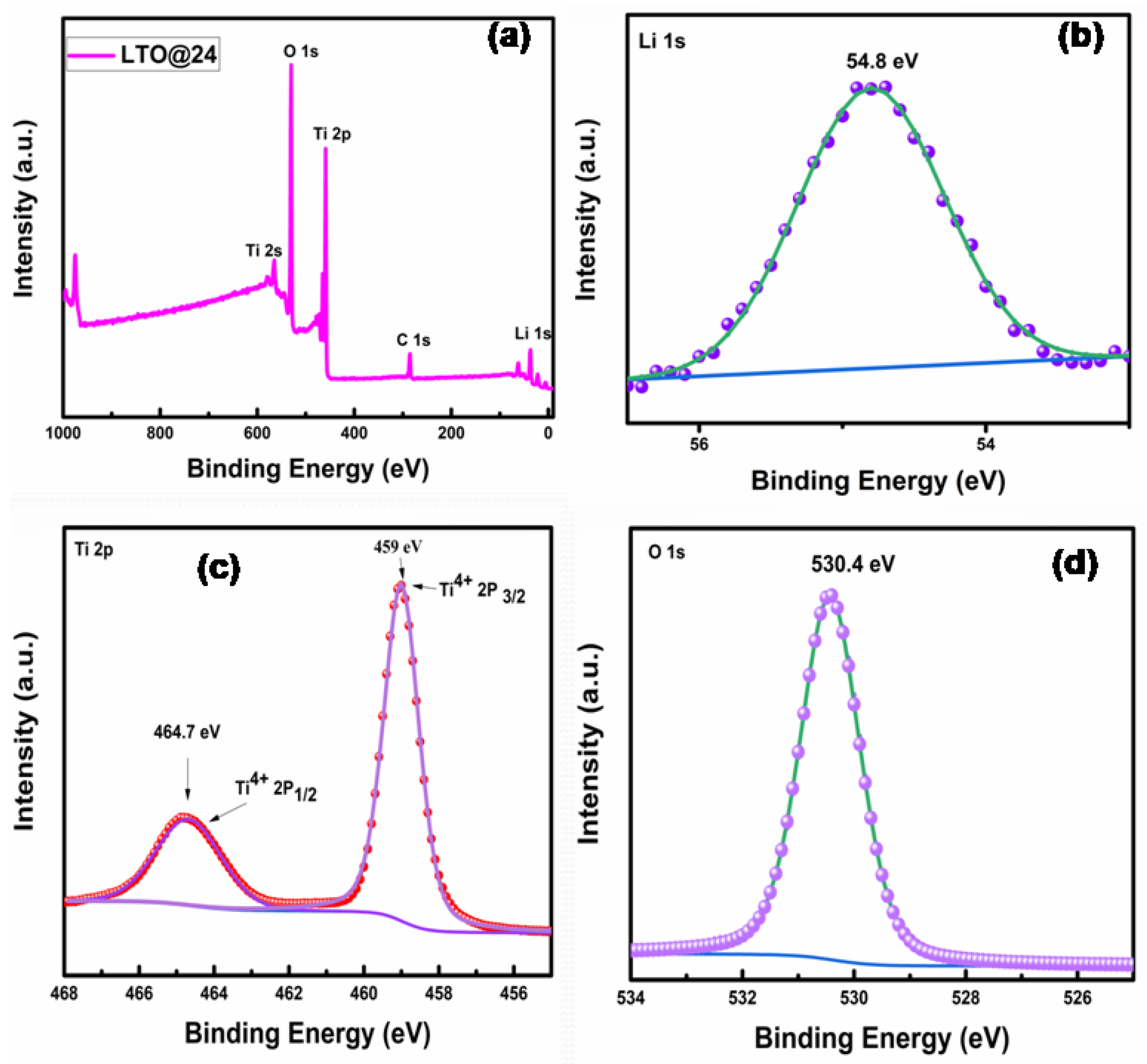
2.1.3. XPS Analysis
2.1.4. Electrochemical Analysis
2.2. Vanadium-Doped Li4Ti5O12 (V-LTO@24)
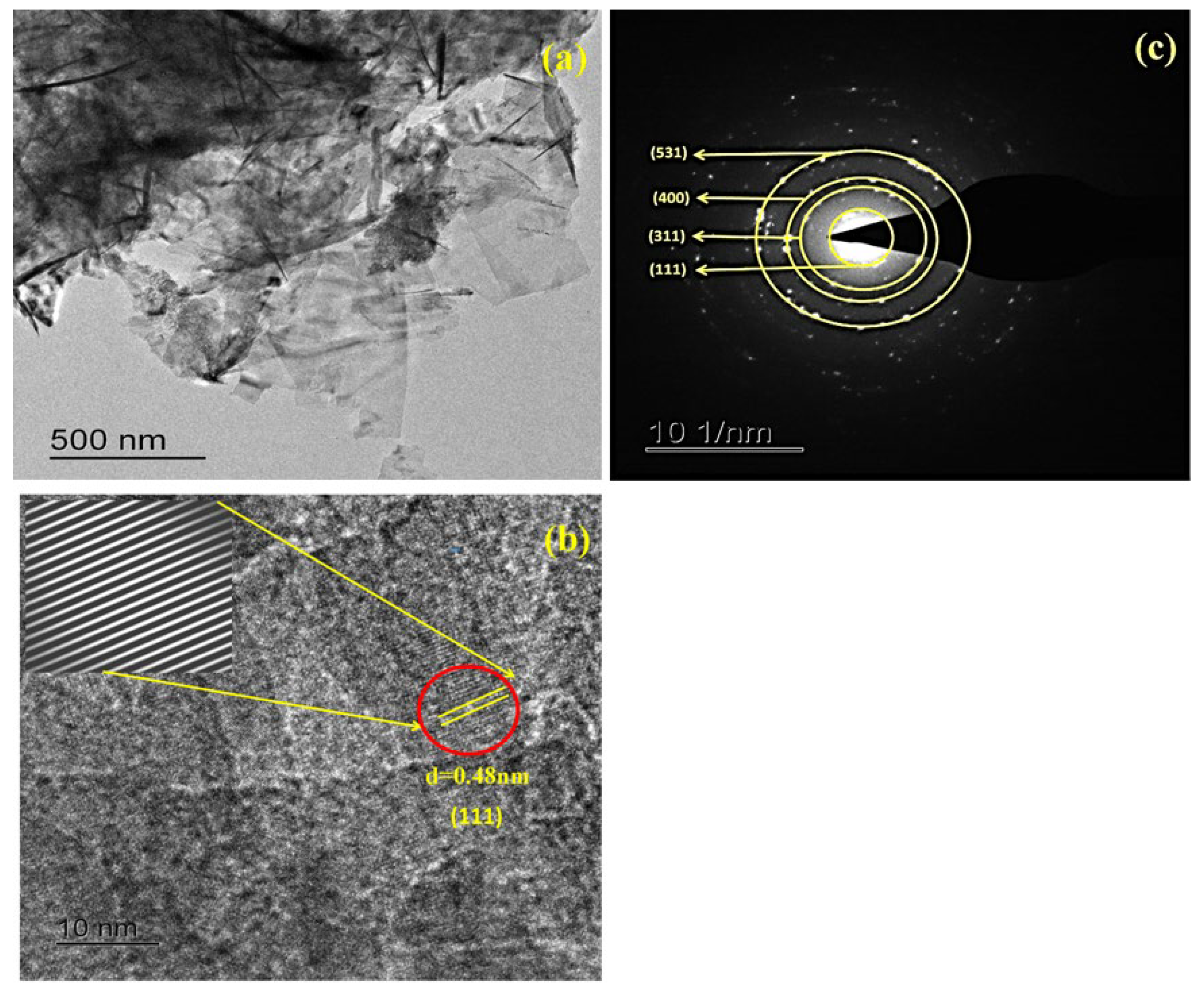
2.2.1. Structural Studies
2.2.2. Surface Morphology
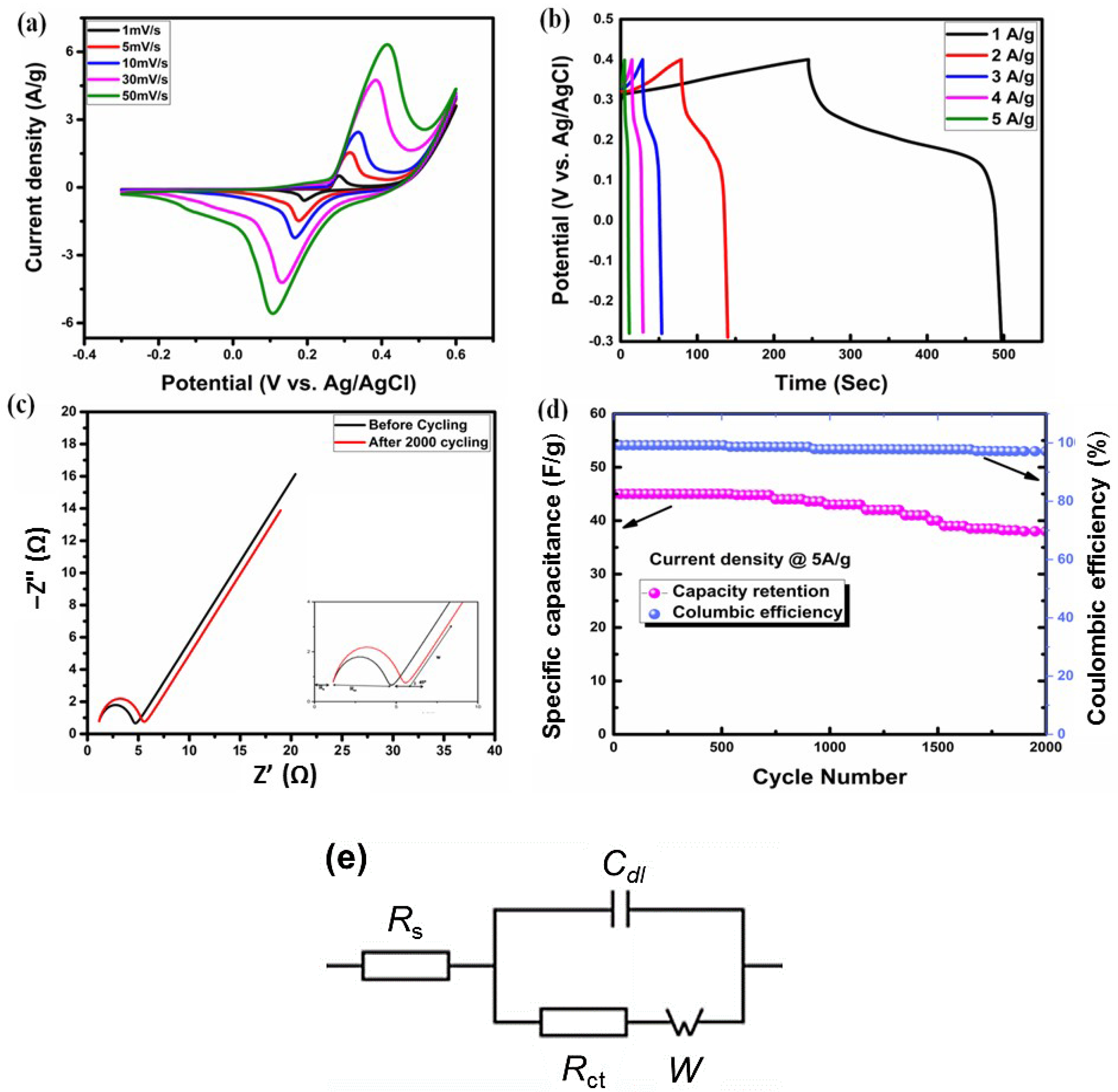
2.2.3. Electrochemical Analysis of V-LTO@24
2.3. Charge Storage Mechanism in LTO-Based Electrodes
3. Materials and Methods
3.1. Materials
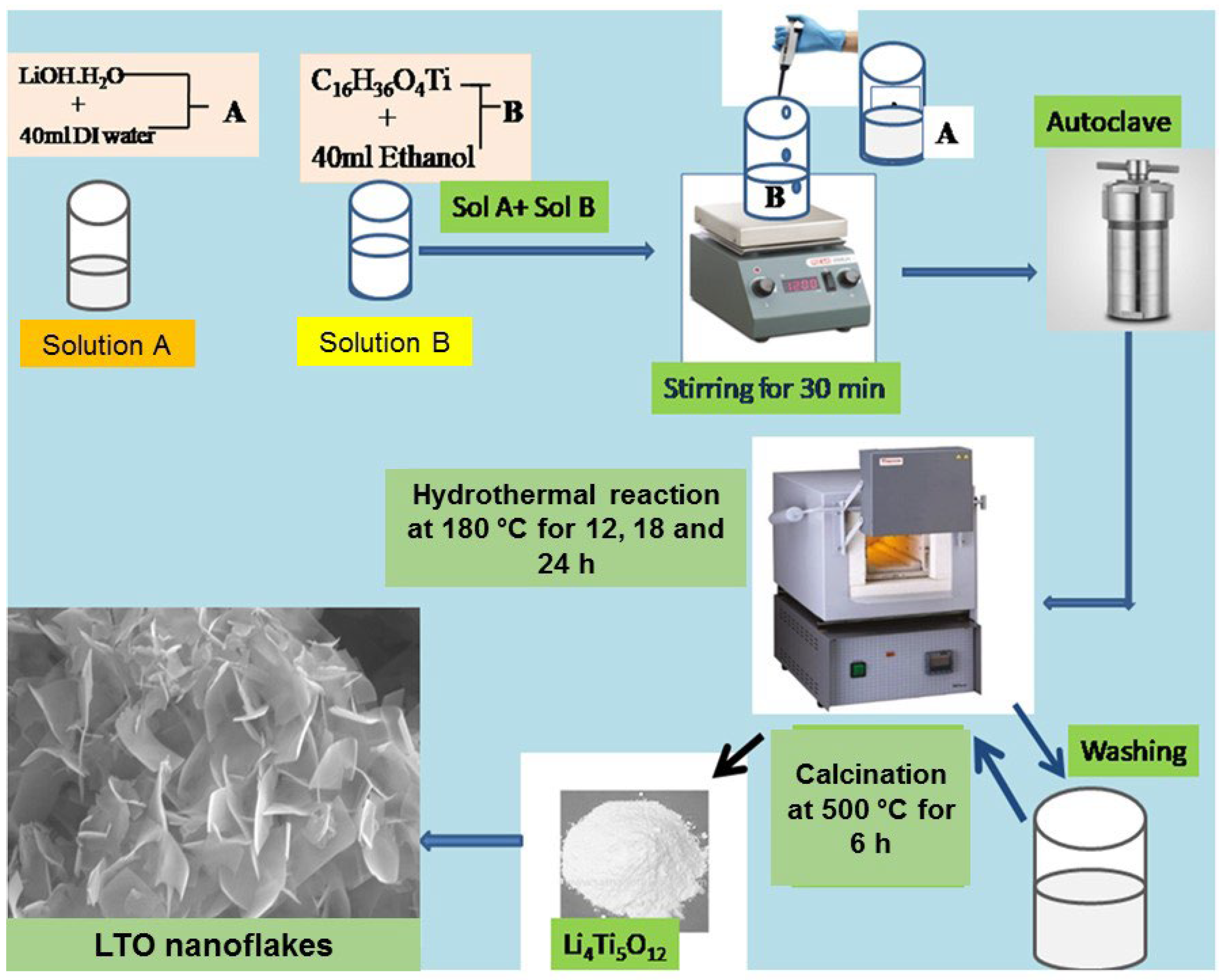
3.2. Preparation of Li4Ti5O12Nanomaterial
3.3. Preparation of V-LTO@24 (Li4Ti5−xVxO12)
3.4. Material Characterizations
3.5. Li4Ti5O12Electrode Preparation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, X.; Peng, L.; Wu, C.; Xie, Y. Two dimensional nanomaterials for flexible supercapacitors. Chem. Soc. Rev. 2014, 43, 3303–3323. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Casalongue, H.S.; Liang, Y.; Dai, H. Ni(OH)2nanoplates grown on graphene as advanced electrochemical pseudocapacitor materials. J. Am. Chem. Soc. 2010, 132, 7472–7477. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Katugampalage, T.R.; Khalid, M.; Ahmed, W.; Kaewsaneha, C.; Sreearunothai, P.; Opaprakasit, P. Microwave assisted synthesis of Mn3O4nanograins intercalated into reduced graphene oxide layers as cathode material for alternative clean power generation energy device. Sci. Rep. 2022, 12, 19043. [Google Scholar] [CrossRef]
- Li, H.; Tao, Y.; Zheng, X.; Luo, J.; Kang, F.; Cheng, H.M.; Yang, Q.H. Ultra-thick graphene bulk supercapacitor electrodes for compact energy storage. Energy Environ. Sci. 2016, 9, 3135–3142. [Google Scholar] [CrossRef]
- Lv, W.; Li, Z.; Deng, Y.; Yang, Q.H.; Kang, F. Graphene-based materials for electrochemical energy storage devices: Opportunities and challenges. Energy Storage Mater. 2016, 2, 107–138. [Google Scholar] [CrossRef]
- Sun, J.; Luo, B.; Li, H. A review on the conventional capacitors, supercapacitors, and emerging hybrid ion capacitors: Past, present, and future. Adv. Energy Sustain. Res. 2022, 3, 2100191. [Google Scholar] [CrossRef]
