Enhanced Photocatalytic Performances and Mechanistic Insights for Novel Ag-Bridged Dual Z-Scheme AgI/Ag3PO4/WO3 Composites
Abstract
1. Introduction
2. Results and Discussion
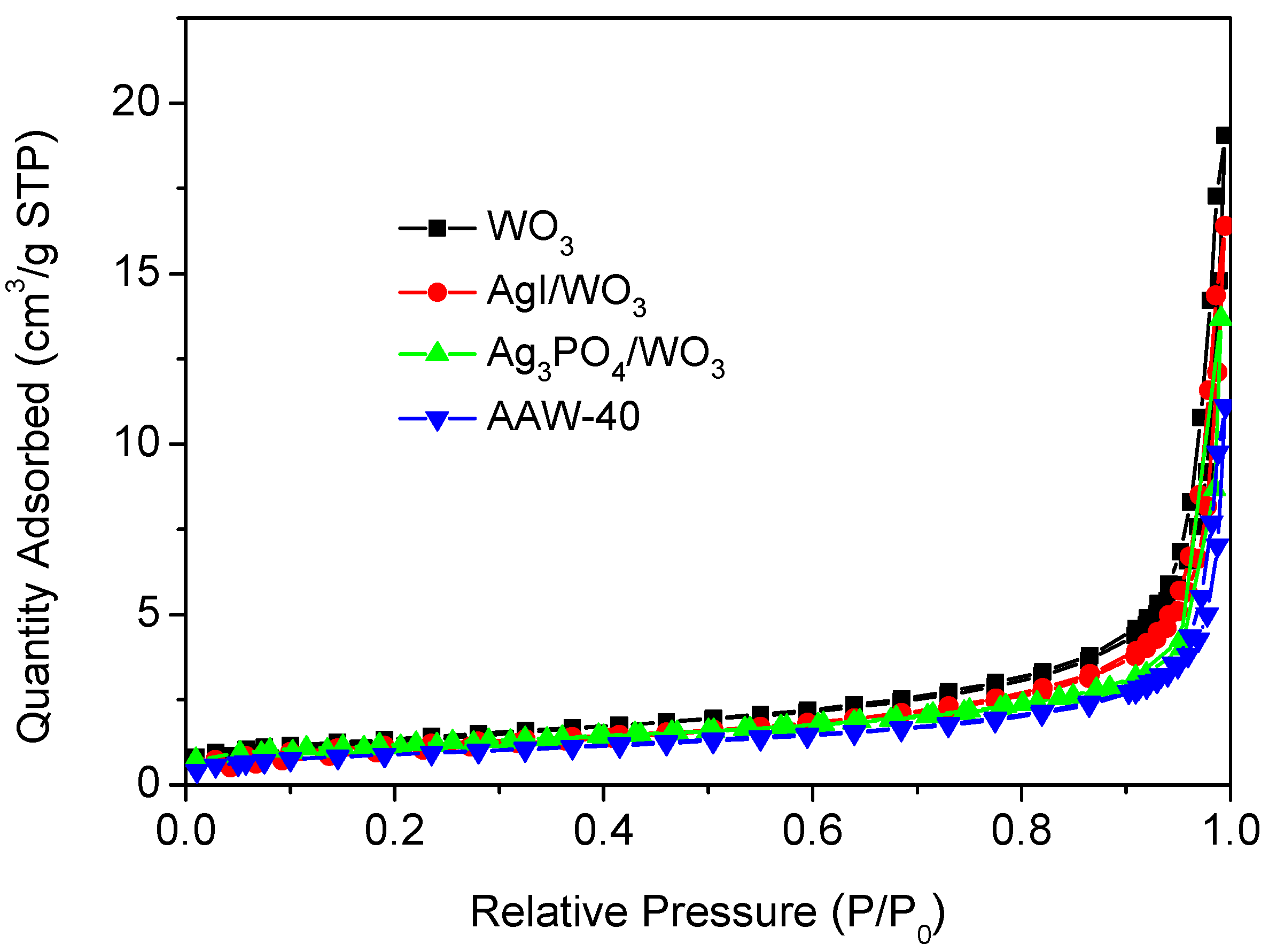
2.1. Characterization of Photocatalysts
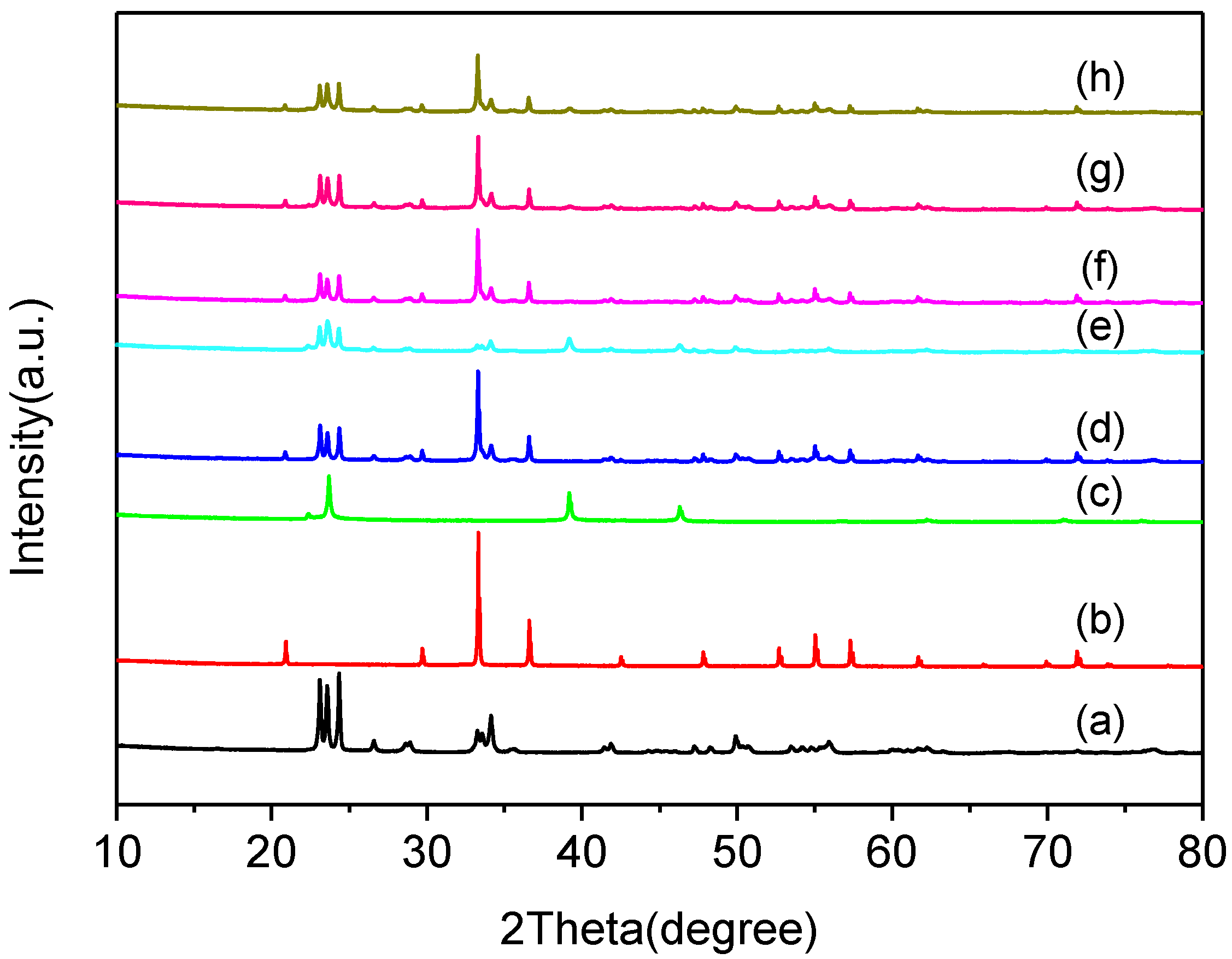
2.1.1. XRD Analysis
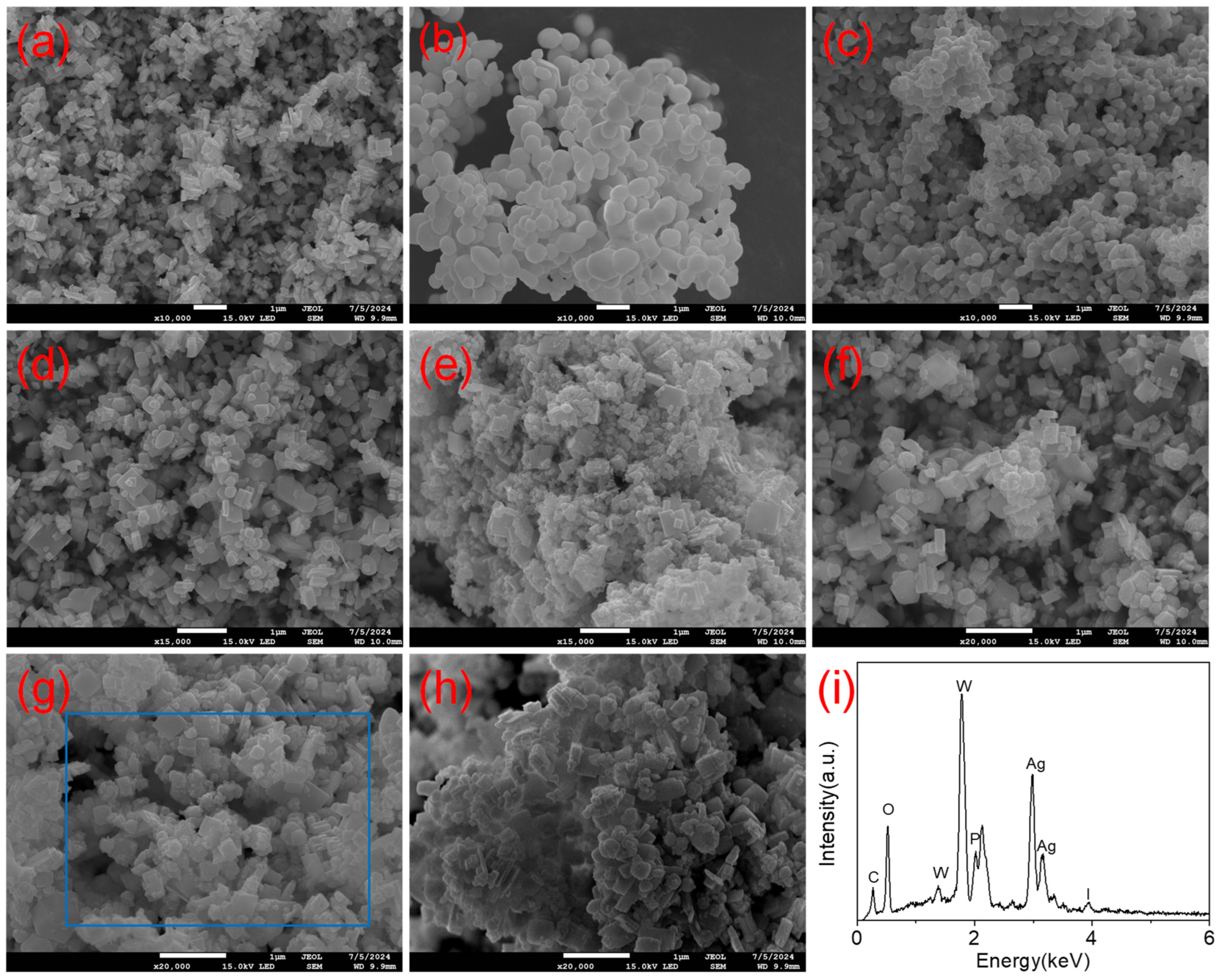
2.1.2. SEM Analysis
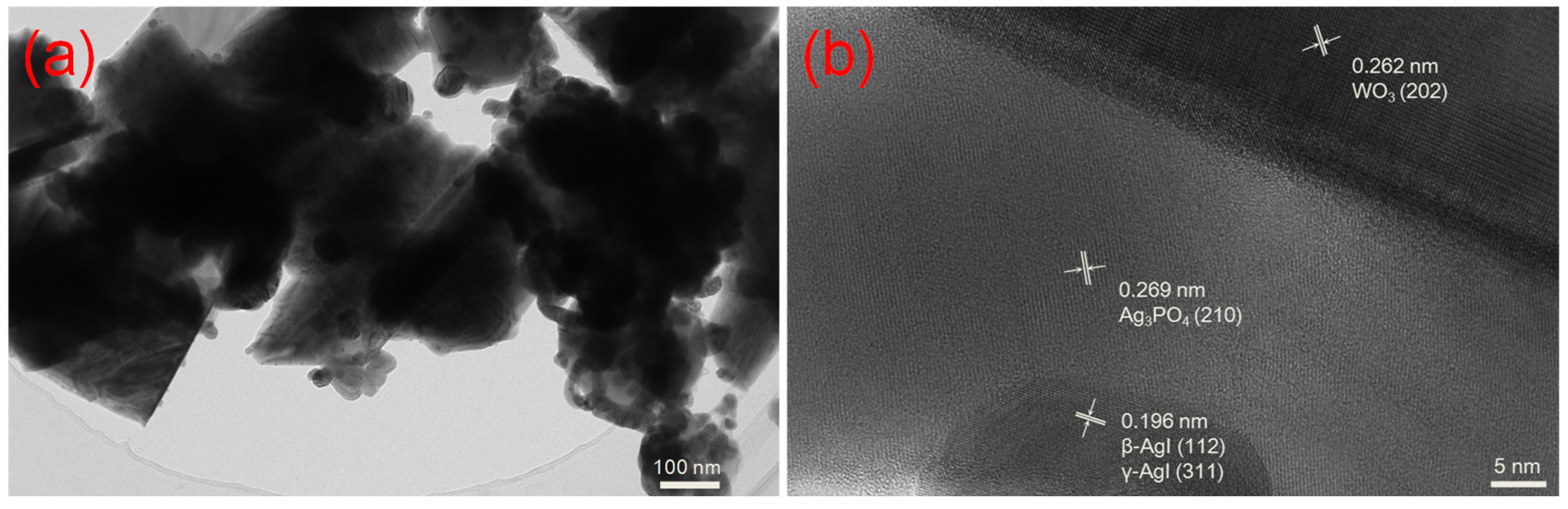
2.1.3. TEM Analysis
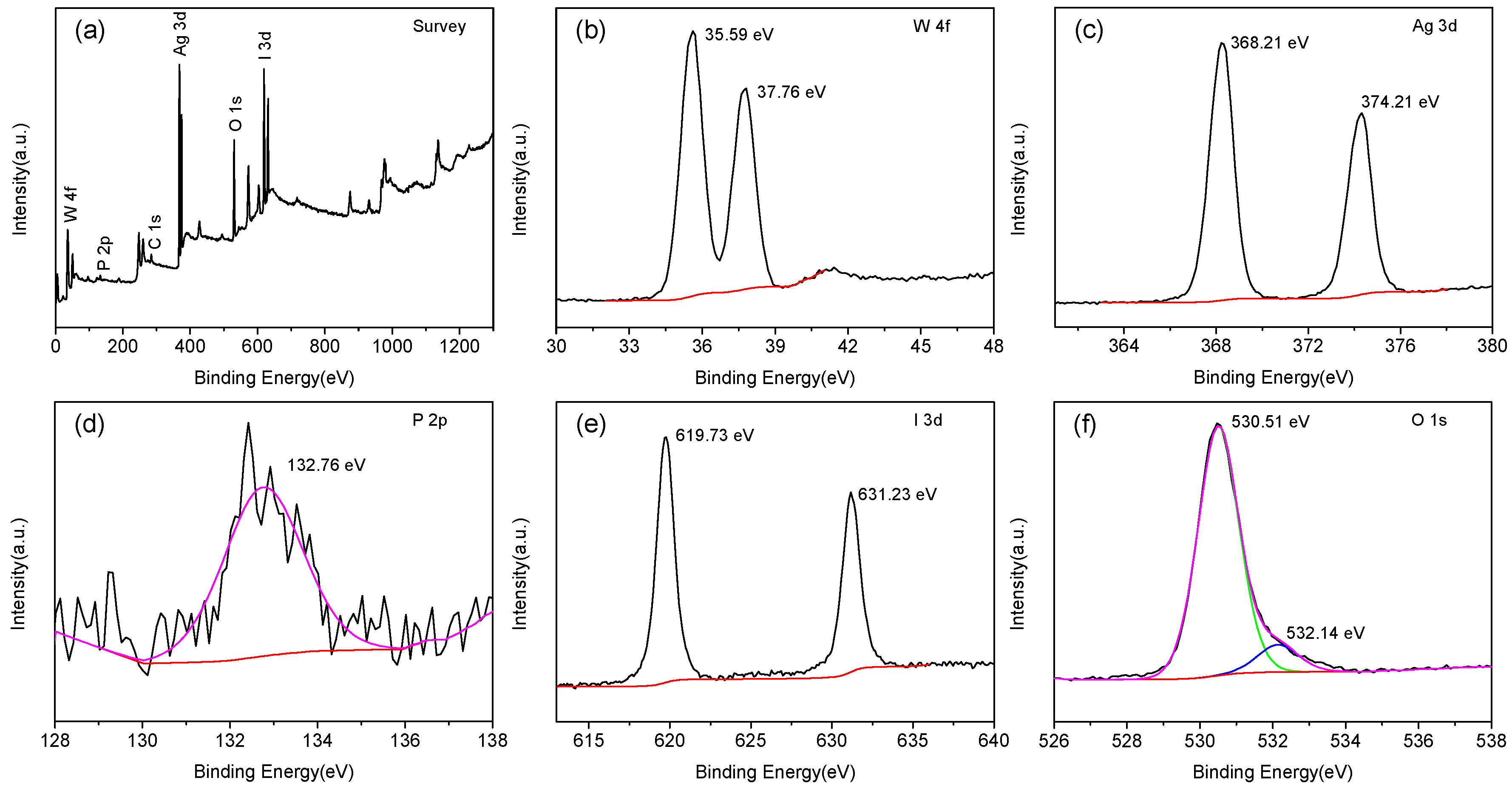
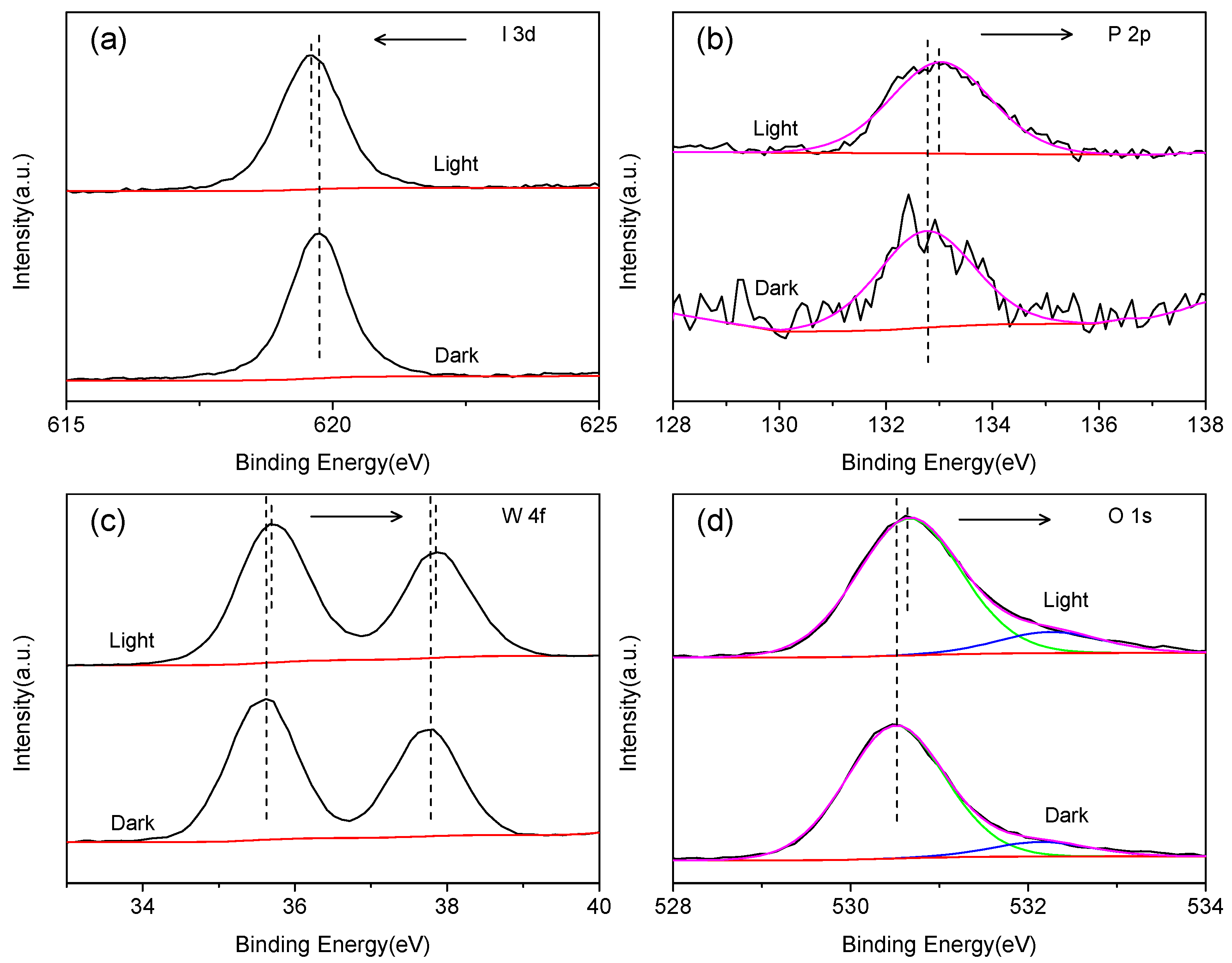
2.1.4. XPS Analysis
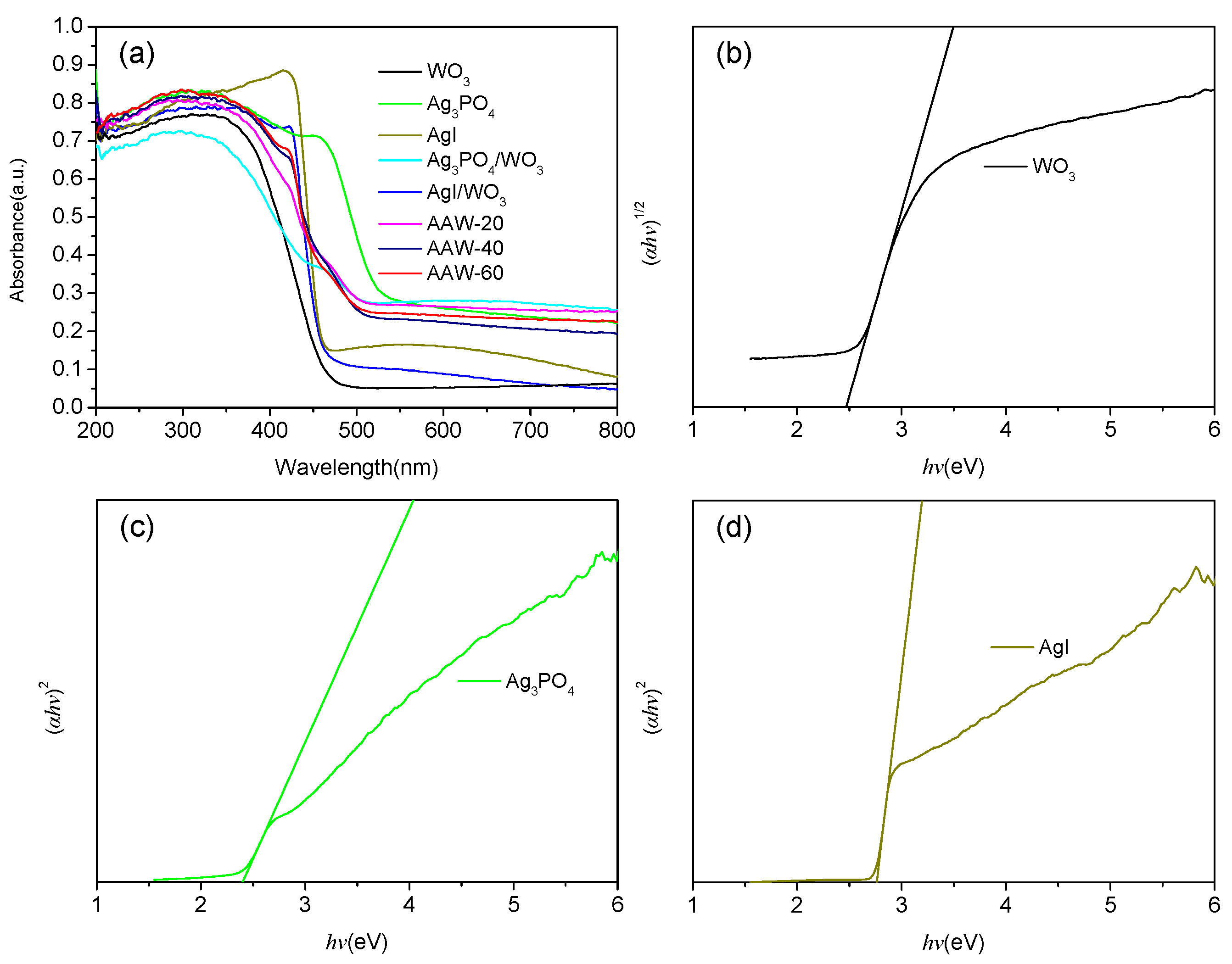
2.1.5. UV–Vis DRS Analysis
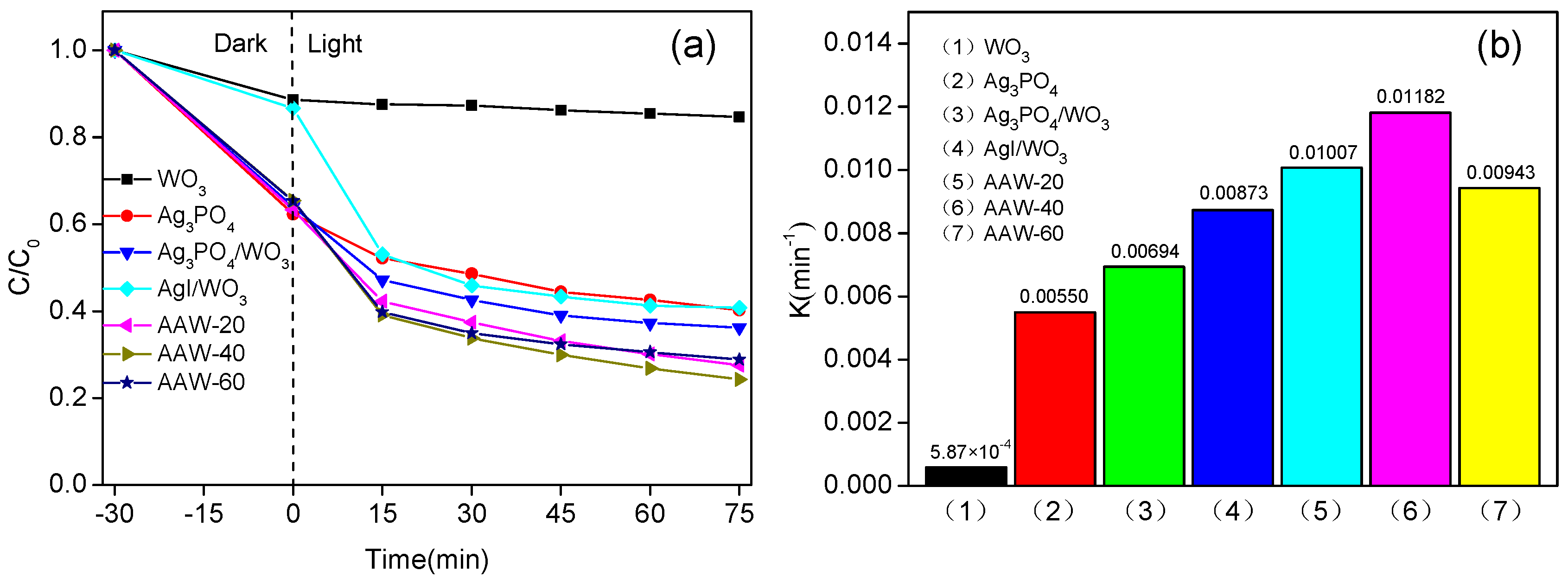
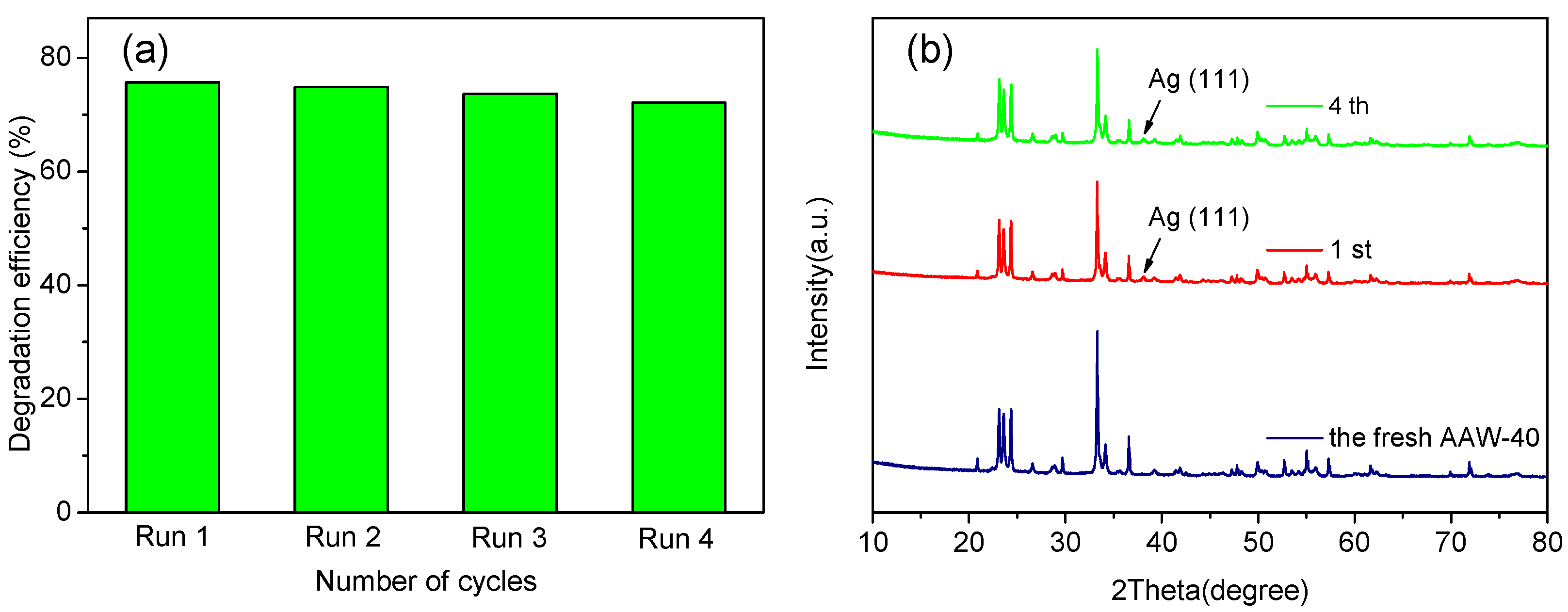
2.2. Photocatalytic Activity and Stability
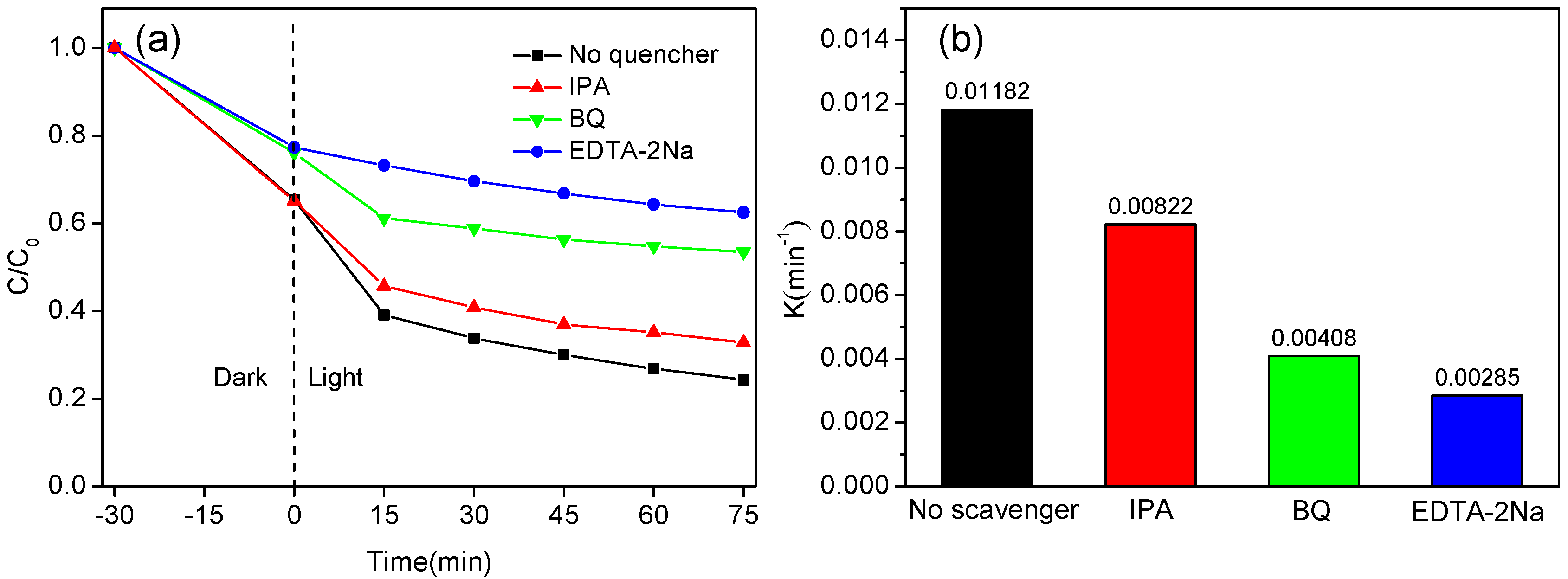
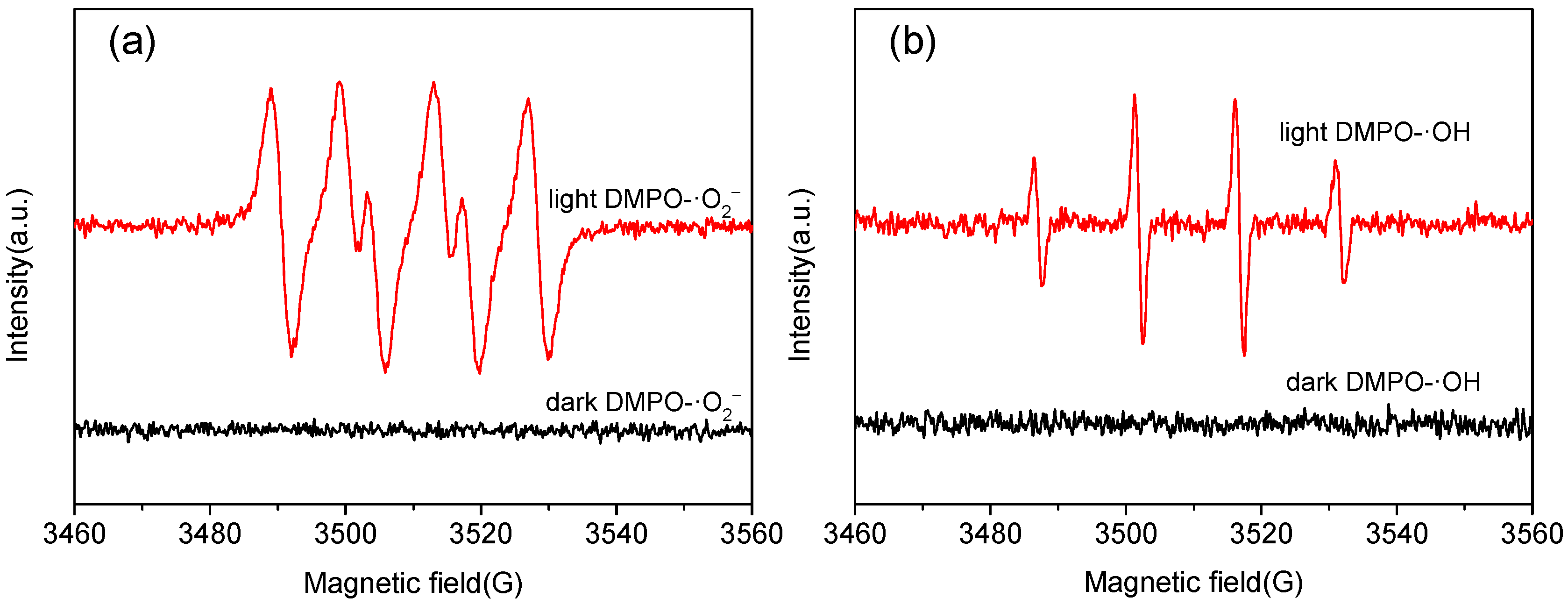
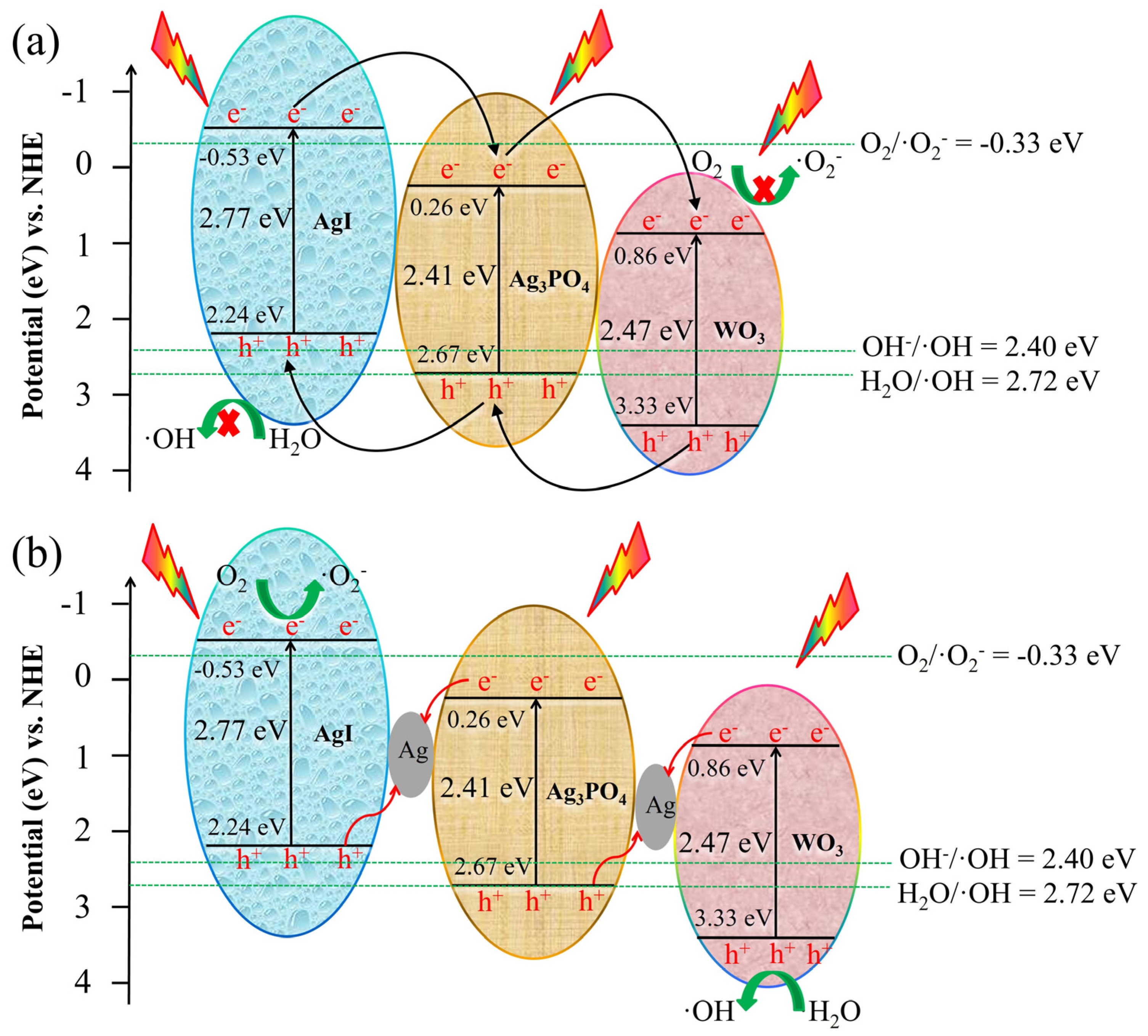
2.3. Photocatalytic Mechanism
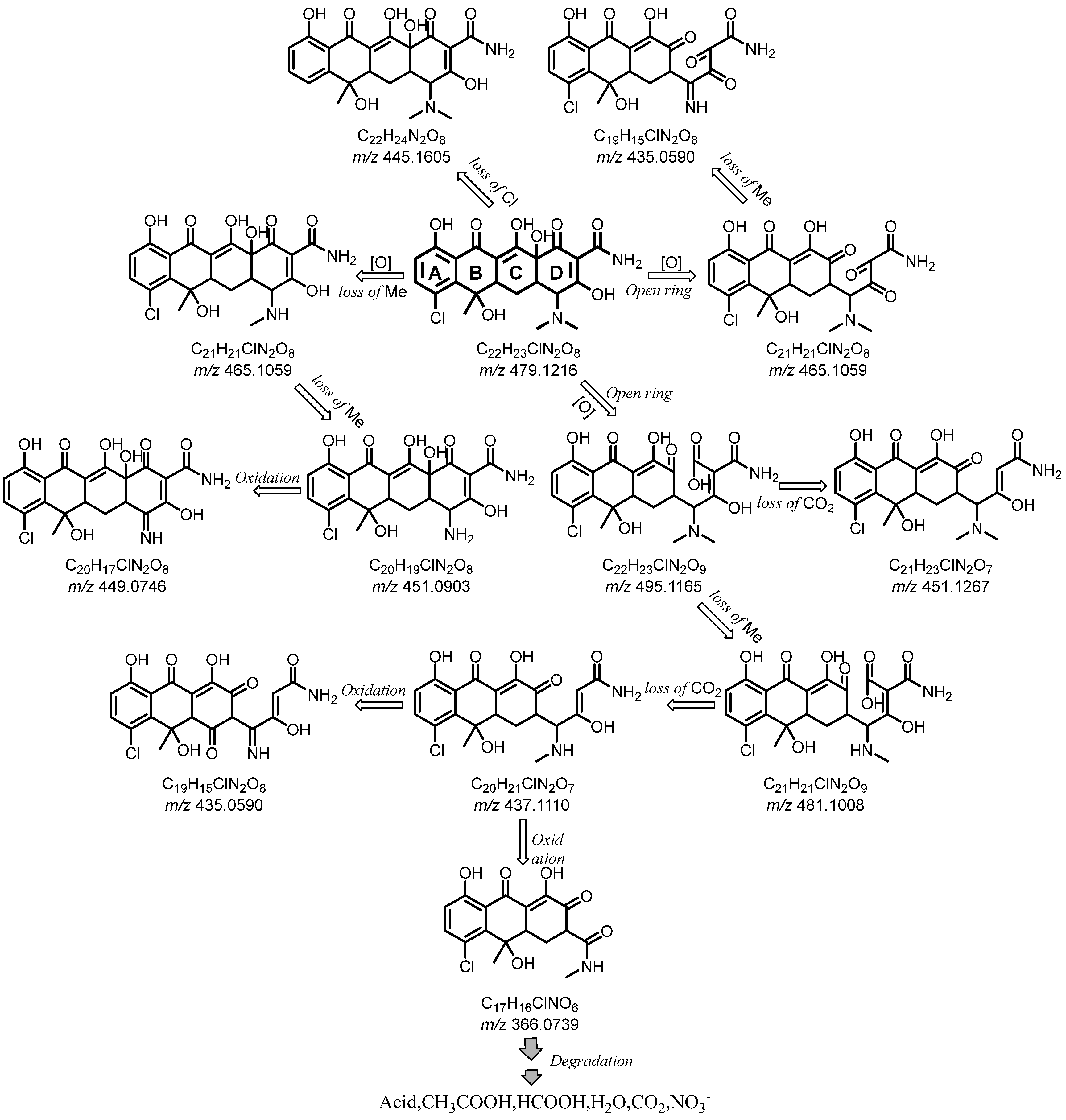
2.4. Possible Degradation Pathway of CTC
3. Materials and Methods
3.1. Synthesis of WO3 Nanosheets
3.2. Synthesis of the AgI/Ag3PO4/WO3 Photocatalysts
3.3. Characterization
3.4. Photocatalytic Degradation of CTC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, X.; Chen, Z.; Zhao, W.; Liu, C.; Qian, X.; Zhang, M.; Wei, G.; Khan, E.; Ng, Y.H.; Ok, Y.S. Recent advances in photodegradation of antibiotic residues in water. Chem. Eng. J. 2021, 405, 126806. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Lin, Y.; Hu, Y.H. Strategies of tuning catalysts for efficient photodegradation of antibiotics in water environments: A review. J. Mater. Chem. A 2021, 9, 2592–2611. [Google Scholar] [CrossRef]