- Wang, J.; Dong, S.; Ding, B.; Wang, Y.; Hao, X.; Dou, H.; Xia, Y.; Zhang, X. Pseudocapacitive materials for electrochemical capacitors: From rational synthesis to capacitance optimization. Natl. Sci. Rev. 2017, 4, 71–90. [Google Scholar] [CrossRef]
- Pallavolu, M.R.; Nallapureddy, J.; Nallapureddy, R.R.; Neelima, G.; Yedluri, A.K.; Mandal, T.K.; Pejjai, B.; Joo, S.W. Self-assembled and highly faceted growth of Mo and V doped ZnOnanoflowers for high-performance supercapacitors. J. Alloys Compd. 2021, 886, 161234. [Google Scholar] [CrossRef]
- Mai, L.Q.; Yang, F.; Zhao, Y.L.; Xu, X.; Xu, L.; Luo, Y.Z. Hierarchical MnMoO4/CoMoO4heterostructured nanowires with enhanced supercapacitor performance. Nat. Commun. 2011, 2, 381. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A. Fabrication of Li4Ti5O12 (LTO) as anode material for Li-ion batteries. Micromachines 2024, 15, 310. [Google Scholar] [CrossRef]
- Yu, L.; Chen, G.Z. Supercapatteries as high-performance electrochemical energy storage devices. Electrochem. Energy Rev. 2020, 3, 271–285. [Google Scholar] [CrossRef]
- Raut, B.; Ahmed, M.S.; Kim, H.Y.; Rahman Khan, M.M.; Bari, G.A.R.; Islam, M.; Nam, K.W. Battery-type transition metal oxides in hybrid supercapacitors: Synthesis and applications. Batteries 2025, 11, 60. [Google Scholar] [CrossRef]
- Devadas, A.; Baranton, S.; Napporn, T.W.; Coutanceau, C. Tailoring of RuO2 nanoparticles by microwave assisted “Instant method” for energy storage applications. J. Power Sources 2011, 196, 4044–4053. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A. Nanostructured MnO2 as electrode materials for energy storage. Nanomaterials 2017, 7, 396. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, S.S.; Gopalakrishnan, A.; Subashchandrabose, R.; Badhulika, S. Phytogenic generation of NiO nanoparticles as green-electrode material for high performance asymmetric supercapacitor applications. J. Energy Storage 2021, 37, 102412. [Google Scholar] [CrossRef]
- Haritha, B.; Deepak, M.; Hussain, O.M.; Julien, C.M. Morphological engineering of battery-type cobalt oxide electrodes for high-performance supercapacitors. Physchem 2025, 5, 11. [Google Scholar] [CrossRef]