- Zhao, M.; Bai, X.; Zhang, Y.; Yuan, Y.; Sun, J. Enhanced photodegradation of antibiotics based on anoxygenic photosynthetic bacteria and bacterial metabolites: A sustainably green strategy for the removal of high-risk organics from secondary effluent. J. Hazard. Mater. 2022, 430, 128350. [Google Scholar] [CrossRef]
- Liu, X.; Wang, F.; Wang, Q. Nanostructure-based WO3 photoanodes for photoelectrochemical water splitting. Phys. Chem. Chem. Phys. 2012, 14, 7894–7911. [Google Scholar] [CrossRef]
- Shan, Y.; Tang, X.; Shen, D.; Zhou, Z.; Wang, M. A review of tungsten trioxide (WO3)-based materials for antibiotics removal via photocatalysis. Ecotox. Environ. Safe. 2023, 259, 114988. [Google Scholar]
- Adhikari, S.; Mandal, S.; Sarkar, D.; Kim, D.-H.; Madras, G. Kinetics and mechanism of dye adsorption on WO3 nanoparticles. Appl. Surf. Sci. 2017, 420, 472–482. [Google Scholar] [CrossRef]
- Pereira, M.F.G.; Nascimento, M.M.; Cardoso, P.H.N.; Oliveira, C.Y.B.; Tavares, G.F.; Araújo, E.S. Preparation, microstructural characterization and photocatalysis tests of V5+-doped TiO2/WO3 nanocomposites supported on electrospun membranes. Inorganics 2022, 10, 143. [Google Scholar] [CrossRef]
- Dutta, V.; Sharma, S.; Raizada, P.; Thakur, V.K.; Khan, A.A.P.; Saini, V.; Asiri, A.M.; Singh, P. An overview on WO3 based photocatalyst for environmental remediation. J. Environ. Chem. Eng. 2021, 9, 105018. [Google Scholar] [CrossRef]
- Singh, S.A.; Madras, G. Photocatalytic degradation with combustion synthesized WO3 and WO3–TiO2 mixed oxides under UV and visible light. Sep. Purif. Technol. 2013, 105, 79–89. [Google Scholar] [CrossRef]