- Nunna, G.P.; Merum, D.; Ko, T.J.; Choi, J.; Hussain, O.M. High-performance MoO3supercapacitor electrodes: Influence of reaction parameters on phase, microstructure, and electrochemical properties. Int. J. Energy Res. 2022, 46, 5973–5987. [Google Scholar] [CrossRef]
- Zhang, J.C.; Liu, Z.D.; Zeng, C.H.; Luo, J.W.; Deng, Y.D.; Cui, X.Y.; Chen, Y.N. High-voltage LiCoO2 cathodes for high-energy-density lithium-ion battery. Rare Met. 2022, 41, 3946–3956. [Google Scholar] [CrossRef]
- Abou-Rjeily, J.; Bezza, I.; Laziz, N.A.; Autret-Lambert, C.; Sougrati, M.T.; Ghamouss, F. High-rate cyclability and stability of LiMn2O4 cathode materials for lithium-ion batteries from low-cost natural β− MnO2. Energy Storage Mater. 2020, 26, 423–432. [Google Scholar] [CrossRef]
- Borhani-Haghighi, S.; Kieschnick, M.; Motemani, Y.; Savan, A.; Rogalla, D.; Becker, H.W.; Meijer, J.; Ludwig, A. High-throughput compositional and structural evaluation of a Lia(NixMnyCoz)Or thin film battery materials library. ACS Comb. Sci. 2013, 15, 401–409. [Google Scholar] [CrossRef]
- Lakshmi-Narayana, A.; Hussain, O.M.; Mauger, A.; Julien, C.M. Transport properties of nanostructured Li2TiO3 anode material synthesized by hydrothermal method. Sci 2019, 1, 56. [Google Scholar] [CrossRef]
- Ohzuku, T.; Ueda, A.; Yamamoto, N. Zero-strain insertion material of Li[Li1/3Ti5/3]O4 for rechargeable lithium cells. J. Electrochem. Soc. 1995, 142, 1431–1435. [Google Scholar] [CrossRef]
- Ge, H.; Xie, L.; Wang, C.; Pan, R.; Huang, B.; Sun, Z.; Cao, X.; Yang, T.; Wu, G. Advanced pseudocapacitive lithium titanate towards next-generation energy storage devices. J.Energy Chem. 2025, 103, 773–792. [Google Scholar] [CrossRef]
- Sun, C.; Ji, X.; Weng, S.; Li, R.; Huang, X.; Zhu, C.; Xiao, X.; Deng, T.; Fan, L.; Chen, L.; et al. 50C fast-charge Li-ion batteries using a graphite anode. Adv. Mater. 2022, 34, 2206020. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Z.; Cheng, Y.; Zhang, Y.; Liu, W.; Su, J.; Liu, N.; Gao, Y. Revealing the phase-transition dynamics and mechanism in a spinel Li4Ti5O12 anode material through in situ electron microscopy. ACS Appl. Mater. Interfaces 2020, 12, 20874–20881. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.L.; Wu, X.; Huang, K.J. High-performance supercapacitor based on three-dimensional flower-shaped Li4Ti5O12-graphene hybrid and pine needles derived honeycomb carbon. J. Colloid Interface Sci. 2018, 529, 171–179. [Google Scholar] [CrossRef]
- Lee, B.; Yoon, J.R. Preparation and characteristics of Li4Ti5O12 with various dopants as anode electrode for hybrid supercapacitor. Curr. Appl. Phys. 2013, 13, 1350–1353. [Google Scholar] [CrossRef]
- Saxena, S.; Sil, A. Role of calcination atmosphere in vanadium doped Li4Ti5O12 for lithium ion battery anode material. Mater. Res. Bull. 2017, 96, 449–457. [Google Scholar] [CrossRef]
- Khairy, M.; Bayoumy, W.A.; Qasim, K.F.; El-Shereafy, E.; Mousa, M.A. Ternary V-doped Li4Ti5O12-polyaniline-graphene nanostructure with enhanced electrochemical capacitance performance. Mater. Sci. Eng. B 2021, 271, 115312. [Google Scholar] [CrossRef]
- Batsukh, I.; Lkhagvajav, S.; Adiya, M.; Galsan, S.; Bat-Erdene, M.; Myagmarsereejid, P. Investigation of structural, optical, and electrochemical properties of niobium-doped Li4Ti5O12 for high-performance aqueous capacitor electrode. Ceram. Int. 2023, 49, 26313–26321. [Google Scholar] [CrossRef]
- Tian, Q.; Chen, P.; Zhang, Z.; Yang, L. Achievement of significantly improved lithium storage for novel clew-like Li4Ti5O12 anode assembled by ultrafine nanowires. J. Power Sources 2017, 350, 49–55. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, J.M. Improved performances of hybrid supercapacitors using granule Li4Ti5O12/activated carbon composite anode. Mater. Lett. 2018, 228, 220–223. [Google Scholar] [CrossRef]
- Putjuso, T.; Putjuso, S.; Karaphun, A.; Swatsitang, E. Influence of Li concentration on structural, morphological and electrochemical properties of anatase-TiO2 nanoparticles. Sci. Rep. 2024, 14, 11200. [Google Scholar] [CrossRef]
- Khanna, S.; Marathey, P.; Paneliya, S.; Chaudhari, R.; Vora, J. Fabrication of rutile–TiO2 nanowire on shape memory alloy: A potential material for energy storage application. Mater. Today Proc. 2022, 50, 11–16. [Google Scholar] [CrossRef]