- Shandilya, P.; Sambyal, S.; Sharma, R.; Mandyal, P.; Fang, B. Properties, optimized morphologies, and advanced strategies for photocatalytic applications of WO3 based photocatalysts. J. Hazard. Mater. 2022, 428, 128218. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, J.; Yu, S.; Zhou, Y.; Yan, X. Ag loaded WO3 nanoplates for efficient photocatalytic degradation of sulfanilamide and their bactericidal effect under visible light irradiation. J. Hazard. Mater. 2016, 318, 407–416. [Google Scholar] [CrossRef]
- Zhao, B.; Shao, N.; Chen, X.; Ma, J.; Gao, Y.; Chen, X. Construction of novel type II heterojunction WO3/Bi2WO6 and Z-scheme heterojunction CdS/Bi2WO6 photocatalysts with significantly enhanced photocatalytic activity for the degradation of rhodamine B and reduction of Cr(VI). Colloids Surf. A 2023, 663, 131072. [Google Scholar] [CrossRef]
- Xiao, Y.; He, Z.; Wang, R.; Tao, X.; Li, B. Synthesis of WO3 nanofibers decorated with BiOCl nanosheets for photocatalytic degradation of organic pollutants under visible light. Colloids Surf. A 2019, 580, 123752. [Google Scholar] [CrossRef]
- Zhi, L.; Zhang, S.; Xu, Y.; Tu, J.; Li, M.; Hu, D.; Liu, J. Controlled growth of AgI nanoparticles on hollow WO3 hierarchical structures to act as Z-scheme photocatalyst for visible-light photocatalysis. J. Colloid Interf. Sci. 2020, 579, 754–765. [Google Scholar] [CrossRef]
- Wang, T.; Quan, W.; Jiang, D.; Chen, L.; Li, D.; Meng, S.; Chen, M. Synthesis of redox-mediator-free direct Z-scheme AgI/WO3 nanocomposite photocatalysts for the degradation of tetracycline with enhanced photocatalytic activity. Chem. Eng. J. 2016, 300, 280–290. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Y.; Xiong, Y.; Zhou, D.; Dong, S. An environmentally friendly Z-scheme WO3/CDots/CdS heterostructure with remarkable photocatalytic activity and anti-photocorrosion performance. J. Catal. 2017, 356, 1–13. [Google Scholar] [CrossRef]