- Chandra Sekhar, J.; Merum, D.; Hussain, O.M. Microstructural and supercapacitive performance of cubic spinel Li4Ti5O12nanocomposite. Eur. J. Mater. Sci. Eng. 2020, 5, 222–233. [Google Scholar]
- Xing, L.L.; Huang, K.J.; Cao, S.X.; Pang, H. Chestnut shell-like Li4Ti5O12 hollow spheres for high-performance aqueous asymmetric supercapacitors. Chem. Eng. J. 2018, 332, 253–259. [Google Scholar] [CrossRef]
- Sha, Y.; Zhao, B.; Ran, R.; Cai, R.; Shao, Z. Synthesis of well-crystallized Li4Ti5O12nanoplates for lithium-ion batteries with outstanding rate capability and cycling stability. J. Mater. Chem. A 2013, 1, 13233–13243. [Google Scholar] [CrossRef]
- Kalawa, O.; Sichumsaeng, T.; Kidkhunthod, P.; Chanlek, N.; Maensiri, S. Ni-doped MnCo2O4 nanoparticles as electrode material for supercapacitors. J. Mater. Sci. Mater. Electron. 2022, 33, 4869–4886. [Google Scholar] [CrossRef]
- Aldon, L.; Kubiak, P.; Womes, M.; Jumas, J.C.; Olivier-Fourcade, J.; Tirado, J.L.; Corredor, J.I.; Pérez Vicente, C. Chemical and electrochemical Li-insertion into the Li4Ti5O12 spinel. Chem. Mater. 2004, 16, 5721–5725. [Google Scholar] [CrossRef]
- Yan, G.; Fang, H.; Zhao, H.; Li, G.; Yang, Y.; Li, L. Ball milling-assisted sol–gel route to Li4Ti5O12 and its electrochemical properties. J. Alloys Compd. 2009, 470, 544–547. [Google Scholar] [CrossRef]
- Zaghib, K.; Simoneau, M.; Armand, M.; Gauthier, M. Electrochemical study of Li4Ti5O12 as negative electrode for Li-ion polymer rechargeable batteries. J. Power Sources 1999, 81, 300–305. [Google Scholar] [CrossRef]
- Shinde, S.K.; Jalak, M.B.; Ghodake, G.S.; Maile, N.C.; Kumbhar, V.S.; Lee, D.S.; Fulari, V.J.; Kim, D.Y. Chemically synthesized nanoflakes-like NiCo2S4 electrodes for high-performance supercapacitor application. Appl. Surface Sci. 2019, 466, 822–829. [Google Scholar] [CrossRef]
- Xia, H.; Xu, Q.; Zhang, J. Recent progress on two-dimensional nanoflake ensembles for energy storage applications. Nano-Micro Lett. 2018, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Le, P.A.; Le, V.Q.; Tran, T.L.; Nguyen, N.T.; Phung, T.V.B.; Dinh, V.A. Two-dimensional NH4V3O8nanoflakes as efficient energy conversion and storage materials for the hydrogen evolution reaction and supercapacitors. ACS Omega 2022, 7, 25433–25442. [Google Scholar] [CrossRef]
- Pawar, S.M.; Kim, J.; Inamdar, A.I.; Woo, H.; Jo, Y.; Pawar, B.S.; Cho, S.; Kim, H.; Im, H. Multi-functional reactively-sputtered copper oxide electrodes for supercapacitor and electro-catalyst in direct methanol fuel cell applications. Sci. Rep. 2016, 6, 21310. [Google Scholar] [CrossRef]
- Zhu, J.; Song, D.; Pu, T.; Li, J.; Huang, B.; Wang, W.; Zhao, C.; Xie, L.; Chen, L. Two-dimensional porous ZnCo2O4 thin sheets assembled by 3D nanoflake array with enhanced performance for aqueous asymmetric supercapacitor. Chem. Eng. J. 2018, 336, 679–689. [Google Scholar] [CrossRef]
- Naresh, B.; Kuchi, C.; Rajasekhar, D.; Reddy, P.S. Solvothermal synthesis of MnCo2O4 microspheres for high-performance electrochemical supercapacitors. Colloids Surf. A: Physicochem. Eng. Asp. 2022, 640, 128443. [Google Scholar] [CrossRef]
- Saraf, M.; Natarajan, K.; Mobin, S.M. Emerging robust heterostructure of MoS2–rGO for high-performance supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 16588–16595. [Google Scholar] [CrossRef]
- Sheokand, S.; Kumar, P.; Jabeen, S.; Samra, K.S. 3D highly porous microspherical morphology of NiO nanoparticles for supercapacitor application. J. Solid State Electrochem. 2023, 27, 727–738. [Google Scholar] [CrossRef]
- Shaik, D.P.; Kumar, M.S.; Reddy, P.N.K.; Hussain, O.M. High electrochemical performance of spinel Mn3O4 over Co3O4nanocrystals. J. Molecular Struct. 2021, 1241, 130619. [Google Scholar] [CrossRef]
- Pawar, S.M.; Inamdar, A.I.; Gurav, K.V.; Jo, Y.; Kim, H.; Kim, J.H.; Im, H. Effect of oxidant on the structural, morphological and supercapacitive properties of nickel hydroxide nanoflakes electrode films. Mater. Lett. 2015, 141, 336–339. [Google Scholar] [CrossRef]
- Xi, Y.; Xiao, Z.; Lv, H.; Sun, H.; Zhai, S.; An, Q. Construction of CuO/Cu-nanoflowers loaded on chitosan-derived porous carbon for high energy density supercapacitors. J. Colloid Interface Sci. 2023, 630, 525–534. [Google Scholar] [CrossRef]