- You, L.; Gao, M.; Li, T.; Guo, L.; Chen, P.; Liu, M. Investigation of the kinetics and mechanism of Z-scheme Ag3PO4/WO3 p–n junction photocatalysts with enhanced removal efficiency for RhB. New J. Chem. 2019, 43, 17104–17115. [Google Scholar] [CrossRef]
- Wang, C.; Wu, M.; Yan, M.; Shen, H.; Cai, F.; Hu, B.; Shi, W. Enhanced visible-light photocatalytic activity and the mechanism study of WO3 nanosheets coupled with Ag3PO4 nanocrystals. Ceram. Int. 2015, 41, 6784–6792. [Google Scholar] [CrossRef]
- Duan, Y.; Deng, L.; Shi, Z.; Zhu, L.; Li, G. Assembly of graphene on Ag3PO4/AgI for effective degradation of carbamazepine under Visible-light irradiation: Mechanism and degradation pathways. Chem. Eng. J. 2019, 359, 1379–1390. [Google Scholar] [CrossRef]
- Yan, M.; Wu, Y.; Zhu, F.; Hua, Y.; Shi, W. The fabrication of a novel Ag3VO4/WO3 heterojunction with enhanced visible light efficiency in the photocatalytic degradation of TC. Phys. Chem. Chem. Phys. 2016, 18, 3308–3315. [Google Scholar] [CrossRef]
- Tang, M.; Ao, Y.; Wang, C.; Wang, P. Rationally constructing of a novel dual Z-scheme composite photocatalyst with significantly enhanced performance for neonicotinoid degradation under visible light irradiation. Appl. Catal. B 2020, 270, 118918. [Google Scholar] [CrossRef]
- Tang, M.; Ao, Y.; Wang, C.; Wang, P. Facile synthesis of dual Z-scheme g-C3N4/Ag3PO4/AgI composite photocatalysts with enhanced performance for the degradation of a typical neonicotinoid pesticide. Appl. Catal. B 2020, 268, 118395. [Google Scholar] [CrossRef]
- Lv, J.; Dai, K.; Lu, L.; Geng, L.; Liang, C.; Zhu, G. Cu/Ag/Ag3PO4 ternary composite: A hybrid alloy-semiconductor heterojunction structure with visible light photocatalytic properties. J. Alloy. Compd. 2016, 682, 778–784. [Google Scholar] [CrossRef]
- Huang, H.; Li, Y.X.; Wang, H.L.; Jiang, W.F. In situ fabrication of ultrathin-g-C3N4/AgI heterojunctions with improved catalytic performance for photodegrading rhodamine B solution. Appl. Surf. Sci. 2021, 538, 148132. [Google Scholar] [CrossRef]
- Shen, Y.; Li, T.; Zhong, X.; Li, G.; Li, A.; Wei, D.; Zhang, Y.; Wei, K. Ppb-level NO2 sensing properties of Au-doped WO3 nanosheets synthesized from a low-grade scheelite concentrate. Vacuum 2020, 172, 109036. [Google Scholar] [CrossRef]
- Bi, W.; Huang, L.; Akbar, N.; Wu, Y. Interfacial ionic transport in natural palygorskite-Na0.60CoO2 nanocomposite mineral materials. Int. J. Hydrog. Energ. 2022, 47, 24439–24451. [Google Scholar] [CrossRef]
- Bulter, M.A. Photoelectrolysis and physical properties of the semiconducting electrode WO2. J. Appl. Phys. 1977, 48, 1914–1920. [Google Scholar]
- Zeng, J.; Wang, H.; Zhang, Y.; Zhu, M.K.; Yan, H. Hydrothermal synthesis and photocatalytic properties of pyrochlore La2Sn2O7 nanocubes. J. Phys. Chem. C 2007, 111, 11879–11887. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, X.; Ma, L.; Ning, X.; Zhan, L.; Xu, X.; Xu, L.; Zhang, L.; Ruan, H.; Zhang, Z. Fabrication of WO3/Ag2CrO4 composites with enhanced visible-light photodegradation towards methyl orange. Adv. Powder Technol. 2017, 28, 1018–1027. [Google Scholar] [CrossRef]
- Chen, X.J.; Dai, Y.Z.; Wang, X.Y.; Guo, J.; Liu, T.H.; Li, F.F. Synthesis and characterization of Ag3PO4 immobilized with graphene oxide (GO) for enhanced photocatalytic activity and stability over 2,4-dichlorophenol under visible light irradiation. J. Hazard. Mater. 2015, 292, 9–18. [Google Scholar] [CrossRef]