- Merum, D.; Nallapureddy, R.R.; Pallavolu, M.R.; Mandal, T.K.; Gutturu, R.R.; Parvin, N.; Banerjee, A.N.; Joo, S.W. Pseudocapacitive performance of freestanding Ni3V2O8nanosheets for high energy and power density asymmetric supercapacitors. ACS Appl. Energy Mater. 2022, 5, 5561–5578. [Google Scholar] [CrossRef]
- Pallavolu, M.R.; Banerjee, A.N.; Joo, S.W. Battery-type behavior of Al-doped CuO nanoflakes to fabricate a high-performance hybrid supercapacitor device for superior energy storage applications. Coatings 2023, 13, 1337. [Google Scholar] [CrossRef]
- Ajay, A.; Paravannoor, A.; Joseph, J.; Anopp, A.V.; Nair, S.V.; Balakrishnan, A. 2 D amorphous frameworks of NiMoO4 for supercapacitors: Defining the role of surface and bulk controlled diffusion processes. Appl. Surface Sci. 2015, 326, 39–47. [Google Scholar] [CrossRef]
- Hussain, M.N.; Inayat, A.; Ansir, R.; Naveed, A.; Abbas, S.M.; Haider, A.; Shah, S.M. Probing the synergy of Ni(OH)2/NiO nanoparticles supported on rGO for battery-type supercapacitors. Energy Technol. 2024, 12, 2300854. [Google Scholar] [CrossRef]
- Gao, L.; Huang, D.; Shen, Y.; Wang, M. Rutile-TiO2decorated Li4Ti5O12nanosheet arrays with 3D interconnected architecture as anodes for high performance hybrid supercapacitors. J. Mater. Chem. A 2015, 3, 23570–23576. [Google Scholar] [CrossRef]
- Ni, J.; Yang, L.; Wang, H.; Gao, L. A high-performance hybrid supercapacitor with Li4Ti5O12-C nano-composite prepared by in situ and ex situ carbon modification. J. Solid State Electrochem. 2012, 16, 2791–2796. [Google Scholar] [CrossRef]
- Zuo, W.; Wang, C.; Li, Y.; Liu, J. Directly grown nanostructured electrodes for high volumetric energy density binder-free hybrid supercapacitors: A case study of CNTs//Li4Ti5O12. Sci. Rep. 2015, 5, 7780. [Google Scholar] [CrossRef]
- Gangaja, B.; Nair, S.V.; Santhanagopalan, D. Interface-engineered Li4Ti5O12–TiO2 dual-phase nanoparticles and CNT additive for supercapacitor-like high-power Li-ion battery applications. Nanotechnology 2018, 29, 095402. [Google Scholar] [CrossRef]




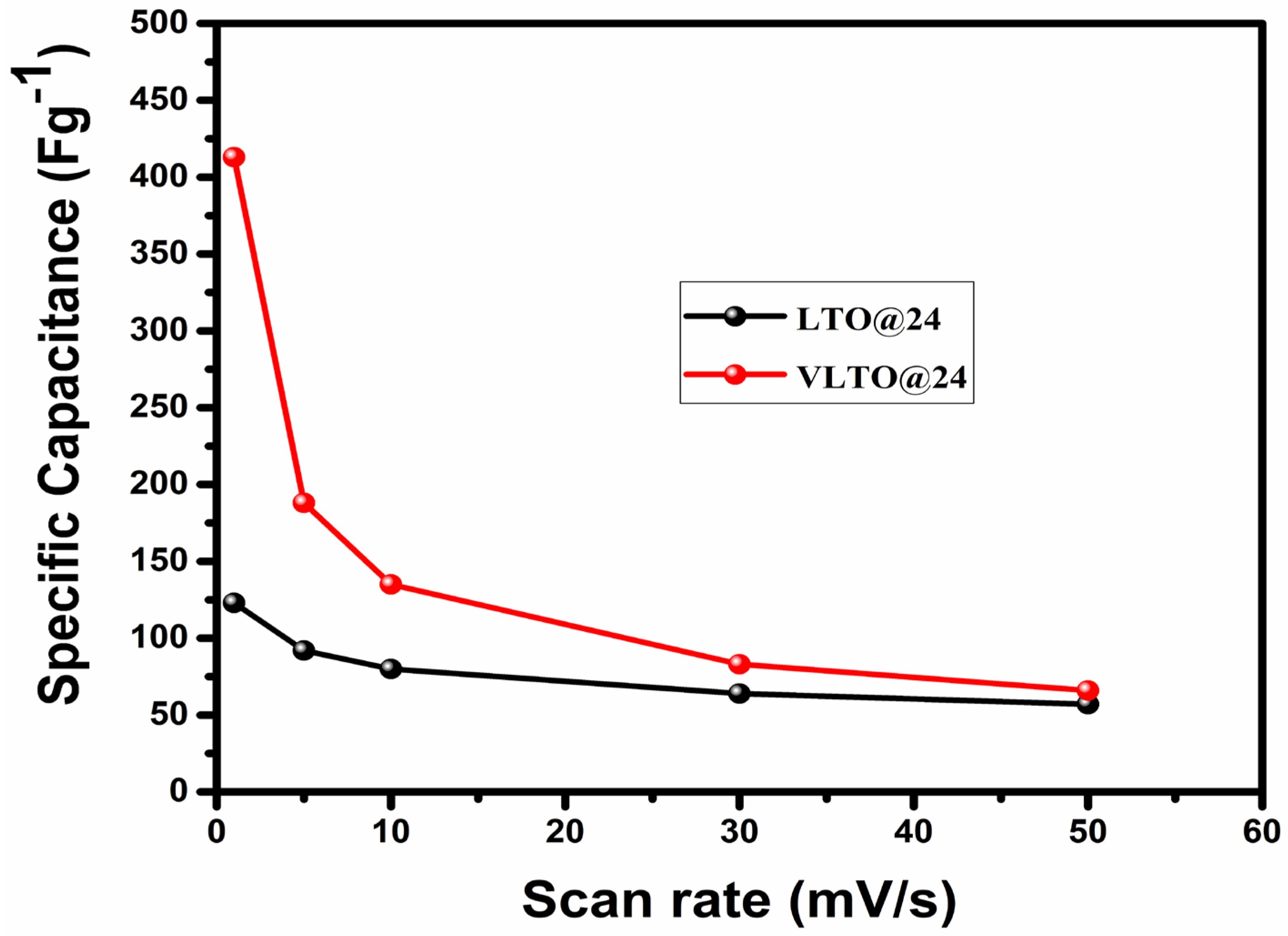

| Sample | Crystallite Size (nm) | Dislocation Density (δ) | Microstrain (rd) | Lattice Parameter (Å) | Unit Volume (Å3) |
|---|---|---|---|---|---|