- Wu, D.; Long, M. Realizing visible-light-induced self-cleaning property of cotton through coating N-TiO2 film and loading AgI particles. ACS Appl. Mater. Interfaces 2011, 3, 4770–4774. [Google Scholar] [CrossRef]
- Shi, S.; Teng, F.; Hao, W.; Gu, W.; Yang, Z.; Zhao, F. Influence of crystal water on crystal structure, electronic structure, band structure, and charge separation of WO3·2H2O nanosheets. Inorg. Chem. 2019, 58, 9161–9168. [Google Scholar] [CrossRef]
- Chen, X.; Dai, Y.; Guo, J.; Liu, T.; Wang, X. Novel magnetically separable reduced graphene oxide (RGO)/ZnFe2O4/Ag3PO4 nanocomposites for enhanced photocatalytic performance toward 2,4-Dichlorophenol under visible light. Ind. Eng. Chem. Res. 2016, 55, 568–578. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, D.; He, T.; Wu, Z.; Chen, M. In-situ ion exchange synthesis of hierarchical AgI/BiOI microsphere photocatalyst with enhanced photocatalytic properties. CrystEngComm 2013, 15, 7556–7563. [Google Scholar] [CrossRef]
- Teng, P.; Li, Z.; Gao, S.; Li, K.; Copner, N.; Zhihai, L.; Yang, X. Flexible PAN-BiOI-AgI heterojunction nanofiber and the photocatalytic degradation property. Opt. Mater. Express 2022, 12, 1031–1042. [Google Scholar] [CrossRef]
- Wen, X.J.; Niu, C.G.; Guo, H.; Zhang, L.; Liang, C.; Zeng, G.M. Photocatalytic degradation of levofloxacin by ternary Ag2CO3/CeO2/AgBr photocatalyst under visible-light irradiation: Degradation pathways, mineralization ability, and an accelerated interfacial charge transfer process study. J. Catal. 2018, 358, 211–223. [Google Scholar] [CrossRef]
- Le, S.; Jiang, T.; Li, Y.; Zhao, Q.; Li, Y.; Fang, W.; Gong, M. Highly efficient visible-light-driven mesoporous graphitic carbon nitride/ZnO nanocomposite photocatalysts. Appl. Catal. B 2017, 200, 601–610. [Google Scholar] [CrossRef]
- Luo, J.; Chen, J.; Chen, X.; Ning, X.; Zhan, L.; Zhou, X. Construction of cerium oxide nanoparticles immobilized on the surface of zinc vanadate nanoflowers for accelerated photocatalytic degradation of tetracycline under visible light irradiation. J. Colloid Interface Sci. 2021, 587, 831–844. [Google Scholar] [CrossRef]
- Muhmood, T.; Xia, M.; Lei, W.; Wang, F. Erection of duct-like graphitic carbon nitride with enhanced photocatalytic activity for ACB photodegradation. J. Phys. D Appl. Phys. 2018, 51, 065501. [Google Scholar] [CrossRef]
- Ling, Q.; Kuang, P.; Zhong, X.; Hu, B. Adsorption-photoreduction behaviors and mechanisms of layered double hydroxide loaded on uranium(VI) removal. Dalton Trans. 2023, 52, 8247–8261. [Google Scholar] [CrossRef]
- Kang, F.; Sheng, G.; Yang, X.; Zhang, Y. Fabrication of two-dimensional Bi2MoO6 nanosheet-decorated Bi2MoO6/Bi4O5Br2 type II heterojunction and the enhanced photocatalytic degradation of antibiotics. Inorganics 2024, 12, 289. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Zeng, G.; Liang, J.; Chen, X.; Yu, H.; Wang, H.; Wu, Z.; Zhang, J.; Xiong, T. In-situ synthesis of direct solid-state dual Z-scheme WO3/g-C3N4/Bi2O3 photocatalyst for the degradation of refractory pollutant. Appl. Catal. B 2018, 227, 376–385. [Google Scholar] [CrossRef]
- Xue, W.; Huang, D.; Li, J.; Zeng, G.; Deng, R.; Yang, Y.; Chen, S.; Li, Z.; Gong, X.; Li, B. Assembly of AgI nanoparticles and ultrathin g-C3N4 nanosheets codecorated Bi2WO6 direct dual Z-scheme photocatalyst: An efficient, sustainable and heterogeneous catalyst with enhanced photocatalytic performance. Chem. Eng. J. 2019, 373, 1144–1157. [Google Scholar] [CrossRef]