| LTO@12 | 11 | 8.27652 × 1015 | 0.009933 | 8.4117(0) | 595.18 |
| LTO@18 | 12 | 6.98751 × 1015 | 0.008899 | 8.4018(2) | 593.08 |
| LTO@24 | 13 | 6.31196 × 1015 | 0.00827 | 8.3671(8) | 585.76 |
| V-LTO@24 | 11.8 | 7.7152 × 1015 | 0.0088 | 8.3401(2) | 580.11 |
| Sample | Capacitance | Retention Over Cycling | Ref. |
|---|---|---|---|
| V-doped LTO | 179 mAh g−1 @ 1C | 95% (300) | [29] |
| Granule-LTO powders | 63 F g−1 @ 0.5 A g−1 | 92.8% (7000) @ 3 A g−1 | [32] |
| SSR synthesized nano-LTO | 265 F g−1 @ 0.5 A g−1 | 81% (5000) @ 0.5 A g−1 | [35] |
| R-TiO2 decorated LTO | 143 mAh g−1 @30C | 92.3% (3000) | [57] |
| 3D chestnut shell-like LTO | 653 F g−1 @ 1 A g−1 | 88.5% (4000) | [36] |
| C-modified LTO | 83 F g−1 @ 2C | 84% (9000) @0.98 A g−1 | [58] |
| LTO nanowire | 125 F g−1 @ 0.55 mA cm−2 | 95% (400) @ 0.4 mA cm−2 | [59] |
| LTO–TiO2 nanoparticles | 174 mAh g−1 @ 2 A g−1 | 85% (3000) @ 2 A g−1 | [60] |
| Hydrothermal LTO@24 | 357 F g−1 @ 1 A g−1 | 98.5% (2000) @ 5 A g−1 | this work |
| Hydrothermal V-LTO@24 | 442 F g−1 @ 1 A g−1 | 99.8% (2000) @ 5 A g−1 | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deepak, M.; Hussain, O.M.; Julien, C.M. Microstructure and Electrochemical Properties of Pure and Vanadium-Doped Li4Ti5O12 Nanoflakes for High Performance Supercapacitors. Inorganics 2025, 13, 223. https://doi.org/10.3390/inorganics13070223
Deepak M, Hussain OM, Julien CM. Microstructure and Electrochemical Properties of Pure and Vanadium-Doped Li4Ti5O12 Nanoflakes for High Performance Supercapacitors. Inorganics. 2025; 13(7):223. https://doi.org/10.3390/inorganics13070223
Chicago/Turabian StyleDeepak, Mudda, Obili M. Hussain, and Christian M. Julien. 2025. "Microstructure and Electrochemical Properties of Pure and Vanadium-Doped Li4Ti5O12 Nanoflakes for High Performance Supercapacitors" Inorganics 13, no. 7: 223. https://doi.org/10.3390/inorganics13070223
APA StyleDeepak, M., Hussain, O. M., & Julien, C. M. (2025). Microstructure and Electrochemical Properties of Pure and Vanadium-Doped Li4Ti5O12 Nanoflakes for High Performance Supercapacitors. Inorganics, 13(7), 223. https://doi.org/10.3390/inorganics13070223