- Arif, M.; Mahsud, A.; Ali, A.; Liao, S.; Xia, J.; Xiao, H.; Azam, M.; Muhmood, T.; Lu, Z.; Chen, Y. Unraveling the synergy of interface engineering α-MnO2/Bi2WO6 heterostructures and defective active sites for superdurable photocatalysis: Mechanistic insights into charge separation/transfer. Chem. Eng. J. 2023, 475, 146458. [Google Scholar] [CrossRef]
- Muhmood, T.; Cai, Z.; Lin, S.; Xiao, J.; Hu, X.; Ahmad, F. Graphene/graphitic carbon nitride decorated with AgBr to boost photoelectrochemical performance with enhanced catalytic ability. Nanotechnology 2020, 31, 505602. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, B.; Zhang, L.; Yu, J. In situ irradiated XPS investigation on S-Scheme TiO2@ZnIn2S4 photocatalyst for efficient photocatalytic CO2 reduction. Small 2021, 17, 2103447. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; Tang, J.; Wang, Q.; Meng, R.; Li, Q. Enhanced Photocatalytic Performances and Mechanistic Insights for Novel Ag-Bridged Dual Z-Scheme AgI/Ag3PO4/WO3 Composites. Inorganics 2025, 13, 222. https://doi.org/10.3390/inorganics13070222
Ma C, Tang J, Wang Q, Meng R, Li Q. Enhanced Photocatalytic Performances and Mechanistic Insights for Novel Ag-Bridged Dual Z-Scheme AgI/Ag3PO4/WO3 Composites. Inorganics. 2025; 13(7):222. https://doi.org/10.3390/inorganics13070222
Chicago/Turabian StyleMa, Chunlei, Jianke Tang, Qi Wang, Rongqian Meng, and Qiaoling Li. 2025. "Enhanced Photocatalytic Performances and Mechanistic Insights for Novel Ag-Bridged Dual Z-Scheme AgI/Ag3PO4/WO3 Composites" Inorganics 13, no. 7: 222. https://doi.org/10.3390/inorganics13070222
APA StyleMa, C., Tang, J., Wang, Q., Meng, R., & Li, Q. (2025). Enhanced Photocatalytic Performances and Mechanistic Insights for Novel Ag-Bridged Dual Z-Scheme AgI/Ag3PO4/WO3 Composites. Inorganics, 13(7), 222. https://doi.org/10.3390/inorganics13070222





